Theoretical Calculation of Optical Properties of Zn2(OH)PO4 and Its Experimental Verification
2021-04-17,,2,,,,,,N
, ,2, , , , , , N
(1.College of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probe for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Normal University, Jinan 250014, China; 2.ASTRO-Century Education and Thchnology Co., Ltd., Zibo 255039, China; 3.Beijing Center for Crystal Research and Development, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China)
Abstract:The crystalline structure of Zn2(OH)PO4(ZPOH)belongs to orthorhombic unit cell structure, space group of P21212, which has no center of symmetry.Based on the plane-wave pseudopotential ab initio method, the electronic structure, linear refractive indices and second harmonic generation(SHG)coefficients of Zn2(OH)PO4 were calculated and the Sellmeier equations were also fitted.To verify the calculated values, ZPOH was synthesized using hydrothermal method, and the measured SHG effect is in accordance with the theoretical calculation.The ultraviolet(UV)cut-off edge and thermal stability of ZPOH were also reported for the first time.
Key words:Zn2(OH)PO4; hydrothermal method; SHG effect; plane-wave pseudopotential; ultraviolet(UV)cut-off edge; linear refractive index; thermal stability
0 Introduction
In the past few years, nonlinear optical(NLO)crystals have attracted much attention because of their important applications including photolithography at 193 nm and 157 nm, high-resolution photo emission spectroscopy, photonic devices fabrications etc[1-4].Up to now, many excellent NLO materials of Na5(B2P3O13)(NBP)[5], BaAlBO3F2(BABF)[6], KTiOPO4(KTP)[7], BaBe2BO3F3[8], Rb3Al3B3O10F[9],CsBe2BO3F2(CBBF)[10], β-Ba2B2O4(BBO)[11-12], LiB3O5(LBO)[13-15], KBe2BO3F2(KBBF)[16-17], Li4Sr(BO3)2[18], NH4B4O6F[19],Li2B6O9F2[20], AB4O6F[21],MB5O7F3[22], M2B10O14F6[23]and so on have been found one after another.Laser sources from ultraviolet to visible light have been realized basically for the requirement of information and communication, image processing, laser diode, optical signal processing and optical computing based on the applications of above NLO crystals.However, there is still a lack of deep ultraviolet(λ<200 nm)and near-infrared lasers at present while the searching of new NLO crystals is a challenging task.
By reviewing the development of NLO materials in the past decade, it′s well known that the way of searching new NLO crystals basically is of a "cooking mode" style, namely, synthesizing a series of compounds with non-centrosymmetric structure at first, then testing their second harmonic generation(SHG)effect, growing crystals, measuring the linear and nonlinear optical properties, and evaluating the potential applications finally."Cooking mode" approach has evident shortcomings.On one hand, we do not know what the SHG effect of the prepared compound is, thus making the synthesis work not target orientated.On the other hand, a promising NLO crystal is not only determined by its NLO effect, but also birefringence, transparent ranges, laser damage threshold, etc.So, the way of searching NLO materials has low work efficiency.Is there another strategy that might work better? The anionic group theory[24-25]can calculate the NLO optical properties in advance before the crystals are grown, which may be the best way forward.
CASTEP[26]is a plane-wave pseudo-potential total energy package, which is the density functional theory(DFT)in the local density approximation(LDA)[27]or gradient-corrected LDA based on the anionic group theory developed by Perew and Wang[28].The electronic band structures and optical properties of many borate crystals including LBO, BBO, CBO, CLBO and KBBF were calculated using CASTEP[29-30], and reasonable results consistent with the experimental data were obtained.
It is well known that phosphate has abundant structures, many NLO crystals such as KH2PO4(KDP), KD2PO4(DKDP), CsH2PO4(CDP), CsD2PO4(DCDP)and so on have been found from phosphate.This kind of compounds are made up of(PO4)3-group,among(PO4)3-groups which are interconnected by hydrogen bond.The SHG effect of these phosphate comes from(PO4)3-and(H2PO4)-groups according to the anionic theory[31-32].

Herein, optical properties of ZPOH were calculated using the plane-wave pseudopotential method, and then their validity through experiments were verified.The work demonstrates how to searching NLO materials by the strategy of theory guiding practice.
1 Experimental
All of the commercially available reagents were purchased and unpurified.Zn3(PO4)2·4H2O(99.9%, Beijing Yili Fine Chemicals Co., Ltd.), CH3COOH(AR, Beijing Daxing Chemical Plant).
1.1 Synthesis of ZPOH
ZPOH was synthesized by using the hydrothermal method reported by Akira Kawahara[33].At first, 2 g Zn3(PO4)2·4H2O is dissolved into dilute acetic acid solution(20 mL, pH=5), then the mixture is transferred into a stainless steel autoclave.After heating at 300 ℃ for 24 h to 72 h, the autoclave is cooled down to room temperature by the rate of 3 ℃·h-1.Finally, the microcrystals of ZPOH are obtained after the precipitate is washed three times with pure water.
1.2 Characterization
The as-prepared samples were characterized by scanning electron microscopy(JSM6301F)and X-ray powder diffractometer(XRD)(Bruker D8-advance X-ray Diffractometer with Cu Kαradiation), respectively.
The reflectance spectrum of ZPOH was measured using Cary5000 UV/VIS/NIR spectrophotometer, the measured band range is from 200 nm to 2 000 nm.
Thermogravimetric analyses were accomplished on a TA Instrument SDT2960 simultaneous DTA-TGA at the heating rate of 10 ℃·min-1under N2.
2 Results and discussion
Firstly, the theoretical first-principles calculations for ZPOH are performed by the plane-wave pseudopotential method implemented in the CASTEP package[34].The ion-electron interactions are modeled by the optimized normal-conserving pseudopotentials[35]for all elements.The local density approximation(LDA)[27]is adopted to describe the exchange and correlation(XC)potentials.The kinetic energy cutoffs of 900 eV and Monkhorst-Packk-point meshes[36]with a density of(2×2×3)points in the Brillouin zone are chosen.
As is known, the energy band gap of crystals is always underestimated by the DFT calculation with the XC functional.A scissors operator[37]is usually introduced to shift up all the conduction bands to agreeing with the measured band gap in order to calculate the optical coefficients.Then the second-order susceptibility[38]such as the SHG coefficientdijcan be calculated by the formula developed by Lin et al[29]according to the electronic structures.
2.1 Electronic structures and properties
Fig.1 shows the electronic band structures of ZPOH along the lines of high symmetry points in the Brillouin zone.It is clear that ZPOH is an indirect gap crystal with a calculated band gap of 2.28 eV.The partial density of state(PDOS)of ZPOH is shown in Fig.2, which is projected on the constitutional atoms, from which several electronic characteristics are shown:(i)The valence bands(VB)lower than-10 eV are mainly consisted of the isolated inner-shell orbitals of P 3s3p, O 2s, which have little interaction with neighbor atoms;(ii)3 d orbitals of zinc are located at about-5 eV;(iii)upper part of VB and the bottom of CB are primarily composed of the p orbitals of oxygen(2p)and phosphorus(3p), so the states on both sides of the band gap mostly consist of those from the P-O group.The optical properties are determined by the P-O group in both crystals owing to the optical response of a crystal primarily derived from the electronic transitions of VB and CB states close to the band gap[39], which agrees with the anionic group theory proposed by Chen[40]for the ultraviolet NLO crystals.

Fig.1 Calculated electronic band structures along the lines of high symmetry points in the Brillouin zone for ZPOH
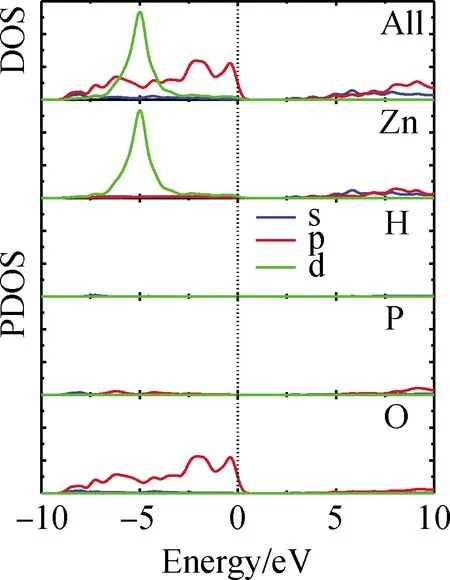
Fig.2 Partial density of state(PDOS)projected on respective species of atoms in ZPOH
2.2 Optical response
The nonlinear and linear optical properties were investigated by the scissors factors corrected by LDA approach.As the results show, the non-zero SHG coefficientd14is-0.05 pm·V-1, which is 0.13 times as that of KH2PO4(KDP,d14=-0.39 pm·V-1).In ZPOH crystal, the(PO4)3-tetrahedra connected by Zn-O polyhedra, the SHG effect mainly comes from the sum of the contributions of(PO4)3-groups.However, the framework consists of infinite straight chains of ZnO8octahedra alongc-axis, connected together by isolated(PO4)3-tetrahedra and distorted Zn-O pyramids, which leads to the crystal structure with large anisotropy.Furthermore, the direction of distortion of every group is almost mutually offsetting, which makes the SHG effect of ZPOH smaller finally.
The calculated refractive index for ZPOH crystal at the wavelength range of 240 nm to 1 500 nm is shown in Fig.3.Calculation shows that the birefringence(Δn)is 0.075 3 at the wavelength of 1 064 nm, indicating that this compound is easy to achieve the phase-matching condition in the UV spectral region.The sellmeier equations are as follows according to the refractive index:

Fig.3 Calculated refractive index for ZPOH crystal at the wavelength range of 240 nm to 1 500 nm
(1)
(2)
(3)
nx、ny、nz:principal refractive index,λ: incident wavelength.
In order to verify the calculation results,ZPOH microcrystals have been synthesized using the reported hydrothermal method[33].Different sizes of ZPOH microcrystals can be obtained by adjusting reaction time.Fig.4 shows the SEM image and XRD pattern of as-prepared ZPOH, respectively.As can be seen, the size of ZPOH microcrystals is on average 40 μm with a polygon prismmorphology, which corresponds to the crystallization rule of orthorhombic system.XRD pattern indicates that the as-prepared microcrystals are crystallized in orthorhombic system(P21212).Taking the same size microcrystalline KDP(KH2PO4)with ZPOH served as the standard, the SHG effect of ZPOH powder is measured using fundamental 1 064 nm light generated with a Q-switched Nd∶YAG laser.The results show that the SHG effect of ZPOH is about 0.2 times as that of KDP, which is in line with the calculation result.

Fig.4 SEM image(a)and XRD patterns(b)of as-grown ZPOH microcrystals, respectively
Fig.5 shows the connection between the particle size and SHG effect of ZPOH.As can be seen, the SHG effect of ZPOH increases gradually with the growth of crystal size, which means that ZPOH crystal could support phase-matching of SHG in the visible region[41].The result also agrees with the theoretical calculation.

Fig.5 Changing trend of SHG effect of ZPOH with particle size
To further evaluate ZPOH crystal, the reflection spectrum and thermo-stability of ZPOH have been measured and shown in Fig.6 and Fig.7, respectively.As is shown in Fig.6, the measured wavelength range is from 200 nm to 2 000 nm, ZPOH has a relatively wide optical transparency range from about 385 nm to 1 250 nm, the average light transmittance is about 90%, and the UV cut-off edge is 250 nm.We noticed that the curve is abnormal in the range of 200 nm to 250 nm, and the reason is twofold: the test light source may be unstable, and secondly, the sample may produce fluorescence.Fig.7 shows that ZPOH begins to break down at 565 ℃ and end at 600 ℃, the total weight loss is about 4.02%.According to the theory of inorganic chemistry, orthophosphate has good thermal stability, so the decomposition equation of ZPOH is as follows:

Fig.6 Measured curve of reflectance spectrum of ZPOH
2Zn2(OH)PO4=ZnO+Zn3(PO4)2+H2O
(4)
To verify above corollary,the pyrolysis experiment of ZPOH has been carried out.As known according to Fig.7, ZPOH begins to break down at 565 ℃ and end at 600 ℃, so we adopt 700 ℃ as the sintering temperature.The detailed process is as follows: Take 1 g ZPOH microcrystals into muffle furnace and heat at 700 ℃ for 10 h.After sintered residue cools down into room temperature, they are weighted and characterized by XRD, respectively.The residue is 0.960 1 g, namely, lost 3.99% which is in line with the TG test.XRD data confirm that the residue is mixture of ZnO(PDF#79-2205)and Zn3(PO4)2(PDF#76-0518)(see Fig.8), the results are consistent with the decomposition equation.

Fig.7 Thermal gravity analysis curve of ZPOH

Fig.8 XRD patterns of the decomposition product of ZPOH
The weight loss is mainly H2O if ignoring the volatilization of ZnO at high temperature.The loss in theory is 4%, which agrees with above experiment result.Considering the high decomposition temperature, ZPOH has good thermal stability and meets the requirement of NLO material.
An electric analytical balance is employed to determine the density of ZPOH by using the drainage method, and the measured density is 4.012 g/cm3, in line with the cell parameters reported by Akira Kawahara[33].
3 Conclusion
The optical properties of ZPOH and deduced its Sellmeier equations were calculated by using the plane-wave pseudopotential ab initio method.The theoretical predictions have been confirmed by the experimental data successfully.The results provide the strongest confirmation that the way of searching NLO materials from theory to practice is an effective shortcut.
