Comparative filtration and dewatering behavior of vitrinite and inertinite of bituminous coal:Experiment and simulation study
2021-04-08AnPingWenchengXiaYaoliPengGuangyuanXie
An Ping,Wencheng Xia,Yaoli Peng,Guangyuan Xie
School of Chemical Engineering and Technology,China University of Mining and Technology,Xuzhou 221116,Jiangsu,China
Keywords:Coal maceral groups Filtration Dewatering Simulation
ABSTRACT The filtration and dewatering of fine clean coal not only ensure industrial water recycle in coal washing plant,but also reduce the moisture of coal product in order to meet the requirements of combustion or coking industry.Fine clean coal is mainly composed by organic matter,and the property difference of different organic matter determines the filtration and dewatering behavior.In this investigation,vitrinite and inertinite were separated from a clean bituminous coal,and the comparative filtration and dewatering behavior of vitrinite and inertinite were conducted.The results showed that inertinite has lower dewatering rate and higher filter cake moisture than vitrinite.The analysis of filter cake structure showed that inertinite particle is easier to be broken into small particles due to the difference of mechanical properties,thus forming more compact filter cake than vitrinite.The analysis of particle surface properties showed that vitrinite is more hydrophobic than inertinite,which makes water easier drained from filter cake.The simulation study showed that the structure of inertinite is more porous than that of vitrinite,and the interaction between inertinite and water is stronger than that between vitrinite and water.This study provides a theoretical basis for improving coal dewatering by selectively improving coal maceral hydrophobicity.
1.Introduction
Wet coal beneficiation is widely used in coal preparation plants.After separation,wet coal is usually dewatered before utilization,except coal used as coal-water slurry.The coarse coal are usually dehydrated by centrifugation,and the fine coal are usually dewatered by filtration [1,2].With a large-scale application of mechanized mining,the content of fine particles in the raw coal is increasing[3],and the dewatering problem of fine coal is becoming more and more serious.For coking coal,the increase of moisture content will lead to the prolongation of coking formation time and the decrease of coke output.For steam coal,the moisture will cause heat loss and increase the boiler failure rate [4-8].
In order to meet the moisture requirement of commercial coal,fine clean coal is generally mixed with other coarse clean coal products in different proportion and then forwarded to industrial usage.If the moisture content is still too high,the seller has to reduce the blending proportion to meet the commercial requirements.A large amount of fine coal can only be sold cheaply or be discarded because the moisture content cannot meet commodity standard.In addition,during transportation of coal products to other areas,the moisture in coal will increase transportation costs,pollute the environment along the railway,and easily freeze in the train container in winter [9-11].Therefore,efficient fine coal dewatering technology is very important in coal preparation plants.
In the dewatering industry,reducing the content of fine particles in the slurry is considered to be the most direct method to solve the problem of ultra-fine slurry filtration.However,mixing coarse coal directly into slurry will make the production process complicated [12].Hence,adding flocculants in the slurry seems more friendly,and flocculants can promote the formation of fine particles into large-diameter particles,which can improve the filtration and dewatering efficiency [13-17].
Surfactants can enhance the dewatering efficiency by improving the wettability of particle surface and reducing the surface tension of the slurry [1,18,19].In order to make this method more economical,the combination of surfactants and flocculants has been studied to improve the efficiency of dewatering [20-23].
For the dewatering of fine clean coal,scholars have different schemes [3,23,24].However,current research focuses more on the use of filter aids,while ignoring the influence of coal properties on dewatering.It is well-known that coal is a mixture of organic and inorganic components.The properties of each component are quite different [25-27].The content of minerals in fine clean coal is small,so the difference in organic properties (coal maceral groups) dominates the filtration and dewatering processes.In this paper,vitrinite and inertinite were separated from a clean bituminous coal,and the comparative filtration and dewatering behavior of vitrinite and inertinite were conducted.The results of this paper may be useful for understanding the existing dewatering theory and providing new ideas for the development of new filter reagent.
2.Experiment
2.1.Samples
The two samples,vitrinite-riched (named group V) and inertinite-riched (named group I) used for the study were separated from a clean bituminous coal of Shanghai Miao coal preparation plant in Inner Mongolia,China.According to the density difference between the maceral groups,the two samples with different contents of maceral groups were obtained for experiments.The contents of maceral groups in the two samples are shown in Table 1.The two samples are approximately considered to be pure maceral groups as vitrinite and inertinite.The proximate and ultimate analysis of maceral groups are shown in Table 2.
2.2.Dewatering tests
The dewatering tests were conducted on a self-made laboratory dewatering device,as shown in Fig.1.First,the 6 g sample and reverse osmosis water were stirred at a mass concentration of 20% for 5 min to prepare slurry.Then,the slurry was added into the filter through the feeding port above the filter.Finally,the feed valve was closed quickly and the pump started to inflate.The air pressure is 98 kPa (1 kg/cm2).The filtrate is collected by a beaker placed on an electronic balance and the mass is recorded at regular intervals.The total filtration time is 150 s.After filtration,the flange in the middle of the filter was opened to remove the filter cake.The filter cake was dried at 90 and 105°C for 2 h to calculate the free moisture and absorbed moisture,respectively(These temperature takes into account the boiling point of water at 100°C and the heterogeneity of the temperature inside the oven).
2.3.Filter cake structure analysis
The dried filter cake was made into thin slices with epoxy resin to observe the pore structure of filter cake.Zeiss-DMRXP polarizing microscope,which can emit ultraviolet,was used to observe the filter cake at a magnification of 500x.
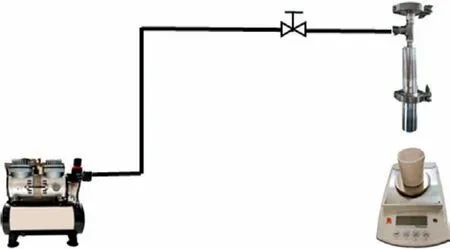
Fig.1.Schematic diagram of filtering device.
2.4.Particle size analysis
The samples were analyzed by GSL-1000 laser particle size analyzer (Liaoning Instrument Research Institute Co.,Ltd.).Ethanol was used as the dispersion medium in the experiment,and the shading rate of the solution was between 25% and 35%.
2.5.Fourier transform infrared spectroscopy (FTIR) experiments
Nicolet iS5 infrared spectrometer was used for the FTIR analysis of vitrinite and inertinite.The sample and KBr were ground in a ratio of approximately 1:100,which were subjected to a pressure of 20 MPa to obtain the slices of samples.The samples were examined in the chamber of spectrometer.The FTIR spectrum was scanned 16 times in the range 4000-400 cm-1,with the resolution of 4 cm-1.
2.6.Contact angle test
The JC2000D1 contact angle measuring instrument was used to measure the sample contact angle.The 1-2 g coal samples and boric acid were pressed into thin sections at 60 MPa.The contact angle was measured by the sessile drop method.Each sample was measured three times,and the final results were averaged.
3.Results and discussion
3.1.Dewatering results
The samples were dehydrated and the cumulative mass-time curve of filtrate is shown in Fig.2.
In the first half of filtration,with the increase of time,the cumulative mass of filtrate increased rapidly.After reaching the inflec-tion point,the cumulative mass of filtrate basically did not change with time.The slope of the rising curve can be used to represent the filtration rate.Although the slope is always changing,it can be seen that the dehydration rate of group V is much higher than that of group I,which shows that the type of maceral group has an obvious effect on the dewatering process.In this paper,the time corresponding to the inflection point is regarded as the starting time of the process of pressure air replacing the liquid in the cake capillary.It can be seen that the group V started the replacement process at 27 s due to its faster dewatering rate,and replaced 1.77 g filtrate in the following time,while the group I started the process at 45 s,and only 0.32 g filtrate was replaced in the whole process.The free moisture and adsorbed moisture of group I are 45.02% and 0.9% respectively,which are significantly higher than that of vitrinite group and further prove that the type of maceral group has an obvious effect on the dewatering process.

Table 1 Maceral groups content of experimental samples.

Table 2 Proximate and ultimate analysis of group V and I.
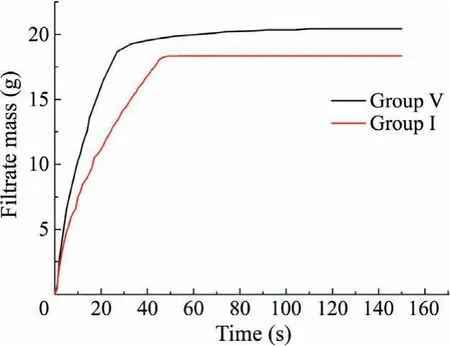
Fig.2.Cumulative mass-time curve of filtrate.

Fig.3.Particle diameter-volume fraction curve of the samples.
3.2.Particle size composition analysis
In the process of filtration,the coarse particles form the basic skeleton structure,and the fine particles fill the pore of skeleton continuously until the end of filtration [28].The high content of fine particles will form a dense filter cake,which will hinder the water exhausted.Therefore,the particle size composition significantly affects the dewatering process.Fig.3 and Table 3 show the particle size composition and distribution of groups V and I.It can be seen that the vitrinite particles are coarser than the inertinite,which is caused by the different mechanical properties of the maceral groups because the inertinite is more likely to be broken into fine particles during crushing and grinding [29].
3.3.Analysis of filter cake structure
Further understanding of the influence of coal maceral groups on the dewatering was gained by studying the structure of filtercake and the distribution of maceral groups in the filter cake.The vitrinite is dark gray under the oil lens reflection light,while the inertinite is light gray or white [30].Under the ultraviolet light,the vitrinite and inertinite are black,and the epoxy resin in the filter cake pore is green,which can be used for distinguishing the distribution of the maceral groups and pores in the filter cake.

Table 3 Particle size of samples.
From the representative picture (Figs.4 and 5),it can be seen that there are more large-scale vitrinite and a small part of vitrinite-inertinite intergrowth in group V,and there is basically no significant inertinite.The macerals clasts dominated by the vitrinite fill in the gaps between these large particles.However,the large particles in group I is less than that in group V,which is due to the poor mechanical properties of inertinite.Besides,a small amount of vitrinite in the form of large particles is found in group I.
The difference of filter cake structure can be better understood by calculating the average pore proportion,the average pore diameter,and the average pore perimeter of the cake sample (Table 4).The results show that the inertinite owns a bigger average pore perimeter in the filter cake and a smaller average pore diameter compared with the vitrinite.The average pore proportion of two samples are similar.It means the maceral groups have a significant effect on the structure of filter cake.For this study,the coal sample was fine enough to form a similar dense filter cake,which caused the porosity to be basically similar.However,the inertinite particles are smaller,making it easier to fill in the pores,which will reduce the pore diameter and increase the average perimeter of pore.A smaller pore diameter and longer pore perimeter will hinder the filtration process,resulting in the decrease of filtration speed and the saving of moisture in filter cake [31,32].
3.4.Analysis of vitrinite and inertinite surface properties
The difference in oxygenated groups between vitrinite and inertinite can be determined by peak fitting of 1800-950 cm-1absorption band in FTIR spectrum[33-36].The fitting result were shown in Fig.6 and Table 5.
The 1800-950 cm-1absorption band is divided into seven parts:carboxyl C=O,aromatic C=C,alcohol OH,CH3or CH2,phenol OH,alkyl ether C-O-C and aryl ether C-O-C.The content of phenol OH in vitrinite was significantly higher than that in inertinite.The content of alcohol OH and alkyl ether C-O-C in inertinite were 5.52% and 4.12%,which are slightly lower than that in vitrinite.Meanwhile,in inertinite,the content of carboxyl C=O and aryl ether C-O-C is almost double of that in vitrinite.
The hydrophilicity of oxygenated groups in coal is-COOH>-OH>-C-O-C->-C=O [37,38].The oxygenated groups on the surface of coal can affect the surface properties.These oxygenated groups will form hydrogen bonds with water molecules and form a dense hydration film on coal particle surface [39],which will strongly impact the hydrophobicity of coal particles and its dewatering process.In addition,the water adsorption on coal surface is very tight,which makes it difficult to remove by conventional mechanical dewatering.
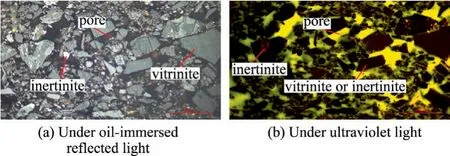
Fig.4.Group V under oil-immersed reflected light and ultraviolet light.
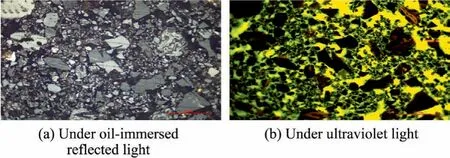
Fig.5.Group I under oil-immersed reflected light and ultraviolet light.
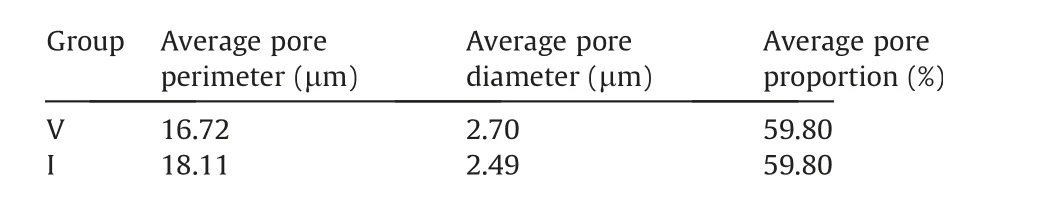
Table 4 Structural parameters of the filter cakes.
The contact angle was further used to quantitatively evaluate the wettability of groups V and I.The contact angle measurements results indicate that the contact angle of groups V and I were 81.24° and 56.41°,respectively.The contact angle of vitrinite is higher than that of inertinite,which can be attributed to the difference of oxygen content and the oxygenated groups on the coal surface between the vitrinite and inertinite.The vitrinite and inertinite have different wettability,which will also affect the dewatering process [19,40].
3.5.Molecular simulation
The molecular simulation is to use the maceral groups molecular model to simulate the coal-water interaction process,which can eliminate the influence of minerals on the experiment,and can more clearly understand the differences in the interaction of maceral groups and water.In this investigation,the models used in the simulation were studied in the authors’ previous study,which can reflect the characteristics of the two maceral groups[41].The molecular formulas of vitrinite and inertinite are C126H99-O12N3S2and C131H98O17,respectively.In order to simulate the real solution environment,the O-H bond on the carboxyl group of the inertinite was broken to form H+and cation of the inertinite.
Coal has no crystal structure,so an amorphous substrate was constructed using twenty molecular models to represent coal.To reduce the computation time,the bottom of amorphous substrate was constrained [42].Amorphous substrate optimization uses smart algorithm and a constant particle number,pressure,and temperature (NPT) ensemble.The water environment is represented by 2000 water molecules placed above the coal model.At the top of water molecular,a vacuum slab with a thickness of 200 Å and 2000 fixed water were placed to eliminate the boundary condition in z axis.
The Forcite in Material Studio is used to calculate the coal water interaction process,and the DREIDING force field is used to calculate the interaction.The constant particle number volume and temperature (NVT) ensemble using a Nosé-Hoover thermostat was used in calculation [43-45].The calculation time step is 1 fs and the total calculation time is 500 fs.Ewald summation and atom-based method are used to calculate the long electrostatic interaction and van der Waals interaction.As an example,Fig.7 shows the inertinite and water before and after simulation and the top water layer was omitted.

Fig.6.Curve-fitted FTIR spectrum for 1800-950 cm-1.
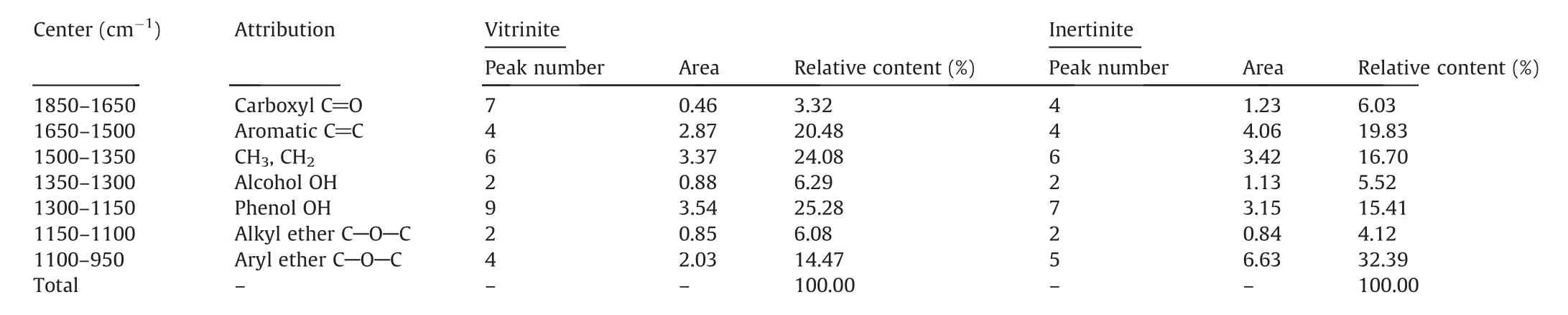
Table 5 Curve-fitted FTIR spectrum for 1800-950 cm-1 band.
3.5.1.Free volume fraction
Free-volume theory [46]treats the voids inside the material that probe molecules can freely enter and exit as free volume,which was originally used to characterize the properties of amorphous polymer materials.Using the solvent accessible surface as the surface and water molecules as molecular probes,the free volume and surface area of the vitrinite and inertinite models were calculated.The grid resolution was selected to ultra-fine and the grid interval,van der Waals scale factor and max solvent radius are 0.15,1,and 2 Å,respectively.Water molecular radius was regarded as 1.4 Å.The solvent accessible surfaces of the two maceral groups are shown in Fig.8,and the calculation results are shown in Table 6.
The results show that the surface area of inertinite particle is larger and contains more internal pores,compared with vitrinite.The larger specific surface area will make the water contact with inertinite more easily,and these internal pores will retain more water after fully wetting,resulting in the increase of water content in filter cake.
3.5.2.Coal-water interaction energy and hydrogen bond
The combining ability of water and maceral groups can be reflected by the interaction energy and the number of hydrogen bonds.Interaction energy can be calculated by Eq.(1).

where Eintis the interaction energy between coal and water;Etthe total potential energy of coal-water system;Ecoalthe total potential energy of coal;and Ewaterthe total potential energy of water.
A high negative value of Eintindicates a more favorable interaction between coal and water.Table 7 shows that the interaction energy in inertinite-water system is significantly higher than that in vitrinite-water system,which indicates that the inertinite has stronger interaction with water than the vitrinite.
The distance angle algorithm is used to calculate the number of hydrogen bonds.When hydrogen bond is formed,the distance between hydrogen atom and acceptor atom should be less than 2.5 Å,and the angle between donor,hydrogen atom,and donor atom should be exceeded than 120° [47],and the hydrogen bond was regarded as formed.The number of hydrogen bonds in inertinite-water system is 51 more than that in vitrinite-water system.On the one hand,it is determined by the type and quantity of oxygenated groups in inertinite.On the other hand,inertinite has a larger solvent accessible surface,which can better contact with water and form more hydrogen bonds.These conclusions can prove that inertinite is more hydrophilic than vitrinite and has a more negative effect on the filtration process.
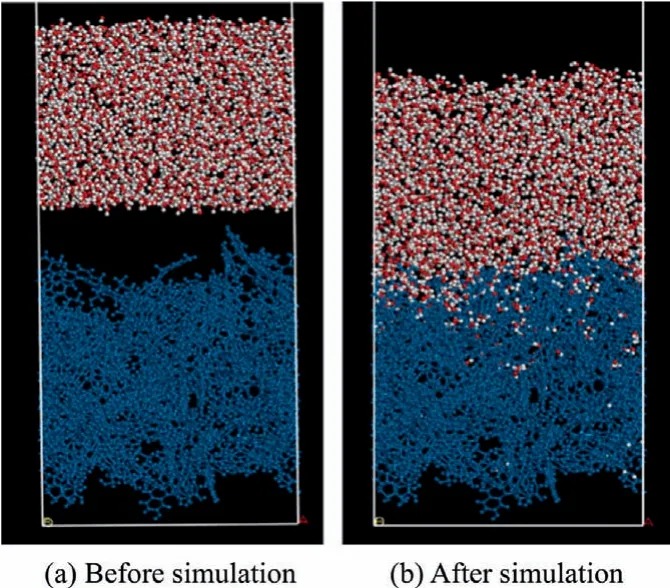
Fig.7.Inertinite-water model before and after simulation.
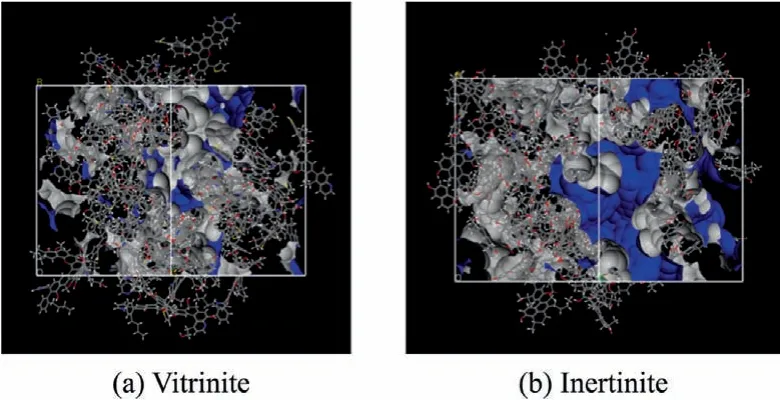
Fig.8.Solvent accessible surface of vitrinite and inertinite (blue is visible outer surface and gray is inner surface).

Table 6 Free volume and surface area of vitrinite and inertinite models.
3.5.3.Dynamics behavior of water molecules
Water has different behaviors on the surface of different maceral groups.To determine the effect of maceral groups on the diffusion of water molecules,the diffusion coefficient (D) was calculated according Eqs.(2)-(4) [48].
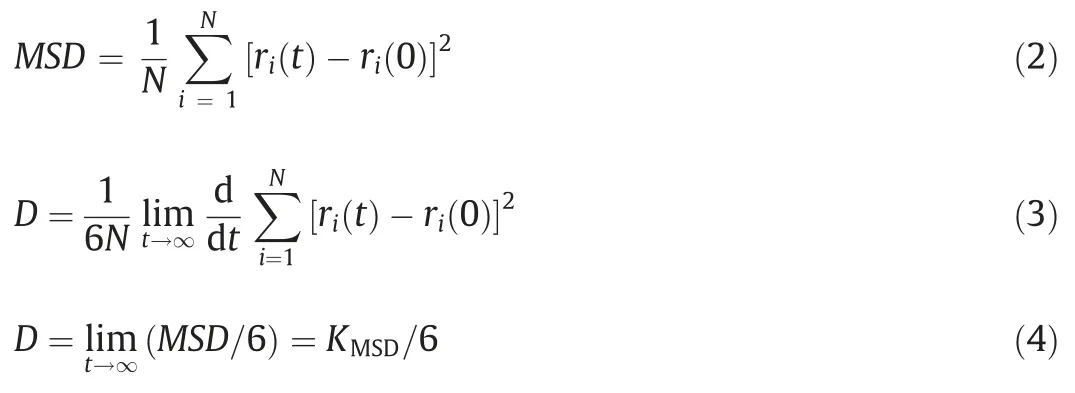
where MSD is the mean square displacement;N the number of diffusion molecules;ri(t) and ri(0)the position vector of a molecule at the t and initial time,respectively;and KMSDthe slope of the MSD curve.
For the whole system,the higher the D value is,the stronger the diffusion of water molecules in the system is.The MSD-time curve of water molecule is shown in Fig.9.The Dvitriniteand Dinertiniteis 4.7 × 10-5and 7.6 × 10-5,respectively.The results show that the diffusion of water molecules on the surface of vitrinite is not as intense as that on the surface of inertinite,because there are fewer pores,less oxygenated groups and better hydrophobicity in vitrinite.If water molecules are not easy to diffuse on the surface of coal,it can be quickly eliminated under the effect of pressure.
3.6.Mechanism of the difference in dewatering behavior between vitrinite and inertinite
Compared with the vitrinite,the inertinite sample has lower filtration speed and higher moisture content in filter cake.With thegradual progress of filtration,the filter cake becomes more and more dense,and the dewatering becomes more and more difficult.The average mass specific resistance is an index used to characterize the macro filtration characteristics of filter cake.Its physical meaning is the resistance of unit mass particles on unit filtration area when they are filtered under a certain pressure.The worse filtration performance corresponds to the larger calculated specific resistance.According to the constant pressure incompressible Darcy equation (Eq.(5)) [49,50],the average mass specific resistance of filter cake in the filtration process was calculated (The density of filtrate ≈1 g/cm3and filtrate viscosity 0.001 N·S/m2).

Table 7 Interaction energy and hydrogen bond number between vitrinite/inertinite model and water.

where t is the time of filtrate;V the filtrate cumulative volume;α the average mass specific resistance;μ the filtrate viscosity;c the suspension concentration;A the filter cake cross-sectional area;R the resistance of filter cloth;and Δp the pressure drop on both sides of filter cake.
Through the calculation,the specific resistance of group V and I is 233512 and 474653 m/kg,respectively,which can explain why the dewatering rate of group V is higher than that of group I.A higher content of inertinite in the filter cake will increase the specific resistance of filter cake and decrease the filtration speed.
Huang et al.[51]deduced Eq.(6) based on Navier-Stokes formula and Darcy filtration equation (Eq.(7)).
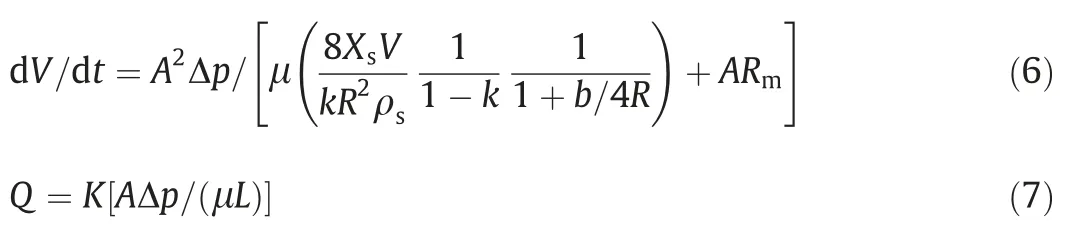
where Q is the filtration velocity;K the cake permeability;Xsthe solids content;k the cake surface porosity;Rmthe medium resistance;b the slip length;and L the cake thickness.
Eq.(6) shows that the rate of filtration can be influenced by pressure drop(Δp),solids content(Xs),cross sectional area of filter cake(A),cake surface porosity(k),pore(capillary)radius(R),medium resistance (Rm),and slip length (b).
According to the previous analysis,it can be seen that due to the difference in the mechanical properties of maceral groups,the vitrinite particles obtained under the same crushing and grinding procedure are relatively coarser than the inertinite.The difference of particle size composition leads to the difference of filter cake structure.The filter cake formed by vitrinite has smaller pore perimeter,smaller specific resistance,and larger average pore diameter,which is conducive to the rapid water removal during the filtration and dewatering.The slip length b is considered to increase with the contact angle of particles[52].The contact angle of vitrinite is higher than that of inertinite,which makes the slip length of water in capillary longer,and is also conducive to the rapid water removal.
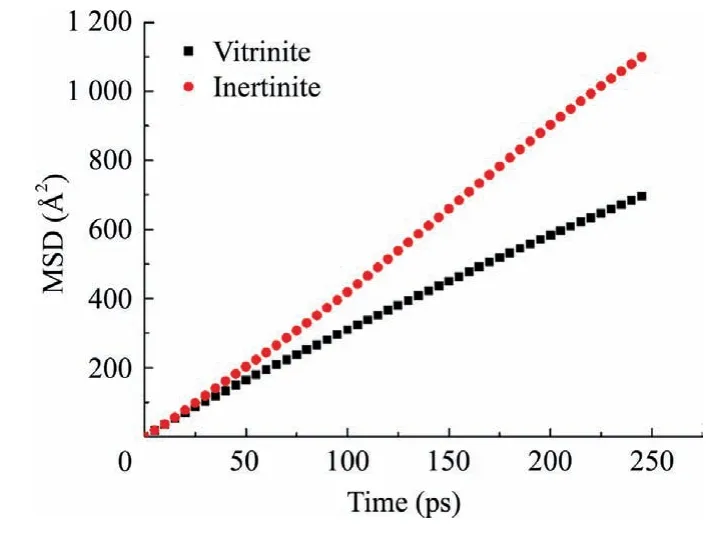
Fig.9.MSD-time curves of water molecular in the vitrinite and inertinite.
After the dewatering process,the water in the filter cake exists in the form of chemical water,adsorbed water,and capillary water[53,54].The chemical water cannot be removed by thermal drying or mechanical dewatering,so it is generally not considered in the solid-liquid separation industry.The formation of adsorbed water is related to the nature of the particles.The more hydrophilic the particles and the greater the specific surface area,the more water they can adsorb.The high hydrophilicity,large surface area,and abundant pore structure of inertinite make it easier to adsorb water,which may be one of the reasons for the high moisture of inertinite filter cake.
Capillary water is stored in the pores between the gaps of particles.When the pressure drop at both ends of the capillary satisfies Eq.(8) [1],the water in the capillary is in equilibrium.When the pressure drop is greater than Δp,water begins to flow out of the capillary.

where γ is the surface tension of the filtrate;cosθ the contact angle of the sample which is related to the hydrophobicity of particles;and r the capillary radius between particles.
It can be seen that under the condition of constant pressure,the vitrinite can generate a filter cake with a larger capillary pore size,plus its higher hydrophobic surface,which can exhaust more water and reduce the filter cake moisture.The inertinite in the samples significantly affects the pore structure and contact angle,which makes the average mass specific resistance and filter cake moisture worse,and finally affects the dewatering process.Compared with the effects of pore structure in the filtration and dewatering processes,the effects of surface wettability difference of coal maceral groups seem more significant,which needs further in-depth study in our future research.
4.Conclusions
After a series of tests and analysis,there are obvious differences between vitrinite and inertinite samples in the dewatering process,which is mainly due to the different properties of vitrinite and inertinite.The following conclusions were obtained from this paper.
(1) Compared with inertinite,vitrinite sample has faster filtration speed and lower cake moisture.Under the same treatment conditions,inertinite is easier to grind into fine particles than vitrinite,which makes inertinite filter cake have smaller average pore diameter and longer average pore perimeter.
(2) Inertinite contains more oxygen,and more carboxyl C=O and aryl ether C-O-C functional groups than vitrinite.A higher content of oxygen-containing functional groups in inertinite leads to smaller contact angle and more hydrophilic for inertinite surface.
(3) On the molecular scale,inertinite particles contain more pores,and water is difficult to lose after entering these pores,which may be the reason for the high adsorbed water content of inertinite.Moreover,the interaction energy between vitrinite and water is small,and the number of hydrogen bonds formed is also small.Water molecules are not easy to diffuse on the surface of vitrinite,which indicates that the interaction between water and vitrinite is weak,and water is easier to be removed under pressure.
(4) The difference of dewatering behavior between vitrinite and inertinite is mainly determined by the cake structure and particle surface properties.Loose cake structure and hydrophobic surface are conducive to rapid water removal and reduce water residue.In this paper,the effect of wettability on the dehydration process seems to be more obvious.
The above results show that the lower content of inertinite in coal water slurry is very beneficial to the dewatering of fine coal.Although it is impractical to improve the filtration performance by reducing the content of inertinite in the factory,the conclusion can explain that the dewatering problem of flotation clean coal often occurs in low-rank coal which is rich in inertinite.In addition,since the inertinite will impact the filtration effect,it can be considered to increase the hydrophobicity of the inertinite to improve the filtration of flotation clean coal.Choosing appropriate crushing and processing procedures also helps to reduce the generation of inertinite debris and improve the dewatering of flotation products.The shortcoming of this article is that it only qualitatively investigated the effect of maceral groups content on the dewatering process,and its quantitative analysis needs further studies in the future.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (U2003125).
杂志排行
矿业科学技术学报的其它文章
- Evaluation of the use of sublevel open stoping in the mining of moderately dipping medium-thick orebodies
- Stability control of gob-side entry retained under the gob with close distance coal seams
- A novel coating technology for fast sealing of air leakage in underground coal mines
- A robust approach to identify roof bolts in 3D point cloud data captured from a mobile laser scanner
- Physical model test and numerical simulation on the failure mechanism of the roadway in layered soft rocks
- Deformation response of roof in solid backfilling coal mining based on viscoelastic properties of waste gangue
