miR-29a对MPP+诱导PC12细胞凋亡影响及机制
2021-05-08谢帅刘欣欣王晓雪
谢帅 刘欣欣 王晓雪
[摘要]目的研究微小RNA-29a(miR-29a)对1-甲基-4-苯基吡啶离子(MPP+)诱导的PC12细胞氧化应激和凋亡的影响以及作用机制。方法不同浓度MPP+(0、100、300、500 μmol/L)诱导PC12细胞24 h,荧光定量PCR(qPCR)检测miR-29a的表达量,噻唑蓝比色法(MTT)检测细胞活力。PC12细胞分别采用0和500 μmol/L MPP+干预并转染anti-miR-29a或对照质粒,采用流式细胞术检测细胞的凋亡率。观察细胞活性氧(ROS)水平。TargetScan软件预测、双荧光素酶报告基因验证miR-29a与重组人转化生长因子诱导因子同源框2(TGIF2)的靶向关系。结果随着MPP+浓度的增加miR-29a表达量随之增加(F=590.067,P<0.05),而细胞活力则随之降低(F=153.561,P<0.05)。下调miR-29a表达可抑制PC12细胞凋亡(F=301.044,P<0.05),降低ROS水平(F=254.120,P<0.05)。TGIF2是miR-29a下游靶基因。结论miR-29a可能通过调控TGIF2抑制MPP+诱导的PC12细胞氧化应激和凋亡。
[关键词]帕金森病;微RNAs;PC12细胞;细胞凋亡;TGFB引导因子2
[中图分类號]R742.5;R342.2[文献标志码]A[文章编号]2096-5532(2021)01-0100-05
[ABSTRACT]ObjectiveTo investigate the effect and mechanism of action of microRNA-29a (miR-29a) on 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis of PC12 cells. MethodsPC12 cells were induced by different concentrations of MPP+ (0,100,300, and 500 μmol/L) for 24 hours, and then quantitative real-time PCR was used to measure the expression of miR-29a and MTT assay was used to measure cell viability. PC12 cells were treated with MPP+ (0 and 500 μmol/L) and transfected with anti-miR-29a or control plasmids, and flow cytometry was used to measure the apoptosis rate of the cells. The level of reactive oxygen species (ROS) in cells was observed. TargetScan software was used to predict and dual-luciferase reporter genes were used to verify the targeting relationship between miR-29a and recombinant human TGFB-induced factor homeobox 2 (TGIF2). ResultsThe expression level of miR-29a increased with the increase in the concentration of MPP+ (F=590.067,P<0.05), while cell viability decreased with the increase in the concentration of MPP+ (F=153.561,P<0.05). Downregulation of miR-29a expression inhibited the apoptosis of PC12 cells (F=301.044,P<0.05) and reduced the level of ROS (F=254.120,P<0.05). TGIF2 was a downstream target gene of miR-29a. ConclusionmiR-29a may inhibit MPP+-induced oxidative stress and apoptosis of PC12 cells by regulating TGIF2.
[KEY WORDS]Parkinson disease; microRNAs; PC12 cells; apoptosis; TGFB-induced factor 2
帕金森病(PD)是一种多发于中老年的慢性神经退行性疾病[1]。研究表明,PD的病理进展与细胞氧化应激[2]、凋亡率增加[3]、线粒体功能障碍[4]等紧密相关。微小RNA(microRNA,miRNA)是一类长度为18~25 nt的短链小RNA,目前已被证实在人类多种疾病包括PD的发生发展中发挥重要的调控作用[5-7]。研究表明,miR-29a在PD病人血浆中表达量明显减少[8-10],表明miR-29a可能参与PD的发生发展和恶化过程。然而,miR-29a在PD中的作用及其具体的作用机制尚不明确。因此,本文通过1-甲基-4-苯基吡啶离子(MPP+)诱导PC12细胞构建PD细胞模型,观察miR-29a对细胞中活性氧(ROS)以及凋亡率的影响,为阐明PD发病的病理机制提供理论基础。
1材料与方法
1.1实验材料
PC12细胞由北纳生物公司提供,胎牛血清、DMEM培养基购自美国Gibco公司;MPP+、2′,7′-二氯二氢荧光素二乙酸酯(DCFH-DA)和噻唑蓝比色法(MTT)试剂购自美国Sigma公司;Trizol试剂、PCR逆转录试剂盒、荧光定量PCR(qPCR)试剂盒、荧光素酶试剂盒购自上海吉玛制药技术有限公司;细胞凋亡检测试剂盒购自美国BD公司;Lipofectamine 2000购自美国Life Technologies公司;对照质粒、anti-miR-29a质粒(5′-UAACCGAUUUCAGAUGGUGCUA-3′)均购自广州锐博生物科技公司。7500荧光定量 PCR 仪购自美国ABI公司,荧光显微镜购自日本OLYMBUS公司。
1.2实验方法
1.2.1细胞培养方法以及模型构建将PC12细胞培养在含体积分数0.10胎牛血清和青霉素-链霉素的DMEM培养基中,培养条件设为体积分数0.05 CO2、37 ℃,待细胞生长达到90%融合时加入胰蛋白酶按1∶3传代。取对数期的PC12细胞以5×105个接种到6孔板中,过夜分别培养,加入终浓度为0、100、300、500 μmol/L的MPP+,放置在37 ℃培养箱继续培养24 h。
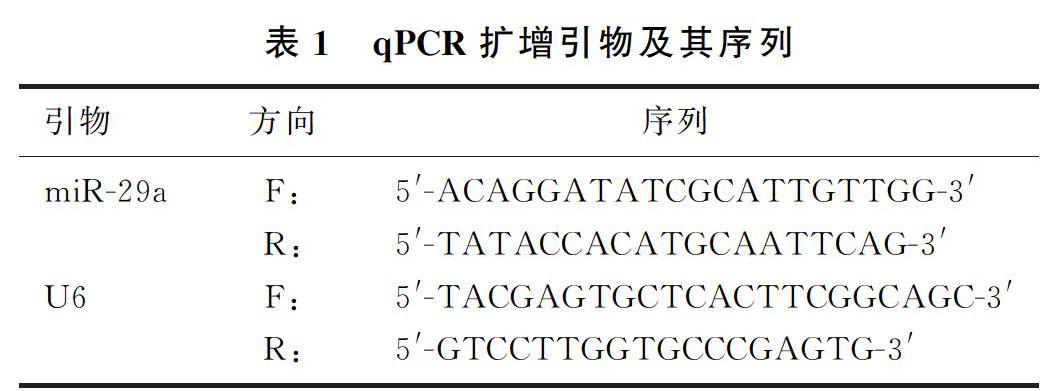
1.2.2qPCR实验收集PC12细胞,采用Trizol试剂提取细胞总RNA,根据逆转录试剂盒说明书操作,合成cDNA。以cDNA为模板,由上海生工生物技术公司设计、合成miR-29a和U6引物(见表1),进行qPCR扩增实验。以U6作为参照。反应条件:95 ℃、8 min;95 ℃、20 s,60 ℃、30 s,35个循环。使用2-ΔΔCt法计算miR-29a 的相对表达量。
1.2.3MTT实验分别向不同浓度MPP+溶液孵育24 h后的PC12细胞中添加100 μL MTT溶液(500 mg/L),37 ℃孵育4 h,除去MTT溶液,再加入150 μL二甲基亚砜,反应10 min,使用酶标仪测定490 nm波长处吸光度值(A),计算各组PC12细胞的存活率,细胞存活率(%)=(实验组A-空白组A)/(对照组A-空白组A)×100%。
1.2.4细胞的转染选取对数期PC12细胞,根据前期实验结果随机分为4组:0 μmol/L MPP++anti-NC组(A组)、0 μmol/L MPP++anti-miR-29a组(B组)、500 μmol/L MPP++anti-NC组(C组)、500 μmol/L MPP++anti-miR-29a组(D组)。A组、B组PC12细胞中加入0 μmol/L的 MPP+孵育24 h,并在Lipofectamine 2000介导下分别转染对照质粒和anti-miR-29a质粒。C组、D组PC12细胞中分别加入500 μmol/L 的MPP+孵育24 h后,再分别转染入对照质粒和anti-miR-29a质粒。继续培养48 h。
1.2.5细胞ROS的检测收集各組PC12细胞接种至24孔板中,每孔加入10 μmol/L 的DCFH-DA,37 ℃孵育30 min。荧光显微镜下随机选取5个视野分析平均绿色荧光强度。
1.2.6流式细胞术实验收集细胞,用PBS冲洗3次,调整细胞密度至1×109/L。根据细胞凋亡试剂盒说明书,在室温下每孔分别加入膜联蛋白 V-FITC和碘化丙啶各5 μL,混合均匀,孵育15 min。采用流式细胞仪检测凋亡率。
1.2.7双荧光素酶报告基因实验用TargetScan(http://www.targetscan.org/vert_71/)软件预测miR-29a与重组人转化生长因子诱导因子同源框2(TGIF2)基因的靶向关系,荧光素酶进一步验证。野生型(TGIF2-wt)荧光素酶报告载体(含有与miR-29a结合位点)以及突变位型(TGIF2-mut)荧光素酶报告载体均购买自广州锐博生物科技公司。将TGIF2-wt和TGIF2-mut分别与对照质粒以及anti-miR-29a质粒共转染入PC12细胞,37 ℃培养48 h,采用荧光素酶试剂盒测定PC12细胞相对荧光素酶活性。
1.3统计学分析
采用SPSS 20.0统计学软件进行数据处理,计量资料数据以±s表示,两组间比较采用t检验;多组均数间比较采用单因素方差分析,组间两两比较使用SNK-q检验。以P<0.05表示差异具有统计学意义。
2结果
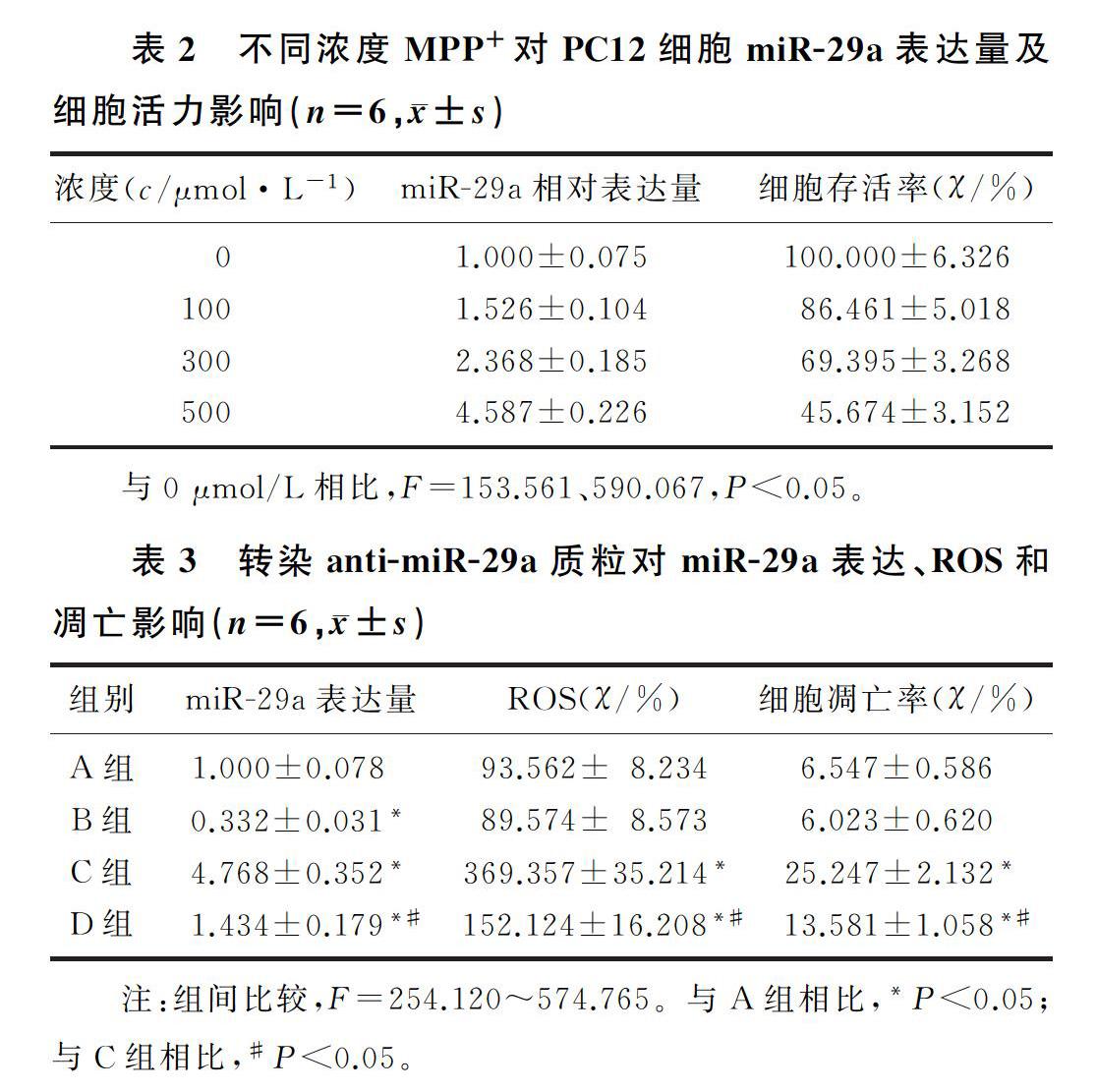
2.1不同浓度MPP+对PC12细胞miR-29a表达量及细胞活力的影响与0 μmol/L相比较,100、300、500 μmol/L 的MPP+诱导PC12细胞中miR-29a的表达量显著上升,差异具有统计学意义(F=590.067,P<0.05),PC12细胞存活率明显降低,差异具有统计学意义(F=153.561,P<0.05)。见表2。
2.2转染anti-miR-29a质粒对细胞miR-29a表达量的影响
与A组相比,B组细胞中miR-29a的表达量明显降低,C组、D组细胞中miR-29a的表达量明显增加,差异具有统计学意义(F=574.765,q=8.106~45.723, P<0.05);与C组相比,D组PC12细胞中miR-29a的表达量明显降低,差异具有统计学意义(q=40.457,P<0.05)。见表3。
2.3下调miR-29a对细胞ROS、凋亡的影响
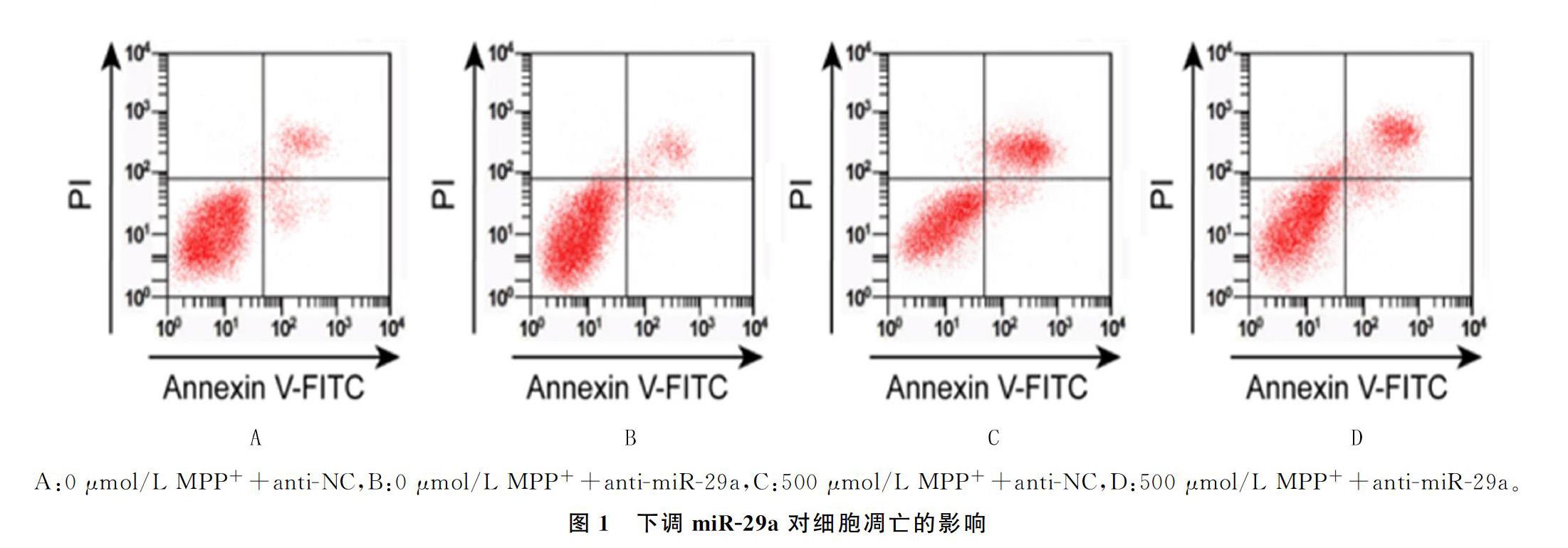
与A组相比较,B组PC12细胞中ROS水平、细胞凋亡率无显著差异, C组、D组PC12细胞中ROS水平、凋亡率明显增加(F=254.120、301.044,q=7.076~36.233,P<0.05);与C组相比较,D组ROS的水平、凋亡率显著降低,差异均具有显著性(q=13.629、26.247, P<0.05)。见图1和表3。
2.4miR-29a靶基因的预测和验证
TGIF2基因3′端非翻译区域与miR-29a靶向结合;与anti-NC与TGIF2-wt共转染相比,anti-miR-29a与TGIF2-wt共转染提高PC12细胞荧光素酶相对活性(t=21.266,P<0.05);但anti-miR-29a与TGIF2-mut共转染对PC12细胞荧光素酶相对活性无明显影响。见图2和表4。
3讨论
PD是一种运动障碍性疾病,随着年龄的增加患病风险增加,严重影响老年人的身体健康。研究表明,细胞凋亡在PD发生、恶化过程中具有重要作用[11-12]。研究证实,miRNA参与神经退行性疾病的发生发展,影响神经细胞的分化、凋亡等,从而影响病人的病理进程[13]。miRNA在PD病人中的表达量显著异常,表明miRNA可能在PD的病理演进过程中发挥重要的作用[14-16]。研究表明,miR-29a表达量异常在人类多种疾病中发挥重要作用,如结直肠癌、胰腺癌、胃癌、子宫内膜癌等[17-20]。邱峰等[21]通过μParaflo微流体芯片技术发现,miR-29a在PD病人表达量明显上调。韩凯等[5]的研究也进一步证实,miR-29a在PD病人外周血清中表达量较对照组明显增加,可作为PD临床诊断的潜在新血清标志物。但miR-29a在PD发生发展过程中的具体作用尚不清楚。
目前,采用MPP+诱导PC12细胞构建PD细胞模型是国内外公认的研究模型[22-23]。本文研究结果显示,miR-29a表达量随着MPP+浓度的增加逐渐升高,表明miR-29a可能参与PD的发生发展过程。MPP+浓度越高,miR-29a的表达量越高,其中浓度为500 μmol/L的MPP+诱导PC12细胞的存活率为(45.674±3.152)%,因此选择500 μmol/L的MPP+作为后续实验干预浓度。此外,本实验结果还显示,转染anti-miR-29a质粒可明显降低PC12细胞中miR-29a的表达量,表明轉染成功,可用于后续实验。本文进一步研究显示,抑制miR-29a的表达量可降低PC12细胞中ROS水平以及凋亡率,表明抑制miR-29a表达可能通过影响细胞的氧化应激水平和凋亡率,从而参与PD的病理进展。
miRNA主要通过靶向阻碍下游靶基因的转录或翻译,调控细胞的生物学特性,从而抑制或诱导疾病的病理进程[24]。TGIF2蛋白是三胺酸环延伸(TALE)蛋白家族成员之一,参与多种肿瘤如胶质瘤、前列腺癌、胃癌等的发生发展过程,对细胞的增殖、凋亡具有重要的调控作用[25-28]。TGIF2对胶质瘤和胶质瘤干细胞同样具有调控作用[29-33]。此外,TGIF2能够参与神经干细胞的调控,在调节神经系统发育中扮演重要角色。因此,推测TGIF2对神经细胞PC12的凋亡可能存在一定的调控作用[34-36]。在本实验中,在线预测显示miR-29a与TGIF2存在靶向结合位点,表明TGIF2可能是miR-29a的下游靶基因。双荧光素酶进一步验证表明,下调miR-29a与野生型TGIF2荧光素酶报告载体共转染可显著增加PC12细胞的荧光素酶活性,而与突变型TGIF2荧光素酶报告载体共转染对PC12细胞荧光素酶活性无显著影响,证实TGIF2是miR-29a的下游靶基因,表明miR-29a可能通过靶向TGIF2抑制MPP+诱导的PC12细胞凋亡和氧化应激反应,从而阻碍PD的进一步恶化。
综上所述,miR-29a在MPP+诱导的PC12细胞中表达量增加,且具有一定的浓度依赖性;下调miR-29a可能通过靶向TGIF2抑制MPP+诱导的PC12细胞凋亡,阻碍其氧化应激反应,这为PD基因靶向治疗提供新的方向。但本实验仅在细胞水平进行了相关研究,且本实验未涉及干扰TGIF2进行验证,本研究尚显不足,后续实验将对此进行补充。此外未来会进一步通过构建动物模型等深入探究miR-29a在PD发生发展中的作用及机制。
[参考文献]
[1]SARRAFCHI A, BAHMANI M, SHIRZAD H A. Oxidative stress and Parkinsons disease: new hopes in treatment with herbal antioxidants[J]. Current Pharmaceutical Design, 2016,22(2):238-246.
[2]YE Y, ZHU Z. MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinsons disease by targeting to Bim[J]. Brain Pathology, 2016,26(2):167-176.
[3]EXNER N, LUTZ A K, HAASS C A. Mitochondrial dysfunction in Parkinsons disease: molecular mechanisms and pathophysiological Consequences[J]. EMBO Journal, 2012,31(14):3038-3062.
[4]PILETI K, KUNEJ T. MicroRNA epigenetic signatures in human disease[J]. Archives of Toxicology, 2016,90(10):2405-2419.
[5]邱峰,吴越,曹辉,等. 帕金森病异常表达microRNAs的筛选及microRNA-1976作用机制的初步研究[J]. 临床神经病学杂志, 2017,30(3):171-174.
[6]BAN E, KWON T H, KIM A. Delivery of therapeutic miRNA using polymer-based formulation[J]. Drug Deliv Transl Res, 2019,9(6):1043-1056.
[7]SAMEC M, LISKOVA A, KUBATKA P, et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression[J]. J Cancer Res Clin Oncol, 2019,145(7):1665-1679.
[8]BAI X, TANG Y, YU M, et al. Downregulation of blood se-rum microRNA 29 family in patients with Parkinsons disease[J]. Sci Rep, 2017,7(1):5411.
[9]BARBAGALLO C, MOSTILE G, BAGLIERI G, et al. Specific signatures of serum miRNAs as potential biomarkers to discriminate clinically similar neurodegenerative and vascular-related diseases[J]. Cell Mol Neurobiol, 2020,40(4):531-546.
[10]OZDILEK B, DEMIRCAN B. Serum microRNA expression levels in Turkish patients with Parkinsons disease[J]. Int J Neurosci, 2020, https://doi.org/10.1080/00207454.2020. 1784165.
[11]HUANG Q, ZHU X, XU M. Silencing of TRIM10 alleviates apoptosis in cellular model of Parkinsons disease[J]. Biochem Biophys Res Commun, 2019,518(3):451-458.
[12]NAOI M, MARUYAMA W, SHAMOTO-NAGAI M. Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate α-synuclein cytotoxicity in disease-modifying therapy for Parkinsons disease[J]. J Neural Transm (Vienna), 2020,127(2):131-147.
[13]JUZWIK C A, S DRAKE S, ZHANG Y, et al. microRNA dysregulation in neurodegenerative diseases: a systematic review[J]. Prog Neurobiol, 2019,182:101664.
[14]GOH S Y, CHAO Y X, DHEEN S T, et al. Role of microRNAs in Parkinsons disease[J]. Int J Mol Sci, 2019,20(22):5649.
[15]ANGELOPOULOU E, PAUDEL Y N, PIPERI C. miR-124 and Parkinsons disease: a biomarker with therapeutic potential[J]. Pharmacol Res, 2019,150:104515.
[16]PATIL K S, BASAK I, DALEN I, et al. Combinatory microRNA serum signatures as classifiers of Parkinsons disease[J]. Parkinsonism Relat Disord, 2019,64:202-210.
[17]WANG A, DENG S, CHEN X, et al. miR-29a-5p/STAT3 positive feedback loop regulates TETs in colitis-associated colorectal cancer[J]. Inflamm Bowel Dis, 2020,26(4):524-533.
[18]DEY S, KWON J J, LIU S, et al. miR-29a is repressed by MYC in pancreatic cancer and its restoration drives tumor-suppressive effects via downregulation of LOXL2[J]. Mol Cancer Res, 2020,18(2):311-323.
[19]WANG L, SONG W. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer[J]. World Journal of Surgical Oncology, 2015,13(1):1-7.
[20]SUI D, YOU D. MiR-29a-5p inhibits proliferation and invasion and induces apoptosis in endometrial carcinoma via targeting TPX2[J]. Cell Cycle, 2018,17(10):1268-1278.
[21]韩凯. 血清miR-103a、miR-30b、miR-29a相對表达量对帕金森病的诊断效能[J]. 山东医药, 2017,57(11):72-74.
[22]ZHOU F, JU J, FANG Y, et al. Salidroside protected against MPP+-induced Parkinsons disease in PC12 cells by inhibiting inflammation, oxidative stress and cell apoptosis[J]. Biotech-nol Appl Biochem, 2019,66(2):247-253.
[23]ZENG R, LUO DX, LI H P, et al. MicroRNA-135b alleviates MPP+-mediated Parkinsons disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis[J]. J Clin Neurosci, 2019,65:125-133.
[24]ANGIUS A, UVA P, PIRA G, et al. Integrated analysis of miRNA and mRNA endorses a twenty miRNAs signature for colorectal carcinoma[J]. Int J Mol Sci, 2019,20(16):4067.
[25]VINCHURE O S, SHARMA V, TABASUM S, et al. Polycomb complex mediated epigenetic reprogramming alters TGF-β signaling via a novel EZH2/miR-490/TGIF2 axis thereby inducing migration and EMT potential in glioblastomas[J]. Int J Cancer, 2019,145(5):1254-1269.
[26]SHIJUN T, LINHUI W. MiR-181a promotes epithelial to mesenchymal transition of prostate cancer cells by targeting TGIF2[J]. European Review for Medical and Pharmacological Sciences, 2017,21(21):4835-4843.
[27]HU Yang, PU Qingha, CUI Bin, et al. MicroRNA-34a inhi-bits tumor invasion and metastasis in gastric cancer by targeting Tgif2[J]. International Journal of Clinical and Experimental Pathology, 2015,8(8):8921-8928.
[28]XU W, XUE R, XIA R, et al. Sevoflurane impedes the progression of glioma through modulating the circular RNA has_circ_0012129/miR-761/TGIF2 axis[J]. Eur Rev Med Pharmacol Sci, 2020,24(10):5534-5548.
[29]DIAO Y, JIN B, HUANG L, et al. MiR-129-5p inhibits glioma cell progression in vitro and in vivo by targeting TGIF2[J]. J Cell Mol Med. 2018,22(4):2357-2367.
[30]SONG C P, CONG J K, WANG M Z. MicroRNA-129-5p represses the growth and aggressiveness of oral squamous cell carcinoma via suppressing HMGB[J]. Kaohsiung J Med Sci, 2020,36(8):1-11.
[31]YAN L, SUN K, LIU Y, et al. Mir-129-5p influences the progression of gastric cancer cells through interacting with spock1[J]. Tumour Biol, 2017,39:1010428317706916.
[32]ZHANG P, LI J, SONG Y, et al. Mir-129-5p inhibits prolife-ration and invasion of chondrosarcoma cells by regulating sox4/wnt/beta-catenin signaling pathway[J]. Cell Physiol Biochem, 2017,42:242-253.
[33]SHEN N, HUANG X, LI J. Upregulation of mir-129-5p affects laryngeal cancer cell proliferation, invasiveness, and migration by affecting stat3 expression[J]. Tumour Biol, 2016,37:1789-1796.
[34]KURIBAYASHI H, TSUHAKO A, KIKUCHI M, et al. Role of transcription factor Tgif2 in photoreceptor differentiation in the mouse retina[J]. Exp Eye Res, 2016,152:34-42.
[35]LUCY C, LAUREN H, AMANIA S, et al. Assessing the role of the T-box transcription factor Eomes in B cell differentiation during either Th1 or Th2 cell-biased responses[J]. PLoS ONE, 2018:e0208343.
[36]WEN Lei, WEN Yuechun, KE Genjie, et al. TRPV4 regulates migration and tube formation of human retinal capillary endothelial cells[J]. BMC Ophthalmology, 2018,18(1):38-43.
(本文编辑 于国艺)
