新型白三烯A4 水解酶抑制剂的合成及体外活性评价
2021-02-25郝甜甜吕洪彬
郝甜甜 ,杨 尧,付 颖,吕洪彬,苗 爱,郭 娜, ,郁 彭
(1.天津科技大学生物工程学院,天津 300457;2.中国医学科学院/北京协和医学院药物研究所天然药物活性物质与功能国家重点实验室,北京 100050)
早在19 世纪中叶,德国病理学家Rudolf Virchow 就已经证明恶性肿瘤组织中有白细胞浸润,认为肿瘤可能源自慢性炎症[1].肿瘤微环境中存在大量炎症细胞和炎症因子,对肿瘤细胞的增生、生存、转移发挥重要作用,是肿瘤形成、发展和转移过程中的重要促进因素[2].能够证明炎症与癌症间存在确定关系的证据之一是抗炎药物能降低各种癌症的发生风险、减慢肿瘤发展进度、降低患者死亡率.例如流行病学研究表明,使用非甾体抗炎药可将结肠癌风险降低40%~50%[3].
白三烯A4 水解酶(LTA4H)是炎症通路中的一个关键酶,其在多种人类恶性肿瘤中高表达,包括结直肠癌、食管癌、肺癌和甲状腺癌等[4].因此,LTA4H 是抑制炎症和肿瘤的一个潜在靶点.人类白细胞的LTA4H 在1984 年首次被纯化,并被鉴定为相对分子质量为6.8×104~7.0×104的胞质单体环氧水解酶(EH),具有EH 活性和依赖锌离子的氨肽酶(AP)活性.两种活性位点和底物结合口袋大部分重叠,因此很难设计高选择性的抑制剂.尽管LTA4H 的上述两种活性有各自的生理作用,但两种活性都促进肿瘤发生发展,即抑制两种活性均有益于抗肿瘤[4].从21 世纪初期开始,对LTA4H 抑制剂的大部分研究主要涉及天然底物白三烯A4(LTA4)的类似物,深入研究发现其水解酶与氨肽酶的双重活性均依赖锌离子,由此开发了锌离子螯合剂作为抑制剂.随着对LTA4H 晶体结构[5-7]的解析,人们开始基于计算机模拟来设计新型的LTA4H 抑制剂,其中DG-051 是曾进行至二期临床研究的一个抑制剂[8-9],其水解酶活性的IC50达到70 nmol/L[10-11],其与LTA4H 结合的共晶X 射线图清晰展示了二者的作用模式[11].
新型LTA4H 抑制剂在抗炎和炎症相关的肿瘤治疗领域具有重要研究价值,而抗炎药物和传统抗癌药物的组合更有望在癌症治疗中发挥作用.本研究拟以吲哚为母核,在前期构效关系和DG-051 相关研究基础上,设计合成一系列新型LTA4H 抑制剂.挑选LTA4H 抑制活性较好的化合物与抗癌药物紫杉醇共价偶联,所制备的偶联物可以看作“孪药”和“前药”的结合体.LTA4H 的抑制剂既可以通过抑制炎症改善肿瘤治疗效果,还有望通过与肿瘤部位高表达的LTA4H 相结合起到靶向肿瘤部位的作用,使紫杉醇在肿瘤部位富集,起到增效减毒的效果.
1 材料与方法
1.1 材料
癌细胞来自国家实验细胞资源共享服务平台(北京).生物试剂,赛默飞世尔科技公司;白三烯B4(LTB4)检测试剂盒,Cayman 公司;化学试剂均为分析纯,安耐吉化学试剂有限公司;溶剂均为分析纯,天津市江天化工技术有限公司;infinite F50 型基础酶标仪,帝肯(上海)贸易有限公司;AV400 型核磁共振波谱仪,布鲁克光谱仪器公司.
1.2 方法
1.2.1 吲哚衍生物的合成
共设计合成两类吲哚衍生物,两类衍生物的合成方法相似,仅1 位和5 位取代基修饰类型、反应顺序及反应条件略有差异.
第一类吲哚衍生物是以5-羟基吲哚为原料,先用Boc 基团保护1 位,在碱性条件下1 位酚羟基与芳香取代基生成醚键,再将1 位脱除保护基,通过亲核取代反应分别连接各种极性基团,生成化合物2-10 至2-17,其中2-10 和2-11 通过硼氢化钠还原可得化合物2-18 和2-19.
第二类吲哚衍生物是在将1 位用Boc 保护后,先通过亲核取代在5 位连接氯乙基基团,再用各种极性取代基分别取代氯原子,生成5 位取代的吲哚,再脱除1 位保护基,最后在1 位连接各类疏水性芳香取代基,得到化合物2-29 至2-46.
所有化合物经NMR、HRMS 确认结构,并用HPLC 分析纯度,其合成路线图及反应条件如图1 和图2 所示.

图1 第一类吲哚衍生物的合成路线Fig.1 Synthetic route of the first type of indole derivatives
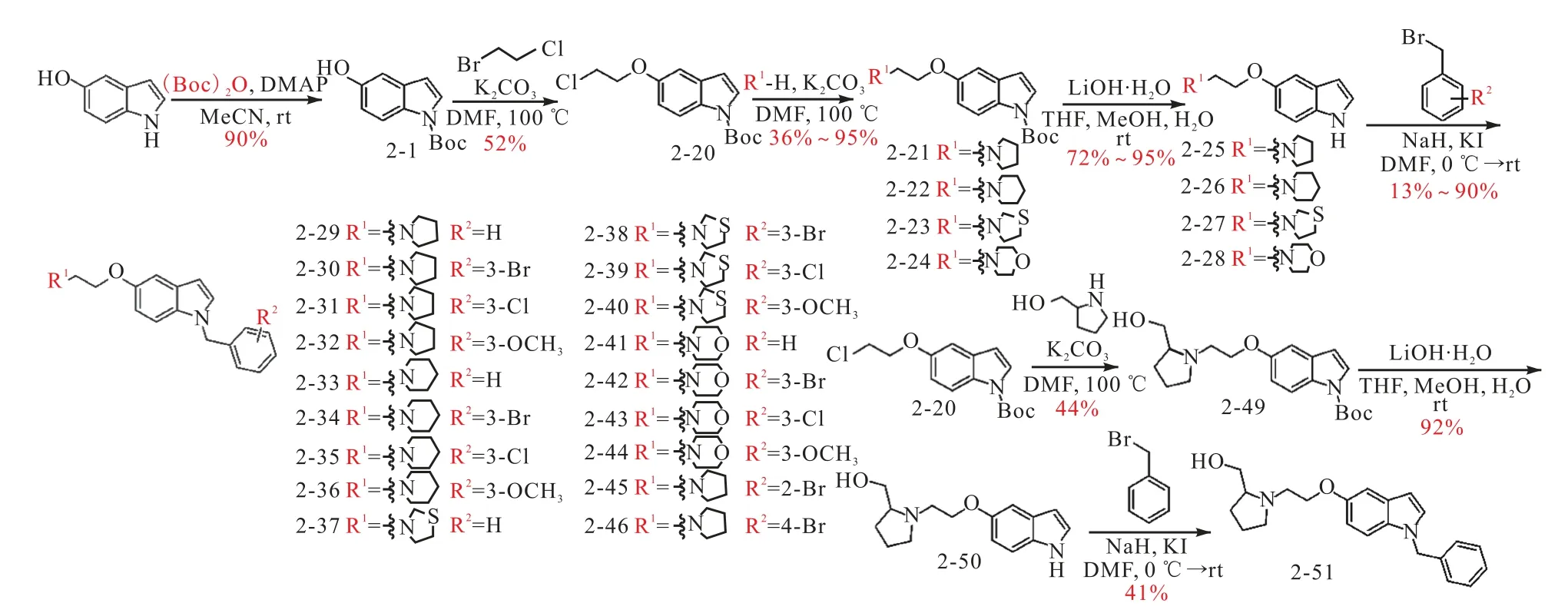
图2 第二类吲哚衍生物的合成路线Fig.2 Synthetic route of the second type of indole derivatives
1.2.2 吲哚衍生物与紫杉醇偶联物的合成
从上述两个系列的吲哚衍生物中分别选择了两个LTA4H 抑制活性较好的化合物(2-18 和2-51)用于与紫杉醇进行偶联,第一类衍生物中的2-18 可以通过分子中的羟基与紫杉醇进行偶联,而选择2-51 是由于与其结构最类似的化合物2-29 具有很好的活性,但分子中缺少可反应的基团,因此在2-20 结构中引入羟甲基获得了化合物2-51(图2).通过图3 和图4 的合成路线,2-18 和2-51 分别与紫杉醇偶联制备了2-48 和2-53.

图3 偶联化合物2-48的合成路线Fig.3 Synthetic route of the conjugate 2-48
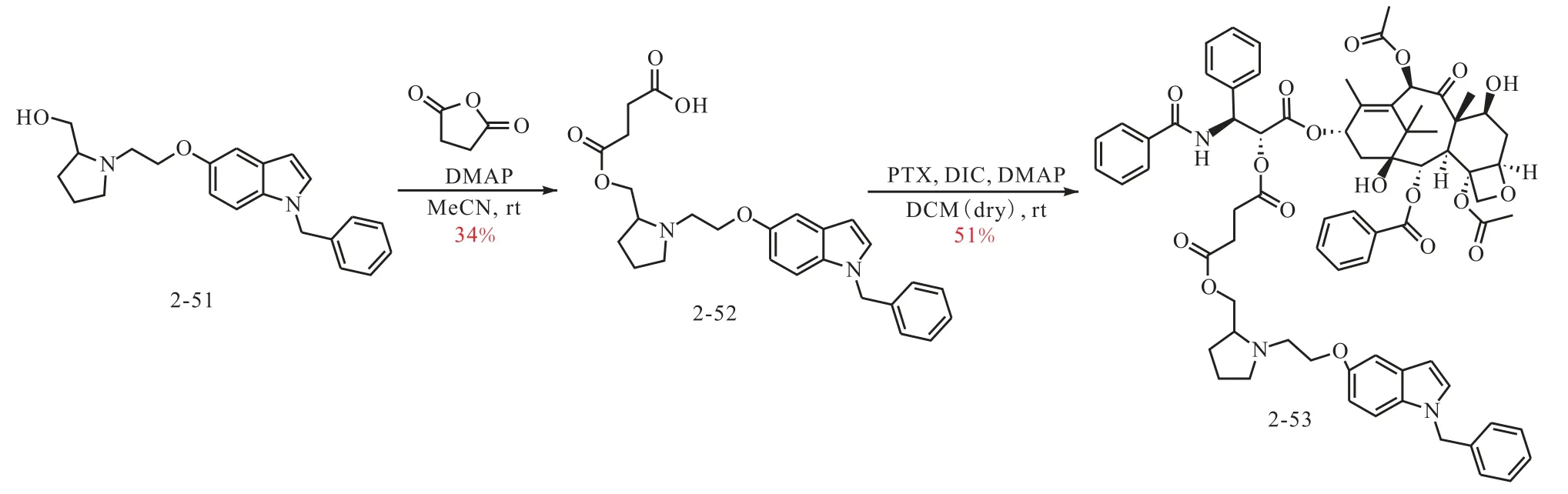
图4 偶联化合物2-53的合成路线Fig.4 Synthetic route of the conjugate 2-53
1.2.3 LTA4H 水解酶抑制活性实验
在化合物抑制 LTA4H 水解酶活性实验中以DMSO 作为阴性对照,Bestatin 为阳性对照,化合物浓度设置为 1µmol/L 和 10µmol/L.使用 LTB4ELISA kit,检测LTA4H 作用于LTA4时LTB4的生成量.当加入LTA4H 抑制剂后,抑制剂抑制酶活性,故可用LTB4生成减少的量评价抑制剂的抑制效果.以化合物组LTB4的生成量相对于DMSO 组LTB4生成量的百分比衡量化合物组对LTA4H 水解酶活的抑制效果,并计算出相应的抑制率.
1.2.4 LTA4H 氨肽酶抑制活性实验
在LTA4H 的氨肽酶活性检测时以DMSO 作为阴性对照,Bestatin 为阳性对照,化合物浓度设置为1µmol/L 和10µmol/L.使用L-丙氨酸-对硝基苯胺为底物,LTA4H 能使底物的C—N 键断裂,生成丙氨酸和对硝基苯胺,在405 nm 下测定对硝基苯胺的吸光度.在一定范围内,对硝基苯胺的生成量与酶浓度呈正比.当加入LTA4H 抑制剂后,抑制剂抑制酶活性,LTA4H 降解底物的能力下降,用对硝基苯胺减少量评价抑制剂的抑制活力.
1.2.5 肿瘤细胞增殖抑制活性实验
采用MTT 法检测合成的2 个偶联化合物以及其分别的前体化合物、PTX 对HCT116(结肠癌细胞)和H460(肺癌细胞)两种肿瘤细胞的增殖抑制活性.以正常细胞为对照,检测在不同浓度化合物处理下肿瘤细胞的存活率,计算化合物抑制肿瘤细胞生长的IC50值.
2 结果与讨论
2.1 两类吲哚衍生物的表征
上述两条合成路线制备的吲哚衍生物,经HPLC检测纯度均达到95%以上,其结构经1H NMR、13C NMR 和HRMS 鉴定,均与预期结构一致,其中终产物的结构表征数据如下:
化合物 2-10 为无色油状物,收率 69%.1H NMR(400 MHz,DMSO-d6)δ 2.18(s,6H),2.50(s,3H),2.61(t,J=6.8 Hz,2H),4.26(t,J=6.8 Hz,2H),6.41(d,J=2.8 Hz,1H),6.90(dd,J=8.8 Hz,1H),6.93~6.97(m,2H),7.28(d,J=2.4 Hz,1H),7.45(d,J =3.2 Hz,1H),7.54(d,J =8.8 Hz,1H),7.90~7.93(m,2H).13C NMR(100 MHz,DMSO-d6)δ 196.8,163.6,148.0,133.8,131.3,131.1,131.0,129.2,116.5,115.0,112.0,111.6,101.1,59.2,45.8,44.3,28.0.HRMS(ESI-TOF)m/z.C20H23N2O2+[M+H]+理论值323.175 4,实测值323.173 8.
化合物 2-11 为无色油状物,收率 95%.1H NMR(400 MHz,DMSO-d6)δ 2.19(s,6H),2.60~2.63(m,5H),4.26(t,J=6.8 Hz,2H),6.39(d,J=2.8 Hz,1H),6.80(d,J=8 Hz,1H),6.94(dd,J=8.8 Hz,J=2.4 Hz,2H),7.12~7.16(m,1H),7.24(d,J=2.4 Hz,1H),7.43~7.48(m,2H),7.54(d,J=8.8 Hz,1H),7.70(dd,J=1.6 Hz,J=6 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 199.0,158.2,149.2,134.2,133.5,130.9,130.2,129.8,129.2,122.8,118.2,114.5,111.5,110.9,101.0,59.2,45.8,44.3,31.8.HRMS(ESI-TOF)m/z.C20H23N2O2+[M+H]+理论值323.175 4,实测值323.175 1.
化合物2-12 为无色油状物,收率70%.1H NMR(400 MHz,DMSO-d6)δ 2.19(s,6H),2.62(t,J =6.8 Hz,2H),4.27(t,J=6.8 Hz,2H),6.42(d,J=2.8 Hz,1H),6.92(dd,J=8.4 Hz,J=2.0 Hz,1H),6.99~7.03(m,2H),7.31(d,J=2.4 Hz,1H),7.47(d,J=3.2 Hz,1H),7.55(d,J=16.8 Hz,1H),7.75~7.79(m,2H).13C NMR(100 MHz,DMSO-d6)δ 162.9,147.0,134.4,133.5,130.6,128.7,118.9,117.0,114.5,111.7,111.2,103.9,100.7,58.7,45.3,43.8.HRMS(ESI-TOF)m/z.C19H20N3O+[M+H]+理论值306.160 1,实测值306.159 7.
化合物 2-13 为无色油状物,收率 33%.1H NMR(400 MHz,DMSO-d6)δ 2.19(s,6H),2.62(t,J=6.8 Hz,2H),4.27(t,J=6.8 Hz,2H),6.43(d,J=3.2 Hz,1H),6.78(d,J=8.8 Hz,1H),6.96(dd,J=2.4 Hz,J=8.8 Hz,2H),7.16~7.20(m,1H),7.33(d,J=2.4 Hz,1H),7.48(d,J=3.2 Hz,1H),7.54~7.58(m,2H),7.83~7.86(m,1H).13C NMR(100 MHz,DMSO-d6)δ 160.8,147.3,135.0,133.9,133.4,130.6,128.6,122.4,116.3,115.9,114.2,111.2,111.2,101.5,100.7,58.7,45.3,43.8.HRMS(ESI-TOF)m/z.C19H20N3O+[M+H]+理论值 306.160 1,实测值306.160 2.
化合物2-14 为无色油状物,收率34%.1H NMR(400 MHz,DMSO-d6)δ 2.44(s,4H),2.67(t,J =6.8 Hz,2H),3.55(t,J =4.4 Hz,4H),4.31(t,J =6.8 Hz,2H),6.43(d,J=2.8 Hz,1H),6.92(dd,J=8.8 Hz,J=2.4 Hz,1H),7.01(d,J=8.8 Hz,2H),7.31(d,J=2.4 Hz,1H),7.49(d,J=2.8 Hz,1H),7.58(d,J=8.8 Hz,1H),7.77(d,J=8.8 Hz,2H).13C NMR(100 MHz,DMSO-d6)δ 163.3,147.5,134.9,133.9,131.1,129.2,119.4,117.5,115.0,112.2,111.8,104.4,101.2,66.7,58.3,53.8,43.5.HRMS(ESI-TOF)m/z.C21H22N3O2+[M+H]+理论值348.170 7,实测值348.169 0.
化合物2-15 为无色油状物,收率75%.1H NMR(400 MHz,DMSO-d6)δ 1.64~1.68(m,4H),2.48~2.50(m,4H),2.80(t,J=6.8 Hz,2H),4.30(t,J=6.8 Hz,2H),6.43(d,J=2.8 Hz,1H),6.92(dd,J=8.8 Hz,J=2.4 Hz,1H),6.99~7.02(m,2H),7.31(d,J=2.0 Hz,1H),7.48(d,J=3.2 Hz,1H),7.56(d,J=8.8 Hz,1H),7.75~7.79(m,2H).13C NMR(100 MHz,DMSO-d6)δ 163.3,147.5,134.9,133.9,131.1,129.2,119.4,117.5,115.0,112.2,111.7,104.4,101.2,55.8,54.1,45.5,23.6.HRMS(ESI-TOF)m/z.C21H22N3O+[M+H]+理论值332.1757,实测值332.1740.
化合物2-16 为无色油状物,收率46%.1H NMR(400 MHz,DMSO-d6)δ 1.65~1.67(m,4H),2.49~2.50(m,4H),2.80(t,J=6.8 Hz,2H),4.30(t,J=6.8 Hz,2H),6.43(d,J=2.8 Hz,1H),6.77(d,J=8.4 Hz,1H),6.96(dd,J=8.8 Hz,J=2.0 Hz,1H),7.16~7.20(m,1H),7.33(d,J=2.0 Hz,1H),7.49(d,J=3.2 Hz,1H),7.54~7.58(m,2H),7.85(dd,J=8.0 Hz,J=1.6 Hz,1H).13C NMR(100 MHz,DMSOd6)δ 161.3,147.8,135.6,134.4,133.9,131.1,129.2,123.0,116.8,116.4,114.7,111.8,111.7,102.0,101.2,55.8,54.1,45.5,23.6.HRMS(ESI-TOF)m/z.C21H22N3O+[M+H]+理论值 332.175 7,实测值332.174 9.
化合物2-17 为无色油状物,收率50%.1H NMR(400 MHz,DMSO-d6)δ 2.43(s,4H),2.67(t,J =6.8 Hz,2H),3.54~3.56(m,4H),4.31(t,J=6.8 Hz,2H),6.44(d,J=2.8 Hz,1H),6.77(d,J=8.4 Hz,1H),6.97(dd,J=8.8 Hz,J=2.4 Hz,1H),7.19(t,J=7.6 Hz,1H),7.33(d,J=2.4 Hz,1H),7.49(d,J=2.8 Hz,1H),7.55~7.60(m,2H),7.85(dd,J =7.6 Hz,J=1.6 Hz,1H).13C NMR(100 MHz,DMSOd6)δ 161.6,147.9,134.1,133.7,133.7,129.7,129.1,121.8,116.5,115.8,115.2,112.3,110.4,102.5,101.5,67.0,58.2,53.9,53.5,44.3.HRMS(ESITOF)m/z.C21H22N3O2+[M+H]+理论值348.170 7,实测值348.170 0.
化合物2-18 为无色油状物,收率92%.1H NMR(400 MHz,DMSO-d6)δ 1.30(d,J =6.4 Hz,3H),2.19(s,6H),2.60(t,J =6.8 Hz,2H),4.24(t,J =6.8 Hz,2H),4.68(m,1H),5.08(d,J=3.6 Hz,1H),6.37(d,J=2.8 Hz,1H),6.86(dd,J=8.8 Hz,3H),7.16(d,J=2.4 Hz,1H),7.28(d,J=8.4 Hz,2H),7.41(d,J =3.2 Hz,1H),7.48(d,J =8.8 Hz,1H).13C NMR(400 MHz,DMSO-d6)δ 157.9,149.8,141.6,133.2,130.6,129.1,127.2,117.2,114.7,111.3,110.8,100.8,68.1,59.2,45.8,44.3,26.4.HRMS(ESITOF)m/z.C20H25N2O2+[M+H]+理论值325.191 1,实测值325.191 0.
化合物2-19 为无色油状物,收率57%.1H NMR(400 MHz,DMSO-d6)δ 1.36(d,J =6.0 Hz,3H),2.19(s,6H),2.61(t,J =6.8 Hz,2H),4.24(t,J =6.8 Hz,2H),5.12(m,1H),6.35(d,J=2.8 Hz,1H),6.66(dd,J=8.0 Hz,J=1.2 Hz,1H),6.84(dd,J=2.0 Hz,J=8.8 Hz,1H),7.05~7.08(m,2H),7.11~7.15(m,1H),7.39(d,J=2.8 Hz,1H),7.47(d,J=8.8 Hz,1H),7.58(dd,J=2.0 Hz,J=7.6 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 154.2,149.9,137.5,132.5,130.0,128.6,127.4,126.2,122.5,116.9,113.6,110.7,109.3,100.3,62.6,58.6,54.9.45.2,43.8,25.0.HRMS(ESI-TOF)m/z.C20H25N2O2+[M+H]+理论值325.191 1,实测值325.189 8.
化合物2-29 为无色油状物,收率51%.1H NMR(400 MHz,DMSO-d6)δ 1.67~1.70(m,4H),2.55(d,J=5.2 Hz,4H),2.82(t,J=6.0 Hz,2H),4.05(d,J=6.0 Hz,2H),5.36(s,2H),6.39(d,J=2.8 Hz,1H),6.74(dd,J =2.4 Hz,J =8.8 Hz,1H),7.07(d,J =2.4 Hz,1H),7.16~7.18(m,2H),7.21~7.31(m,4H),7.44(d,J=3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.1,138.8,131.5,130.0,129.2,128.9,127.7,127.4,112.2,111.3,103.7,101.1,67.5,55.0,54.5,49.7,23.6.HRMS(ESI-TOF)m/z.C21H25N2OH+[M+H]+理论值321.196 1,实测值321.194 7.
化合物2-30 为无色油状物,收率29%.1H NMR(400 MHz,DMSO-d6)δ 1.67~1.70(m,4H),2.57(s,4H),2.83(t,J=6 Hz,2H),4.05(t,J=6 Hz,2H),5.38(s,2H),6.40(d,J=2.8 Hz,1H),6.75(dd,J=8.8 Hz,J=2.4 Hz,1H),7.07(d,J=2.4 Hz,1H),7.15(d,J=7.6 Hz,1H),7.25(t,J=8 Hz,1H),7.31(d,J=8.8 Hz,1H),7.35(s,1H),7.43(d,J=8 Hz,1H),7.46(d,J=3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.1,141.7,131.4,131.1,130.6,130.0,130.0,129.2,126.4,122.2,112.3,111.2,103.8,101.3,67.4,54.9,54.4,48.9,23.5.HRMS(ESITOF)m/z.C21H24N2OBr+[M+H]+理论值399.106 7,实测值399.107 3.
化合物2-31 为无色油状物,收率64%.1H NMR(400 MHz,CDCl3)δ 1.87(s,4H),2.73(s,4H),3.00(t,J=5.6 Hz,2H),4.22(t,J=5.6 Hz,2H),5.21(s,2H),6.52(s,1H),6.91~6.95(m,2H),7.10~7.14(m,3H),7.19~7.26(m,3H).13C NMR(100 MHz,DMSO-d6)δ 153.2,141.5,133.6,131.5,130.9,130.1,129.3,127.7,127.1,126.1,112.3,111.2,103.8,101.4,67.5,54.9,54.5,49.0,23.6.HRMS(ESITOF)m/z.C21H24N2OCl+[M+H]+理论值355.157 2,实测值355.155 5.
化合物2-32 为无色油状物,收率71%.1H NMR(400 MHz,DMSO-d6)δ 1.67~1.70(m,4H),2.54(s,4H),2.80(t,J=6 Hz,2H),3.68(s,3H),4.04(t,J=6 Hz,2H),5.33(s,2H),6.38(d,J=3.2 Hz,1H),6.70~6.75(m,3H),6.80(dd,J=8.8 Hz,J=2.4 Hz,1H),7.07(d,J=2.4 Hz,1H),7.20(t,J=8 Hz,1H),7.30(d,J =9.2 Hz,1H),7.43(d,J =3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 159.8,153.1,140.5,131.6,130.08,130.06,129.2,119.5,113.4,112.8,112.2,111.3,103.7,101.1,67.5,55.4,55.0,54.5,49.6,23.6.HRMS(ESI-TOF)m/z.C22H27N2O2+[M+H]+理论值351.206 7,实测值351.208 4.
化合物2-33 为无色油状物,收率90%.1H NMR(400 MHz,DMSO-d6)δ 1.39(d,J =5.2 Hz,2H),1.49~1.54(m,4H),2.50(d,J=1.6 Hz,4H),2.71(s,2H),4.05(t,J=6 Hz,2H),5.36(s,2H),6.38(d,J=3.2 Hz,1H),6.73(dd,J=2.4 Hz,J=8.8 Hz,1H),7.07(d,J =2 Hz,1H),7.16(d,J =7.2 Hz,2H),7.21~7.24(m,1H),7.29(m,3H),7.44(d,J =2.8 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.1,138.9 131.5,130.1,129.2,128.9,127.7,127.4,112.2,112.3,103.8,101.1,66.3,57.9,54.8,49.7,25.8,24.2.HRMS(ESI-TOF)m/z.C22H27N2O+[M+H]+理论值357.193 7,实测值357.194 0.
化合物2-34 为无色油状物,收率34%.1H NMR(400 MHz,DMSO-d6)δ 1.38(t,J =5.2 Hz,2H),1.47~1.53(m,4H),2.46(s,4H),2.68(t,J=5.2 Hz,2H),4.04(t,J=6 Hz,2H),5.38(s,2H),6.39(d,J=3.2 Hz,1H),6.75(dd,J=2.4 Hz,J=8.8 Hz,1H),7.07(d,J =2 Hz,1H),7.15(d,J =7.6 Hz,1H),7.25(t,J=7.6 Hz,1H),7.31(d,J=8.8 Hz,1H),7.36(s,1H),7.43(d,J=7.6 Hz,1H),7.46(d,J=3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.2,141.8,131.4,131.2,130.6,130.04,130.02,129.2,126.4,122.3,112.3,112.2,103.8,101.4,66.4,58.0,54.8,48.9,25.9,24.3.HRMS(ESI-TOF)m/z.C21H26N2OBr+[M+H]+理论值 413.122 3,实测值413.120 7.
化合物2-35 为无色油状物,收率57%.1H NMR(400 MHz,DMSO-d6)δ 1.34~1.40(m,2H),1.47~1.52(m,4H),2.45(s,4H),2.67(t,J=5.6 Hz,2H),4.04(t,J=5.6 Hz,2H),5.38(s,2H),6.39(d,J=2.8 Hz,1H),6.74(dd,J=2.4 Hz,J=8.8 Hz,1H),7.07(d,J=2.4 Hz,1H),7.11(d,J=6.8 Hz,2H),7.20(s,1H),7.28~7.34(m,3H),7.46(d,J=3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.2,141.5,133.6,131.4,130.9,130.1,129.2,127.7,127.1,126.1,112.4,112.2,103.8,101.4,66.4,58.0,54.9,49.0,26.0,24.4.HRMS(ESI-TOF)m/z.C22H26N2OCl+[M+H]+理论值369.172 8,实测值369.171 3.
化合物2-36 为无色油状物,收率13%.1H NMR(400 MHz,DMSO-d6)δ 1.53(s,2H),1.74(s,4H),2.50(t,J =1.6 Hz,2H),3.45(d,J =4 Hz,4H),3.68(s,3H),4.30(t,J=5.2 Hz,2H),5.35(s,2H),6.41(d,J=2.8 Hz,1H),6.71(d,J=7.6 Hz,1H),6.74(s,1H),6.79~6.83(m,2H),7.16(d,J=2.4 Hz,1H),7.20(t,J=8 Hz,1H),7.36(d,J=8.8 Hz,1H),7.49(d,J=3.2 Hz,1H).13C NMR(100 MHz,CDCl3)δ 159.7,151.5,138.9,131.9,1 297,129.3,128.9,118.8,112.5,112.5,111.8,110.6,104.1,101.2,62.9,56.1,55.1,53.7,50.1,29.5,22.5,21.4.HRMS(ESITOF)m/z.C23H29N2O2+[M+H]+理论值365.222 4,实测值365.220 6.
化合物2-37 为无色油状物,收率36%.1H NMR(400 MHz,CDCl3)δ 2.91(t,J=5.6 Hz,2H),3.02(t,J=6.4 Hz,2H),3.27(t,J=6.4 Hz,2H),4.24(t,J=5.6 Hz,2H),4.28(s,2H),5.36(s,2H),6.57(d,J=2.8 Hz,1H),6.96(dd,J =2 Hz,J =8.8 Hz,1H),7.18~7.24(m,5H),7.33~7.40(m,3H).13C NMR(100 MHz,CDCl3)δ 153.2,137.6,131.9,129.1,129.0,128.8,127.6,126.78,112.7,110.5,104.0,101.3,68.2,61.3,58.7,52.4,50.3,29.7.HRMS(ESITOF)m/z.C20H23N2OS+[M+H]+理论值339.152 6,实测值339.151 1.
化合物2-38 为无色油状物,收率80%.1H NMR(400 MHz,DMSO-d6)δ 2.68(t,J =5.6 Hz,2H),2.81(t,J =6.4 Hz,2H),3.07(t,J =6.4 Hz,2H),4.07(t,J=5.6 Hz,2H),4.11(s,2H),5.38(s,2H),6.40(d,J =2.8 Hz,1H),6.76(dd,J =8.8 Hz,J =2.0 Hz,1H),7.08(d,J=2.0 Hz,1H),7.15(d,J=8.0 Hz,1H),7.26(t,J=8.0 Hz,1H),7.32(d,J=9.2 Hz,1H),7.35(s,1H),7.43(d,J=8.0 Hz,1H),7.47(d,J=2.8 Hz,1H).13C NMR(100 MHz,DMSOd6)δ 153.1,141.7,131.4,131.1,130.6,130.03,129.99,129.2,126.4,122.2,112.3,112.2,103.8,101.3,68.0,61.4,58.6,51.9,48.9,29.3.HRMS(ESITOF)m/z.C20H22N2OSBr+[M+H]+理论值439.045 0,实测值439.042 9.
化合物2-39 为无色油状物,收率65%.1H NMR(400 MHz,DMSO-d6)δ 2.68(t,J =5.6 Hz,2H),2.81(t,J =6.4 Hz,2H),3.07(t,J =6.4 Hz,2H),4.07(t,J=5.6 Hz,2H),4.11(s,2H),5.39(s,2H),6.40(d,J =2.8 Hz,1H),6.76(dd,J =2.4 Hz,J =8.8 Hz,1H),7.08(d,J=2.4 Hz,1H),7.11(d,J=6.8 Hz,1H),7.20(s,1H),7.31(t,J=2 Hz,1H),7.33(d,J =2 Hz,1H),7.47(d,J =3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.2,141.5,133.6,131.5,130.9,130.1,129.2,127.7,127.1,126.1,112.3,112.2,103.8,101.4,68.0,61.4,58.6,52.4,51.9,49.0,29.4.HRMS(ESI-TOF)m/z.C20H22N2OSCl+[M+H]+理论值373.113 6,实测值373.112 4.
化合物2-40 为无色油状物,收率76%.1H NMR(400 MHz,DMSO-d6)δ 2.68(t,J =5.6 Hz,2H),2.81(t,J =6.4 Hz,2H),3.07(t,J =6.4 Hz,2H),3.68(s,3H),4.06(t,J=5.6 Hz,2H),4.11(s,2H),5.33(s,2H),6.38(d,J=2.8 Hz,1H),6.71(d,J=7.6 Hz,1H),6.74~6.76(m,2H),6.80(dd,J =2.4 Hz,J =8.8 Hz,1H),7.07(d,J =2 Hz,1H),7.20(t,J =8 Hz,1H),7.31(d,J =8.8 Hz,1H),7.44(d,J=2.8 Hz,1H).13C NMR(100 MHz,CDCl3)δ 160.0,153.2,139.3,131.9,129.8,129.1,129.0,119.1,112.8,110.5,104.0,101.3,68.2,61.2,58.6,55.2,52.4,50.2,29.6.HRMS(ESI-TOF)m/z.C21H25N2O2S+[M+H]+理论值 369.163 1,实测值369.162 9.
化合物2-41 为无色油状物,收率64%.1H NMR(400 MHz,DMSO-d6)δ 2.47(t,J =4.4 Hz,4H),2.68(t,J =6.0 Hz,2H),3.57(t,J =4.8 Hz,4H),4.05(d,J=6.0 Hz,2H),5.36(s,2H),6.38(d,J=2.4 Hz,1H),,6.74(dd,J=2Hz,J=8.8 Hz,1H),7.07(d,J=2.4 Hz,1H),7.16(d,J=6.8 Hz,2H),7.22~7.24(m,1H),7.27~7.31(m,3H),7.44(d,J=3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.1,138.9,131.5,131,129.2,128.9,127.7,127.4,112.2,111.3,103.8,101.1,66.7,66.3,57.7,54.1,49.7.HRMS(ESI-TOF)m/z.C21H25N2O2+[M+H]+理论值337.191 1,实测值337.189 5.
化合物2-42 为无色油状物,收率73%.1H NMR(400 MHz,DMSO-d6)δ 2.47(t,J=4.0 Hz,4H)2.68(t,J=6.0 Hz,2H),3.58(t,J=4.4 Hz,4H),4.06(d,J=6.0 Hz,2H),5.38(s,2H),6.40(d,J=3.2 Hz,1H),6.75(dd,J=2.4 Hz,J=8.8 Hz,1H),7.08(d,J=2 Hz,1H),7.15(d,J=8.0 Hz,1H),7.25(t,J=8.0 Hz,1H),7.31(d,J=8.8 Hz,1H),7.36(s,1H),7.43(d,J =8.0 Hz,1H),7.46(d,J =2.8 Hz,1H).13C NMR(100 MHz,CDCl3)δ 153.3,140.0,131.6,130.7,130.3,129.6,129.1,128.7,125.2,122.8,112.7,110.3,103.0,101.6,66.9,66.5,57.8,54.1,49.6.HRMS(ESI-TOF)m/z.C21H24N2O2Br+[M+H]+理论值415.101 6,实测值415.100 5.
化合物2-43 为无色油状物,收率82%.1H NMR(400 MHz,DMSO-d6)δ 2.47(s,4H),2.68(t,J=6.0 Hz,2H),3.57(t,J=4.4 Hz,4H),4.06(d,J=6.0 Hz,2H),5.38(s,2H),6.40(d,J=2.8 Hz,1H),6.75(dd,J=2.4 Hz,J=8.8 Hz,1H),7.08(d,J=2 Hz,1H),7.11(d,J=6.8 Hz,1H),7.20(s,1H),7.29(d,J=8 Hz,1H),7.33(d,J=6.8 Hz,1H),7.46(d,J=2.8 Hz,1H).13C NMR(100 MHz,DMSOd6)δ 153.2,141.5,133.6,131.5,130.9,130.1,129.2,127.7,127.1,126.0,112.4,111.2,103.9,101.4,66.7,66.3,57.7,54.1,49.0.HRMS(ESI-TOF)m/z.C21H24N2O2Cl+[M+H]+理论值 371.152 1,实测值371.1505.
化合物2-44 为无色油状物,收率54%.1H NMR(400 MHz,DMSO-d6)δ 2.47(t,J =4.4 Hz,4H),2.68(t,J =6.0 Hz,2H),3.58(t,J =4.4 Hz,4H),3.68(s,3H),4.05(t,J=6.0 Hz,2H),5.32(s,2H),6.38(d,J=3.2 Hz,1H),6.71(d,J=7.6 Hz,1H),6.73~6.75(m,2H),6.80(dd,J=2.4 Hz,J=8.0 Hz,1H),7.07(d,J=2.4 Hz,1H),7.20(t,J=8.0 Hz,1H),7.30(d,J=8.8 Hz,1H),7.43(d,J=2.8 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 159.8,153.1,140.5,131.6,130.1,130.1,129.2,119.5,113.4,112.8,112.2,111.3,103.8,101.1,66.7,66.3,57.7,55.4,54.2,49.6.HRMS(ESI-TOF)m/z.C22H27N2O3+[M+H]+理论值367.197 7,实测值367.201 2.
化合物2-45 为无色油状物,收率34%.1H NMR(400 MHz,DMSO-d6)δ 1.71(t,J =3.6 Hz,4H),2.62(s,4H),2.88(t,J =6.0,Hz,2H),4.07(t,J =6.0 Hz,2H),5.42(s,2H),6.43(d,J=3.2 Hz,1H),6.51~6.53(m,1H),6.76(dd,J=2.4 Hz,J=8.8 Hz,1H),7.11(d,J=2.0 Hz,1H),7.20~7.23(m,2H),7.39(d,J =3.2 Hz,1H),7.65~7.67(m,1H).13C NMR(100 MHz,DMSO-d6)δ 153.2,137.6,133.1,131.6,13.2,129.8,129.7,128.6,128.5,122.2,112.4,111.1,103.9,101.5,67.3,54.9,54.5,49.9,23.5.HRMS(ESI-TOF)m/z.C21H24N2OBr+[M+H]+理论值399.106 7,实测值399.106 1.
化合物 2-46 为无色油状物,收率 46%.1H NMR(400 MHz,DMSO-d6)δ 1.68(s,4H),2.62(s,4H),2.81(t,J=6.0 Hz,2H),4.04(t,J=6.0 Hz,2H),5.34(s,2H),6.39(d,J=2.8 Hz,1H),6.74(dd,J=2.0 Hz,J=8.8 Hz,1H),7.07(d,J=2.0 Hz,1H),7.10(d,J=8.4 Hz,1H),7.28(d,J=8.8 Hz,2H),7.43(d,J=3.2 Hz,1H),7.48(d,J=8.0 Hz,2H).13C NMR(100 MHz,DMSO-d6)δ 153.2,138.3,131.8,131.4,130.0,129.6,129.3,120.8,112.3,111.2,103.8,101.3,67.5,54.9,54.5,49.0,23.6.HRMS(ESITOF)m/z.C21H24N2OBr+[M+H]+理论值399.106 7,实测值399.106 0.
化合物2-51 为无色油状物,收率41%.1H NMR(400 MHz,DMSO-d6)δ 1.50~1.56(m,1H),1.58~1.68(m,2H),1.75~1.84(m,1H),2.30(dd,J =8.8 Hz,J=16.4 Hz,1H),2.53~2.55(m,1H),2.65~2.71(m,1H),3.09~3.19(m,2H),3.22~3.27(m,1H),3.39~3.42(m,1H),4.02(t,J=6.0 Hz,2H),4.37(s,1H),5.36(s,2H),6.38(d,J=2.8 Hz,1H),6.73(dd,J =2.0 Hz,J =8.8 Hz,1H),7.06(d,J =2.0 Hz,1H),7.17(d,J=7.2 Hz,2H),7.21~7.24(m,1H),7.27~7.31(m,3H),7.43(d,J=3.2 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 153.1,138.9,131.5,130.0,129.2,129.0,127.7,127.4,112.2,111.3,103.8,101.1,68.0,65.9,64.8,55.2,54.3,28.3,23.4.HRMS(ESI-TOF)m/z.C22H27N2O2+[M+H]+理论值351.202 8,实测值351.206 2.
2.2 吲哚衍生物的体外LTA4H 抑制活性
对两类吲哚衍生物分别进行了LTA4H 氨肽酶抑制活性测试,结果见表1 和表2.
从表1 可以看出:在两个浓度下,化合物2-10、2-12、2-15、2-18 氨肽酶抑制活性与Bestatin 相当甚至更优,分析其结构发现吲哚1 位的亲水基团为四氢吡咯环或N,N-二甲胺基时,吲哚5 位疏水端的苯环有对位取代基团,尤其是氰基、羟乙基时氨肽酶抑制活性较好.
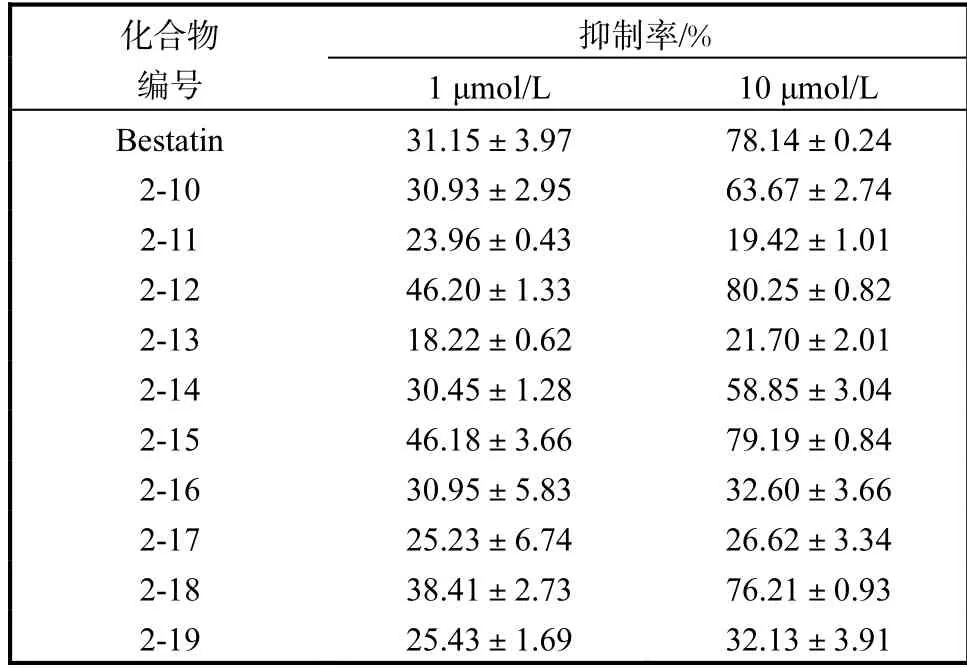
表1 第一类化合物LTA4H 氨肽酶抑制活性Tab.1 The LTA4H aminopeptidase inhibitory activity of the first type compound
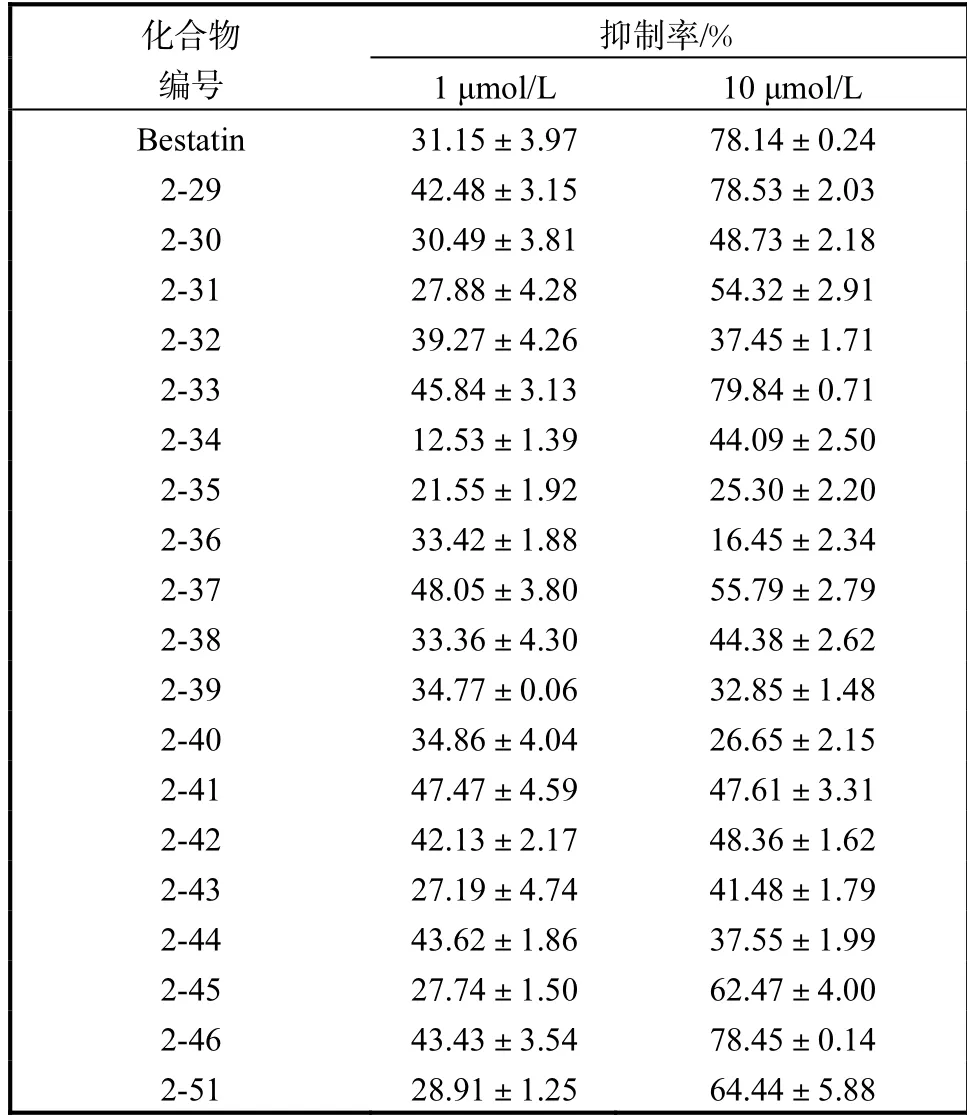
表2 第二类化合物LTA4H 氨肽酶抑制活性Tab.2 The LTA4H aminopeptidase inhibitory activity of the second type compound
从表 2 可以看出:大部分化合物都具有和Bestatin 类似的活性,在1µmol/L 浓度下,化合物2-29、2-33、2-37、2-41、2-44、2-46 的抑制率均高于Bestatin,在10µmol/L 浓度下,化合物2-29、2-33、2-46 抑制率高于Bestatin.结构分析发现:当吲哚5 位的亲水基团为吡咯烷或哌啶环,吲哚1 位的苯环上无取代或间位甲氧基取代、对位溴取代时,化合物对LTA4H 氨肽酶抑制活性较好.
对于抗肿瘤作用来说,LTA4H 的氨肽酶活性和水解酶活性是一致的,即两种活性的抑制效果越好,其对抗肿瘤的预期效果也越好.因此,选取部分氨肽酶抑制活性较好的化合物进行LTA4H 水解酶抑制活性测试,结果见表3.

表3 化合物LTA4H水解酶抑制活性Tab.3 The LTA4H hydrolase inhibitory activity of the compound
从表3 可看出:所选化合物也同样具备LTA4H水解酶抑制活性,尤其是10µmol/L 下活性基本与Bestatin 相似或稍差,而化合物2-10、2-12、2-29、2-33在低浓度下也有较好的水解酶抑制活性.
2.3 吲哚衍生物与紫杉醇偶联物的表征
在与紫杉醇偶联形成孪药时,选择了LTA4H 氨肽酶抑制活性和水解酶抑制活性均较好且结构中有合适官能团进行连接的两个吲哚衍生物(2-18 和2-51),通过丁二酰基与紫杉醇侧链羟基相偶联,制备得到两个偶联物,分别为2-48 和2-53,结构表征如下:
化合物2-48 为白色固体,收率88%.1H NMR(400 MHz,DMSO-d6)δ 1.00(s,3H),1.03(s,3H),1.08~1.14(m,1H),1.23~1.27(m,3H),1.50(s,3H),1.77(d,J=5.2 Hz,2H),2.09(s,3H),2.21~2.23(m,9H),2.29~2.33(m,1H),2.59~2.66(m,6H),3.58~3.59(m,1H),3.98~4.04(m,2H),4.10~4.12(m,1H),4.24~4.28(m,2H),4.63(d,J =4.0 Hz,1H),4.85~4.92(m,2H),5.34~5.37(m,1H),5.42(d,J=5.6 Hz,1H),5.52~5.58(m,1H),5.69~5.74(m,1H),5.80~5.86(m,1H),6.30(s,1H),6.38(d,J=1.6 Hz,1H),6.89(dd,J=1.6 Hz,J=8.8 Hz,3H),7.18~7.20(m,2H),7.26~7.29(m,2H),7.42~7.55(m,9H),7.64~7.67(m,2H),7.71~7.73(m,1H),7.84~7.87(m,2H),7.98(d,J =8.0 Hz,2H),9.22(dd,J=3.2 Hz,J=8.4 Hz,1H).13C NMR(100 MHz,DMSO-d6)δ 202.9,172.0,171.3,170.1,169.5,169.5,169.3,167.0,166.9,165.7,159.1,158.9,157.3,149.4,149.3,140.0,137.8,135.6,135.4,134.8,134.7,134.0,133.9,133.39,133.36,132.0,130.7,130.5,130.1,129.2,128.8,128.7,128.1,128.1,128.0,127.9,117.3,117.2,115.0,114.9,111.4,111.3,111.2,101.1,84.1,80.8,77.2,75.8,75.2,75.0,72.2,71.3,70.9,58.9,58.0,57.9,55.4,54.5,46.6,45.6,44.1,43.5,41.2,26.8,23.8,23.0,22.5,22.3,21.9,21.1,14.4,10.3.HRMS(ESI-TOF)m/z.C71H78N3O18+[M+H]+理论值1 260.527 5,实测值1 260.526 3.
化合物2-53 为白色固体,收率51%.1H NMR(400 MHz,DMSO-d6)δ 1.00(s,3H),1.02(s,3H),1.09~1.15(m,1H),1.23~1.27(m,1H),1.50(s,4H),1.61~1.67(m,3H),1.77(s,3H),1.80~1.84(m,1H),2.08(s,3H),2.23(s,3H),2.31~2.33(m,2H),2.54~2.57(m,2H),2.62~2.64(m,2H),2.71(s,2H),3.08(s,2H),3.58(d,J=6.4 Hz,1H),3.75~3.82(m,1H),3.94~3.95(m,1H),3.98~4.04(m,1H),4.63(s,1H),4.89~4.91(m,2H),5.34~5.36(m,3H),5.42(d,J=7.2 Hz,1H),5.55(t,J=8.8 Hz,1H),5.84(t,J =8.8 Hz,1H),6.30(s,1H),6.37(s,1H),6.72(d,J=8.4 Hz,1H),7.05(s,1H),7.15~7.20(m,3H),7.22~7.24(m,1H),7.26~7.30(m,3H),7.44~7.50(m,7H),7.53~7.55(m,1H),7.64~7.68(m,2H),7.71~7.75(m,1H),7.86(d,J=7.2 Hz,2H),7.98(d,J=7.2 Hz,2H),9.23(d,J=8.0 Hz,1H).13C NMR(100 MHz,DMSOd6)δ 202.9,171.9,170.1,169.5,169.2,166.9,165.7,139.9,138.9,137.8,134.7,134.0,133.9,132.0,131.5,130.4,130.1,129.2,129.0,128.8,128.7,128.1,127.9,127.7,127.4,112.2,111.3,103.8,101.1,84.1,80.7,77.2,75.8,75.2,75.0,71.2,70.9,67.9,67.3,57.9,55.4,54.9,54.4,54.1,49.7,46.5,43.4,28.9,28.3,26.8,23.8,23.5,23.0,21.9,21.1,14.4,10.3.HRMS(ESI-TOF)m/z.C73H80N3O18+[M+H]+理论值1 286.539 2,实测值1 286.543 7.
2.4 吲哚衍生物与紫杉醇偶联物的体外活性
体外评价2 个偶联物及其前体化合物对两种肿瘤细胞增殖的抑制活性,结果见表4.偶联前化合物2-18 和2-51 对HCT116 细胞均显示一定抑制活性.化合物2-18 对H460 细胞未表现出明显活性,而化合物2-51 则有一定的抑制活性,两个吲哚衍生物分别与紫杉醇偶联后所得到的化合物2-48 和2-53有同样的趋势,即对HCT116 细胞的抑制作用更强.对结肠癌细胞HCT116 的这种选择性可能与已报道的LTA4H 在结肠癌细胞中的高表达有关,因为结直肠癌通常是由长期的慢性炎症和溃疡导致,因此本文所设计合成的吲哚衍生物以及与紫杉醇的偶联物对与炎症高度相关的肿瘤具有重要的研究价值.
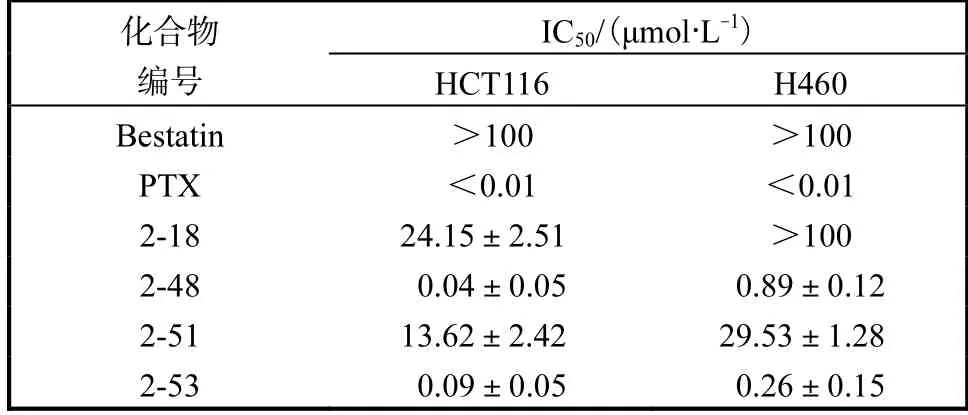
表4 化合物抑制肿瘤增殖活性Tab.4 The antitumor activity of compounds
3 结论
根据LTA4H 抑制剂的研究进展以及炎症与癌症的联系,结合DG-051 的结构和LTA4H 的晶体结构,设计合成两类共计30 个1,5-二取代吲哚衍生物,均为全新化合物.LTA4H 抑制活性测试结果表明,大部分化合物都显示出较好的LTA4H 抑制活性,其中化合物2-12、2-15、2-18、2-29 和2-33 抑制活性尤为显著.初步构效分析发现:以吲哚为母核结构,吲哚环的1 位选择N,N-二甲胺基作为亲水基团,5 位选择苯环、对位羟乙基或氰基取代的苯环作为疏水基团时,或者5 位选择四氢吡咯环、哌啶环作为亲水基团,1 位选择苯环取代时,对LTA4H 的抑制活性更高.为了探索该类化合物在抗肿瘤领域的应用,选取LTA4H 抑制活性较好的2 个化合物与PTX 共价偶联并进行体外抗肿瘤活性测试,偶联化合物2-48 和2-53 表现出对结肠癌细胞HCT116 较好的抑制效果,与预期一致.
