人脐带间充质干细胞培养上清对M1型巨噬细胞的影响及作用机制
2021-03-18廖威张昌林李田
廖威?张昌林?李田


【摘要】目的 通過研究人脐带间充质干细胞(hucMSCs)培养上清对M1型巨噬细胞的影响,探讨其作用机制。方法 选择小鼠单核巨噬细胞白血病细胞系(RAW264.7)巨噬细胞,将其种植于6孔板中,待汇合度约70%时进行处理。配制含50%hucMSCs培养上清的高糖DMEM条件培养基(CM)。巨噬细胞分成3组,blank组用2 ml高糖DMEM培养基培养24 h后更换2 ml高糖DMEM培养基培养24 h,DMEM组用2 ml含200 ng/ml脂多糖(LPS)的高糖DMEM培养基刺激24 h后更换2 ml高糖DMEM培养基培养24 h,CM组用2 ml含200 ng/ml LPS的高糖DMEM培养基刺激24 h后更换2 ml CM培养24 h。收集各组培养上清和巨噬细胞进行后续实验。利用PKH67染色剂进行CM中含细胞膜的成分染色(绿),DAPI染色剂进行巨噬细胞胞核染色(蓝),通过荧光显微镜观察巨噬细胞吞噬hucMSCs-CM中含细胞膜成分的情况。应用实时荧光定量PCR(RT-qPCR)和流式细胞多因子分析技术分析促炎因子TNF-α和抗炎因子IL-10表达水平的改变。应用流式细胞术鉴定经处理后巨噬细胞表型。结果 在hucMSCs上清中,荧光显微镜下观察到RAW264.7吞噬CM中膜性成分且数量随时间增加而增加。在巨噬细胞极化实验中,RT-qPCR结果提示CM组抗炎因子IL-10 mRNA相对表达量低于DMEM组,且促炎因子TNF-α mRNA相对表达量低于blank组和DMEM组(P均< 0.05)。流式细胞术多因子分析结果中,CM组抗炎因子IL-10水平低于DMEM组,促炎因子TNF-α水平低于blank组和DMEM组(P均< 0.05)。流式细胞术鉴定结果中,CM处理的RAW264.7中的F4/80+CD206+CD86-巨噬细胞即M2型巨噬细胞比例高于DMEM组。结论 巨噬细胞可能通过吞噬hucMSCs培养上清中膜性成分诱导M1型巨噬细胞向M2型巨噬细胞极化,从而促进抗炎反应。
【关键词】人脐带间充质干细胞;巨噬细胞极化;炎症;肿瘤坏死因子-α;白介素-10
Effect and mechanism of human umbilical cord mesenchymal stem cell culture supernatant on M1-type macrophages Liao Wei, Zhang Changlin, Li Tian. Department of Gynecology, the Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen 518107, China
Corresponding author, Li Tian, E-mail: sandylitian@ 126. com
【Abstract】Objective To evaluate the effect and investigate the mechanism of human umbilical cord mesenchymal stem cells (hucMSCs) culture supernatant on M1-type macrophages. Methods The mouse macrophage-like cell line RAW264.7 was used as the macrophage cell line, planted in the 6-well plate and treated when the confluence reached approximately 70%. Conditional DMEM medium (CM) containing 50% hucMSCs culture supernatant was prepared. Macrophages were divided into three groups. In the blank group, the cells were cultured in 2 ml DMEM medium for 24 h and then cultured in 2 ml fresh DMEM medium for 24 h. In the DMEM group, the cells were cultured in 2 ml DMEM medium containing 200 ng/ml LPS for 24 h, followed by 24-h culture in 2 ml fresh DMEM medium. In the CM group, the cells were cultured in 2 ml DMEM medium containing 200 ng/ml LPS for 24 h and subsequently cultured in 2 ml fresh CM medium for 24 h. The culture medium and macrophages from each group were gathered for subsequent experiments. Substances having cell membrane components in CM were subject to PKH67 staining (green) and RAW264 with DAPI staining (blue). The phagocytosis of cell membrane components in hucMSCs-CM by macrophages was observed under fluorescence microscope. The expression levels of pro-inflammatory factor TNF-α and anti-inflammatory factor IL-10 were analyzed by real-time fluorescence quantitative polymerase chain reaction (RT-qPCR) and flow cytometry multi-factor analysis. The phenotype of macrophages was identified by flow cytometry. Results Fluorescence microscopy revealed that RAW264.7 phagocytosis substances containing cell membrane components in the CM group were increased with time in the hucMSCs supernanant. In macrophage polarization experiment, RT-qPCR indicated that the expression level of anti-inflammatory factor interleukin-10 (IL-10) mRNA in the CM group was significantly lower than that in the DMEM group, and the expression level of tumor necrosis factor-α (TNF-α) mRNA, a proinflammatory mediator, was significantly lower compared with those in the blank and DMEM groups (all P < 0.05). Flow cytometry multi-factor analysis showed that the expression level of anti-inflammatory factor IL-10 in the CM group was remarkably lower than that in the DMEM group, and the expression level of pro-inflammatory factor TNF-α mRNA was significantly lower than those in the blank and DMEM groups (all P < 0.05). Flow cytometry analysis showed that the proportion of F4/80+CD206+CD86- type M2 macrophages in the CM group was higher compared with that in the DMEM group. Conclusion Macrophages may induce the polarization of M1-type macrophages into M2-type macrophages by phagocytosis of the membranous components in the hucMSCs culture supernanant, thereby promoting the anti-inflammatory response.
【Key words】Human umbilical cord mesenchymal stem cells (hucMSCs);Macrophage polarization;
Inflammation;Tumor necrosis factor-α (TNF-α);Interleukin-10(IL-10)
巨噬细胞源于单核细胞,属于吞噬细胞的一种,介入非特异性免疫和特异性免疫2个免疫过程。巨噬细胞因外界刺激因素的不同而发生不同表型改变或转化。如脂多糖(LPS)和TNF-α可以刺激巨噬细胞向M1型巨噬细胞方向极化,即经典活化型;IL-4和IL-13可以诱导巨噬细胞向M2型巨噬细胞极化即替代活化型,两者生物学功能有着完全不同的作用[1]。一般认为M1型巨噬细胞具有促炎作用,巨噬细胞过度的M1型极化将会引起过度的炎症反应,从而导致组织愈合不良甚至恶化;但也会出现良好的结局,如抗肿瘤作用[2-3]。
M2型巨噬细胞引起的抗炎作用,可促进组织修复[4]。在这些作用中,大家尤其关注M2巨噬细胞的抗炎作用,已有多项研究通过其抗炎作用达到治疗疾病的目的,如肝脏病、肾脏病等[5]。间充质干细胞(MSC)因为来源简单方便,且拥有低免疫原性和调节炎症反应的特性,成为了以细胞为基础的异体移植治疗方法的研究热点[6]。MSC可能主要通过其强大的免疫调节作用改善局部炎症环境,从而促进组织修复[7]。有研究者指出,MSC通过细胞外囊泡途径起到各种效应如炎症环境改变等和治疗各种疾病[8-10]。巨噬细胞是否可以通过吞噬人脐带间充质干细胞(hucMSCs)培养上清里的某些成分如膜性成分,从而影响巨噬细胞极化趋势和促进抗炎反应?目前仍未有定论。为此,本研究探究巨噬细胞是否通过吞噬hucMSCs培养上清组成的条件培养基(CM)中的膜性成分,诱导巨噬细胞发生M1型向M2型极化,为hucMSCs在临床应用上提供基础实验证据。
材料与方法
一、细胞来源
hucMSCs购自博雅(Boyalife)干细胞公司。巨噬细胞RAW264.7由中山大学附属肿瘤医院邓务国课题组赠与。
二、主要试剂
包括DMEM培养基、DMEM-F12培养基(Gibco),MSC专用血清(BI),胎牛血清(FBS,广州信欣生物科技公司),青链霉素双抗溶液(Gibco),Trizol RNA提取试剂盒、逆转录试剂盒、实时荧光定量PCR(RT-qPCR)试剂盒(北京天根生物科技公司),LEGEND plexTM Multi-Analyte Flow Assay Kit流式多因子分析试剂盒和炎症因子TNF-α、IL-10相关抗体,流式细胞术试剂盒、巨噬细胞相关抗体F4/80-PE、CD206-APC和CD86-FITC(北京达科为公司),DAPI即用型染色溶液(索莱宝),PKH67染色试剂盒、脂多糖(广州赛国生物科技有限公司)。
三、方 法
1. hucMSCs-CM制備
待第3~5代的hucMSCs汇合度约80%时,弃培养基,用磷酸盐缓冲液(PBS)清洗2遍,加入无血清DMEM培养基饥饿培养48 h,收集培养上清经4℃、3000×g离心1 h,将离心的上清移至新离心管。配制含50%培养上清的CM:离心的上清和等体积DMEM培养基混匀,加入10%FBS和1%青链霉素双抗溶液,-80℃冻存。需用时,4℃解冻。
2. RAW264.7细胞极化实验
2.1 RAW264.7细胞吞噬含细胞膜成分实验
20 μl PKH67染色溶液加入10 ml CM(不含FBS)配制成工作液,4℃保存备用。将RAW264.7巨噬细胞种植于培养皿中,待汇合度达到约70%时进行处理。更换成2 ml含100 ng/ml LPS常规培养基24 h后,PBS清洗2次,加入2 ml经PKH67标记的CM继续培养。在第1、2、3、4 h行荧光显微镜观察。
2.2巨噬细胞RAW264.7处理
用含10%FBS、1%青链霉素双抗的DMEM培养基培养扩增。将RAW264.7巨噬细胞种植于6孔板中,待汇合度达到约60% ~ 70%时进行处理。将巨噬细胞分成3组,组别:blank组用2 ml常规配制高糖DMEM培养基培养24 h后弃培养基,PBS清洗2次,添加2 ml高糖DMEM培养基培养24 h,DMEM组用2 ml含200 ng/ml LPS的高糖DMEM培养基刺激24 h后,PSB清洗2次,添加2 ml常规配制高糖DMEM培养基培养24 h,CM组用2 ml含200 ng/ml LPS的高糖DMEM培养基刺激24 h后,PBS清洗2次,添加2 ml CM培养24 h。
收集3组培养的上清和RAW264.7细胞进行后续实验。
2.3细胞炎症因子mRNA表达量
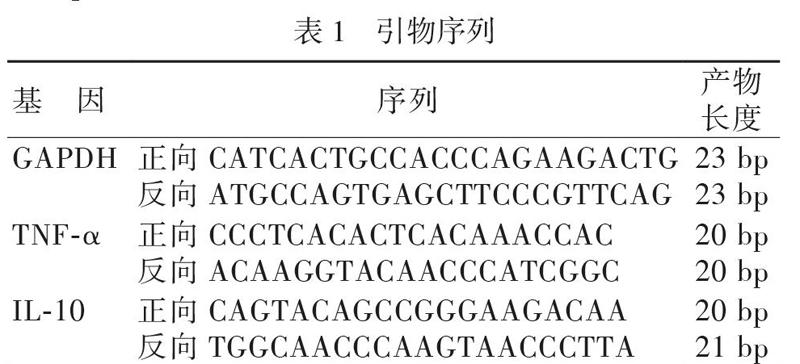
分别按Trizol RNA提取试剂盒说明书、逆转录试剂盒说明书、RT-qPCR试剂盒说明书提取经处理的RAW264.7细胞的总RNA,进行逆转录和RT-qPCR实验。引物序列见表1。
2.4 流式多因子分析术检测上清炎症因子
按Multi-Analyte Flow Assay Kit流式多因子分析试剂盒说明书检测上述组别的RAW264.7细胞上清抗炎因子IL-10和促炎因子TNF-α水平。
2.5 RAW264.7细胞表型鉴定
将RAW264.7巨噬细胞种植于6孔板中,待汇合度达到约70%时进行处理。其中LPS组更换成2 ml含200 ng/ml LPS的DMEM培养基培养24 h后,PBS清洗2次,加入2 ml含200 ng/ml LPS的DMEM培养基继续培养24 h;CM组更换成2 ml含200 ng/ml LPS的DMEM培养基培养24 h后,PBS清洗2次,加入2 ml CM培养24 h。按流式细胞术试剂盒说明书将各组的RAW264.7细胞制作成样品并上机检测。
四、统计学处理
采用SPSS 25.0分析实验数据,正态分布的计量资料以表示,2组间的比较采用两独立样本t检验,多个样本均数采用单因素方差分析,两两比较采用Tukey法。α= 0.05。
结果
一、RAW264.7细胞吞噬hucMSCs条件培养基中膜性成分
荧光显微镜下,CM处理1 h时,细胞核染色剂DAPI标记巨噬细胞RAW264.7细胞核(蓝色,蓝箭头)上未黏附细胞膜染色剂PKH67标记的膜性成分(绿色,绿箭头);2 h时,RAW264.7胞核上开始有膜性成分黏附;至3、4 h时,巨噬细胞RAW264.7胞核上膜性成分黏附数量明显增加,见图1。
二、RAW264.7细胞极化
RT-qPCR顯示,与blank组相比,CM组的IL-10 mRNA相对表达量(t = 6.230,P = 0.003)、TNF-α mRNA相对表达量(t = 12.250,P < 0.001)均较低;与blank组相比,DMEM组的IL-10 mRNA相对表达量较低(t = 10.144,P < 0.001),TNF-α的mRNA相对表达量较高(t = 14.011,P < 0.001);与DMEM组相比,CM组的IL-10 mRNA相对表达量较高(t = 16.620,P < 0.001),TNF-α mRNA相对表达量较低(t = 15.386,P < 0.001),见图2A。流式细胞术多因子分析检测结果显示,3组IL-10水平比较差异有统计学意义(F = 104.006,P < 0.001),其中DMEM组低于CM组低(P = 0.002),CM组低于blank组(P < 0.001),DMEM组低于blank组(P < 0.001);3组TNF-α水平比较差异亦有统计学意义(F = 165.251,P < 0.001),其中DMEM组高于CM组(P < 0.001),CM组高于blank组(P = 0.001),DMEM组高于blank组(P < 0.001),见图2B。流式细胞术细胞鉴定结果显示,LPS组中F4/80+CD86+CD206-细胞(M1)占99.21%、F4/80+CD86-CD206+细胞(M2)占0.01%,CM组中F4/80+CD86+CD206-细胞(M1)占63.23%、F4/80+CD86-CD206+细胞(M2)占20.92%,见图2C。
讨论
MSC可以通过其强大的免疫调节作用诱导巨噬细胞形成具有抗炎作用的M2型巨噬细胞[11]。多项研究应用RAW264.7巨噬细胞成功制备炎症模型[12-14]。因此本研究采用RAW264.7细胞作为研究对象,通过观察RAW264.7细胞经过处理前后细胞表型和炎症因子的改变,从而判断巨噬细胞极性的改变。
RT-qPCR结果中,CM组和DMEM组中IL-10 mRNA相对表达量均下降,说明200 ng/ml LPS刺激RAW264.7细胞24 h后,可以下调RAW264.7细胞表达抗炎因子IL-10,但CM组和DMEM组对比,CM组IL-10 mRNA相对表达量低于DMEM组,说明LPS可以促进炎症反应,而hucMSCs培养上清可以起到抗炎作用,将炎症环境向M2巨噬细胞的炎症环境转换。
流式多因子分析技术检测结果可见CM组和DMEM组分泌的IL-10水平趋势和RT-qPCR相应结果的趋势一致,IL-10分泌均减少但CM组低于DMEM组。对于TNF-α水平,CM组低于DMEM组。由此,流式多因子分析结果进一步验证hucMSCs培养上清的抗炎作用。
流式细胞术鉴定结果中,LPS组的大部分RAW264.7细胞表面抗原表达F4/80+CD86+CD206-,即M1型巨噬细胞,而极少表达F4/80+CD86-CD206+的细胞,即基本没有M2型巨噬细胞。CM组中,细胞表面抗原为F4/80+CD86+CD206-细胞占63.23%,F4/80+CD86-CD206+细胞占20.92%,说明含50% hucMSCs培养上清的CM诱导趋于M1型的RAW264.7向M2型趋势极化。
李志伟等[15]应用骨髓MSCs培养液成功诱导RAW264.7细胞向M2型极化,但该文中IL-10 mRNA表达量是增加的,de Witte等[16]研究结果中IL-10表达量也是增加。但本研究中IL-10并未体现出明显增加效果,可能与LPS处理时间、浓度和hucMSCs-CM处理时间和浓度有关,另外不同来源的间充质干细胞,其作用也有一定差异性[17]。虽然IL-10的表达稍有不同,但其趋势最终都体现出M2型巨噬细胞的抗炎作用。另外,hucMSCs培养上清引起巨噬细胞发生极化改变的具体成分是活性生长因子或细胞外囊泡(EV)或两者共同作用。其中EV的作用受到科研人员的关注。本研究在RAW264.7巨噬细胞吞噬CM中膜性成分实验中,通过PKH67标记CM中的膜性成分,可见在1 h时,DAPI染的RAW264.7胞核未见PKH67标记的膜性成分黏附。随着时间增加,RAW264.7胞核有越来越多的膜性成分黏附。说明RAW264.7吞噬了CM中的膜性成分并呈现时间依赖性。显然,巨噬细胞具有吞噬功能。也有学者报道RAW264.7可以吞噬药物[18]。因此笔者推测其也可能吞噬hucMSCs CM中的膜性成分。Kim等[19]证明了巨噬细胞可以吞噬细胞来源的外泌体,并呈时间、浓度依赖性。所以引起M1型巨噬细胞向M2型巨噬细胞极化的改变可能是因M1型巨噬细胞吞噬了hucMSCs-CM中的膜性成分,该膜性成分有可能是EV。EV按其直径从小到大可分为外泌体、细胞微泡和凋亡小体[20]。其中外泌体最受关注。外泌体可以存在于原核生物和真核生物,具有与母系细胞相似的生物学功能[21]。而且将外泌体注射至体内,不引起溶血、过敏等不良反应,具有更低的免疫原性[22]。也有研究显示外泌体可以改变巨噬细胞极化状态[20]。
綜上所述,巨噬细胞可能通过吞噬hucMSCs培养上清中的膜性成分从M1型状态向M2型状态极化,起到抗炎作用。但本研究并没有证实膜性成分和更深一步去研究具体机制,是研究的不足,今后将开展更进一步的研究探索其作用机制。
参 考 文 献
[1] Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology, 2018, 154(2):186-195.
[2] Seraphim PM, Leal EC, Moura J, Gon?alves P, Gon?alves JP, Carvalho E. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci, 2020, 254:117813.
[3] Eom YW, Akter R, Li W, Lee S, Hwang S, Kim J, Cho MY. M1 macrophages promote trail expression in adipose tissue-derived stem cells, which suppresses colitis-associated colon cancer by increasing apoptosis of CD133+ cancer stem cells and decreasing m2 macrophage population. Int J Mol Sci, 2020, 21(11):3887.
[4] Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials, 2018, 156:16-27.
[5] Chen T, Cao Q, Wang Y, Harris DCH. M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int, 2019, 95(4):760-773.
[6] Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell, 2018, 22(6):824-833.
[7] Klimczak A, Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int, 2016, 2016:4285215.
[8] Fang SB, Zhang HY, Meng XC, Wang C, He BX, Peng YQ, Xu ZB, Fan XL, Wu ZJ, Wu ZC, Zheng SG, Fu QL. Small extracellular vesicles derived from human MSCs prevent allergic airway inflammation via immunomodulation on pulmonary macrophages. Cell Death Dis, 2020, 11(6):409.
[9] Danieli P, Malpasso G, Ciuffreda MC, Cervio E, Calvillo L, Copes F, Pisano F, Mura M, Kleijn L, de Boer RA, Viarengo G, Rosti V, Spinillo A, Roccio M, Gnecchi M. Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Transl Med, 2015, 4(5):448-458.
[10] Guo L, Lai P, Wang Y, Huang T, Chen X, Geng S, Huang X, Luo C, Wu S, Ling W, Huang L, Du X, Weng J. Extracellular vesicles derived from mesenchymal stem cells prevent skin fibrosis in the cGVHD mouse model by suppressing the activation of macrophages and B cells immune response. Int Immunopharmacol, 2020, 84:106541.
[11] Heo JS, Choi Y, Kim HO. Adipose-derived mesenchymal stem cells promote m2 macrophage phenotype through exosomes. Stem Cells Int, 2019, 2019:7921760.
[12] Miao X, Leng X, Zhang Q. The current state of nanoparticle-induced macrophage polarization and reprogramming research. Int J Mol Sci, 2017, 18(2):336.
[13] Essandoh K, Li Y, Huo J, Fan GC. Mirna-mediated macr-ophage polarization and its potential role in the regulation of inflammatory response. Shock, 2016 , 46(2):122-131.
[14] Bardi GT, Smith MA, Hood JL. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine, 2018, 105:63-72.
[15] 李志伟,周号悦,刘湘粤,何漪,丁小凤,胡灵玉. 骨髓间充质干细胞培养液促进STAT3磷酸化诱导Raw264.7细胞向M2型极化. 激光生物学报,2020,29(2):153-160.
[16] de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, Shankar AS, O'Flynn L, Elliman SJ, Roy D, Betjes MGH, Newsome PN, Baan CC, Hoogduijn MJ. Immunomodulation by therapeutic mesenchymal stromal cells(MSC) is triggered through phagocytosis of msc by monocytic cells. Stem Cells, 2018, 36(4):602-615.
[17] Yi X, Chen F, Liu F, Peng Q, Li Y, Li S, Du J, Gao Y, Wang Y. Comparative separation methods and biological characteristics of human placental and umbilical cord mesenchymal stem cells in serum-free culture conditions. Stem Cell Res Ther, 2020, 11(1):183.
[18] 秦露平,呂杰,李建芳,谢良骏,蒋永泺,程木华. 吡格列酮对A549肺癌细胞及RAW264.7巨噬细胞18F-FDG摄取影响的实验研究. 新医学,2019,50(3):188-191.
[19] Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci (Weinh), 2019, 6(20):1900513.
[20] Tong F, Mao X, Zhang S, Xie H, Yan B, Wang B, Sun J, Wei L. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett, 2020, 478:34-44.
[21] Yá?ez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kr?mer-Albers EM, Laitinen S, L?sser C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, L?tvall J, Man?ek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger ?, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles, 2015, 4:27066.
[22] Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, Qian H, Zhu W, Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy, 2016, 18(3):413-422.
(收稿日期:2020-08-12)
(本文编辑:林燕薇)
