维药多脂大戟治疗白内障活性部位化学成分研究
2021-01-27廖东波朱智英范淼崟张亚梅
廖东波 杨 帆 朱智英 范淼崟 张亚梅
江西中医药大学,江西 南昌 330004
多脂大戟EuphorbiaresiniferaBerg.隶属于大戟科Euphorbiaceae,其树脂状分泌物大戟脂(Euphorbium)即维药“排日非云”,具有散寒止痛、燥湿退肿、强筋健骨、消除白内障等功效,主治湿寒性或黏液质性疾病,如瘫痪、关节疼痛、腹水、白内障等疾病[1-2]。课题组前期通过硒性白内障大鼠动物模型筛选多脂大戟治疗白内障活性部位,发现大戟脂95%乙醇提取物的乙酸乙酯萃取部位具有治疗白内障活性。基于此,本实验对此部位进行化学成分研究,以探明多脂大戟治疗白内障的物质基础。
1 仪器与材料
1.1 仪器 Shimazu LC-6AD制备液相色谱仪,Buchi C605中压色谱仪,Agilent 1200液相色谱仪,Buchi R-220SE旋转蒸发仪,TripleTOF5600 质谱仪 (ESI-MS),Bruker AVANCE III 600 核磁共振波谱仪。
1.2 材料 青岛海洋硅胶G (100~200、200~300目),Sephadex LH-20凝胶,反相硅胶C18(50 μ),MCI HP-20,色谱用甲醇、乙腈,其余试剂均为分析纯。多脂大戟树脂购于新疆和田药材市场,经江西中医药大学钟国跃研究员鉴定为大戟科 Euphorbiaceae 植物多脂大戟E.resinifera的树脂状分泌物。标本现存于江西中医药大学中药资源与民族药研究中心标本馆。凭证标本(20180804)存放于江西中医药大学标本馆。
2 提取分离
多脂大戟树脂活性部位(95%乙醇提取物的乙酸乙酯萃取部位)62.5 g,加甲醇溶解后,通过硅胶色谱柱进行初步分离,依次用石油醚-丙酮(50∶1,Fi1),石油醚-丙酮(30∶1,Fi2)、石油醚-丙酮(15∶1,Fi3),石油醚-丙酮(5∶1,Fi4)、石油醚-丙酮(1∶1,Fi5)、乙酸乙酯(Fi6)梯度洗脱,甲醇冲柱(Fi7)。回收溶剂得到7个洗脱部分(Fi1-Fi7)。薄层色谱显色石油醚-丙酮(30∶1),即Fi2部分化合物多,且与其它组分重叠斑点较少。 故将其进一步通过MCI柱层析、凝胶柱层析、中压反相硅胶柱层析、制备反相硅胶柱层析分离,最终得到6个化合物。
3 结构鉴定
各化合物通过TLC、HPLC、香草醛浓硫酸显色、UV、以及MS、NMR等方法确定了结构。
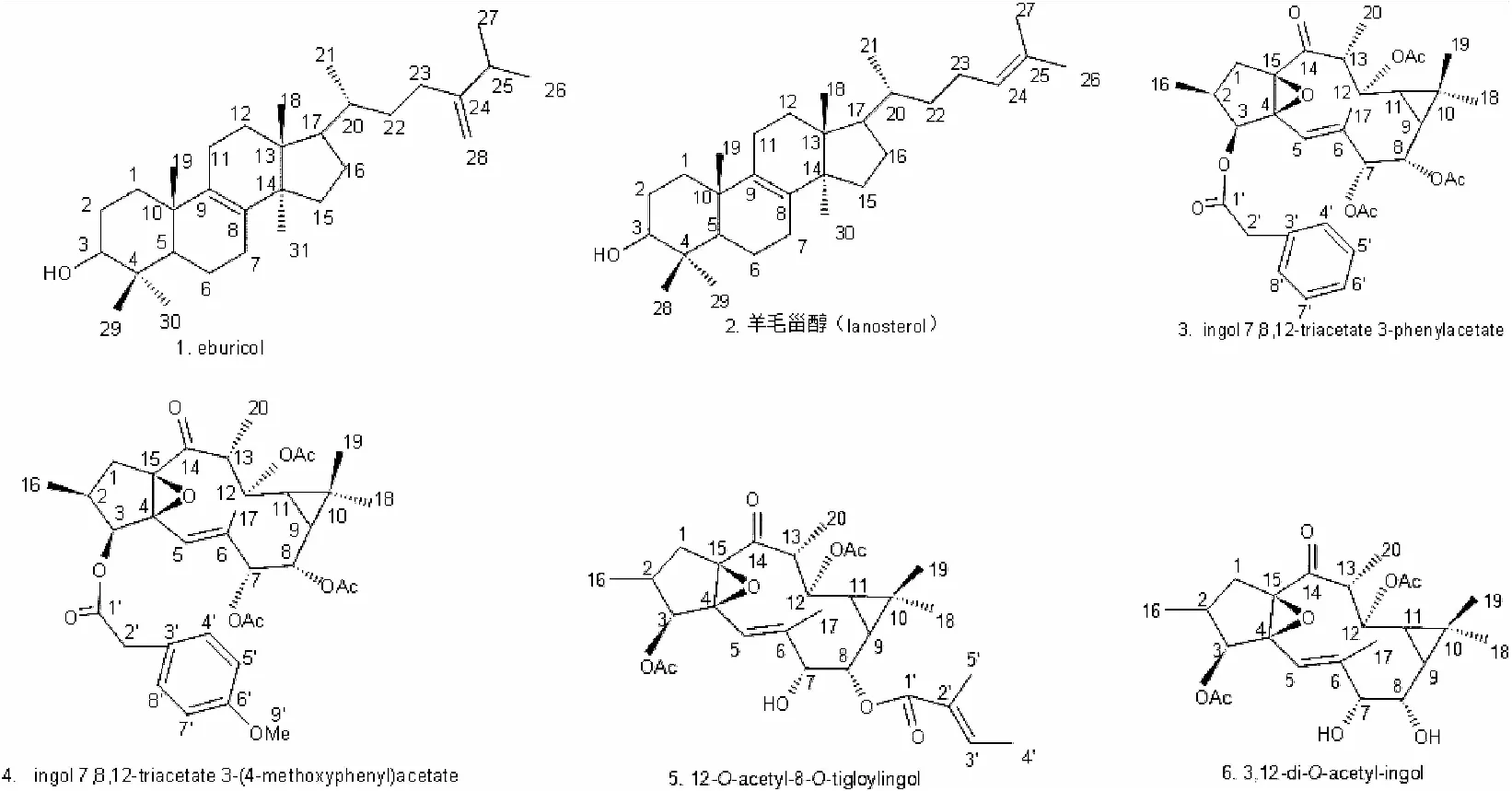
图1 化合物结构式
化合物1(246.9 mg): 白色粉末。HR-ESI-MS:分子量440,分子式C31H52O。1H-NMR (600 MHz, CDCl3) δ: 4.73 (1H, brs, H-28α), 4.68 (1H, brs, H-28β), 3.23 (1H, m, H-3), 1.02 (3H, d,J= 7.1 Hz, H-26), 1.01 (3H, d,J=7.1 Hz, H-27), 0.99 (3H, s, H-29), 0.94 (3H, s, H-19), 0.96 (3H, d,J= 6.3 Hz, H-21), 0.86 (6H, s, H-30, 31), 0.74(3H, s, H-18);13C NMR (150 MHz, CDCl3) δ: 35.5 (C-1), 27.6 (C-2), 76.3 (C-3), 39.2 (C-4), 50.8 (C-5), 17.6 (C-6), 28.1 (C-7), 133.5 (C-8), 134.3 (C-9), 36.6 (C-10), 20.1 (C-11), 25.7 (C-12), 31.2 (C-13),49.6 (C-14), 30.9 (C-15), 30.7 (C-16), 50.1 (C-17), 15.6 (C-18), 18.3 (C-19), 34.9 (C-20), 18.8 (C-21), 33.9 (C-22), 31.2 (C-23), 157.1 (C-24), 31.3 (C-25), 21.6 (C-26), 22.1 (C-27), 106.1 (C-28), 27.9 (C-29), 15.4 (C-30), 24.5 (C-31)上述数据与文献报道一致[3],故鉴定为eburicol。
化合物2(611.2 mg):白色粉末。HR-ESI-MS:分子量426,分子式C30H50O。1H-NMR (600 MHz, CDCl3) δ: 0.75 (3H, s, CH3-30), 0.79 (3H, s, CH3-18), 0.84 (3H, d,J=6.5Hz, CH3-21), 0.87 (3H, s,CH3-28) , 0.91 (3H, s, CH3-29) , 1.59 (3H, s, CH3-26) , 1.68 (3H, s, H-27), 3.22 (1H, dd,J= 4.5, 2.3 Hz, H-3), 5.09 (1H, m, H-24);13C NMR (150 MHz, CDCl3) δ: 35.9 (C-1), 28.2 (C-2), 79.0 (C-3), 38.9 (C-4), 50.1 (C-5), 20.1 (C-6), 28.2 (C-7), 134.0 (C-8), 133.4 (C-9), 37.4 (C-10), 28.2 (C-11), 25.8 (C-12), 44.1 (C-13),49.6 (C-14), 30.9 (C-15), 29.9 (C-16), 50.0 (C-17), 15.6 (C-18), 18.7 (C-19), 35.0 (C-20), 18.8 (C-21), 35.3 (C-22), 24.8 (C-23), 125.4 (C-24), 130.8 (C-25), 24.7 (C-26), 17.7 (C-27), 24.4 (C-28), 27.8 (C-29), 15.6 (C-30)。上述数据与文献报道一致[4],故鉴定为羊毛甾醇。
化合物3(52.2 mg):白色粉末。 HR-ESI-MS:分子量610,分子式C34H42O10。1H-NMR (600 MHz, CHCl3) δ: 2.78 (1H, dd,J= 14.8、9.0 Hz, H-1α), 1.69 (1H, d,J=14.8 Hz, H-1β), 2.51 (1H, m, H-2),5.15 (1H, brs, H-3), 5.39 (1H, brs, H-5), 5.12 (1H, d,J=1.6 Hz, H-7), 4.52 (1H, dd,J= 10.4、1.6 Hz, H-8), 1.09 (1H, dd,J=10.4, 9.3Hz, H-9), 1.01 (1H, m, H-11), 4.82 (1H, dd,J= 10.7、3.8 Hz, H-12), 2.90 (1H, qd,J= 7.4、3.8 Hz, H-13), 0.92 (3H, d,J= 7.4 Hz, H-16), 2.05 (3H, 3, H-17), 1.04 (3H, s, H-18), 0.82 (3H, s, H-19), 1.04 (3H, d,J= 7.0 Hz, H-20), 3.72 (2H, br, H-2′), 7.31 (1H, m, H-4′) , 7.31 (1H, m,J= 8.6 Hz, H-5′), 7.31 (1H, m, H-7′), 7.31 (1H, d,J= 8.6 Hz, H-8′) , 2.09 (3H, s, COCH3), 2.05 (3H, s, COCH3), 1.97 (3H, s,COCH3)。13C NMR (150 MHz, CDCl3) δ: 31.4 (C-1), 29.5 (C-2), 76.3 (C-3), 73.4(C-4), 117.1 (C-5), 139.3 (C-6), 76.9 (C-7), 71.1 (C-8), 24.7 (C-9), 19.2 (C-10), 30.6 (C-11), 70.6 (C-12) , 43.0 (C-13), 207.9(C-14), 71.5 (C-15), 16.9 (C-16), 17.5 (C-17), 29.1 (C-18), 16.1 (C-19), 13.4 (C-20), 170.3 (C-1′), 41.5 (C-2′), 133.8 (C-3′), 129.3 (C-4′), 128.6 (C-5′), 127.4 (C-6′), 128.6 (C-7′), 129.3 (C-8′), 170.8 (COCH3), 170.3(COCH3),170.4(COCH3),21.0 (COCH3), 20.9 (COCH3), 20.6 (COCH3)。以上数据与文献报道一致[5],确定为ingol 7,8,12-triacetate-3-phenylacetate。
化合物4(30.4 mg):白色粉末。 HR-ESI-MS:分子量 640,分子式C35H45O11。1H-NMR (600 MHz, CHCl3) δ: 2.78 (1H, dd,J= 15.0、9.0 Hz, H-1α), 1.68 (1H, d,J=15.0Hz, H-1β), 2.51 (1H, m, H-2),5.16 (1H, d,J= 8.6 Hz, H-3), 5.40 (1H, brs, H-5), 5.14(1H, d,J=1.6 Hz, H-7), 4.54 (1H, dd,J= 10.6、1.6 Hz, H-8), 1.10 (1H, dd,J=10.6、9.1Hz, H-9), 1.04 (1H, m, H-11), 4.83 (1H, dd,J= 10.6、4.0 Hz, H-12), 2.89 (1H, qd,J= 7.3、4.0 Hz, H-13), 0.92 (3H, d,J= 7.3 Hz, H-16), 2.06 (3H, 3, H-17), 1.04 (3H, s, H-18), 0.82 (3H, s, H-19), 1.05 (3H,J=7.0Hz, H-20), 3.80 (3H, s, OCH3), 3.67 (2H, brs, H-2′), 7.20 (1H, m, H-4′) , 6.85 (1H, m, H-5′), 6.85 (1H, m, H-7′), 7.20 (1H, m, H-8′) , 2.06 (3H, s, COCH3), 2.09 (3H, s, COCH3), 1.97 (3H, s,COCH3)。13C NMR (150 MHz, CDCl3) δ: 31.5 (C-1), 29.5 (C-2), 76.9 (C-3), 73.4(C-4), 117.1 (C-5), 139.4 (C-6), 76.9 (C-7), 71.5 (C-8), 24.6 (C-9), 19.3 (C-10), 30.6 (C-11), 70.6 (C-12) , 43.0 (C-13), 207.8 (C-14), 71.1 (C-15), 16.9 (C-16), 17.4 (C-17), 29.1 (C-18), 16.0 (C-19), 13.4 (C-20), 170.4 (C-1′), 40.6 (C-2′), 113.9 (C-3′), 130.4 (C-4′), 125.7 (C-5′), 158.4 (C-6′), 125.7 (C-7′), 130.4 (C-8′), 55.4 (-OCH3), 170.6(COCH3), 20.6(COCH3), 170.5 (COCH3), 20.9 (COCH3), 170.3(COCH3), 21.0(COCH3)。以上数据与文献报道一致[6],确定为ingol 7,8,12-triacetate 3-(4-methoxyphenyl)acetate。
化合物5(21.1 mg):白色粉末。 HR-ESI-MS:分子量 490,分子式C27H38O8。1H-NMR (600 MHz, CHCl3) δ: 1.64 (1H, dd,J=14.9、1.9 Hz, H-1β), 2.76 (1H, dd,J=14.9、9.0 Hz, H-1α), 2.40 (1H, m, H-2),4.36 (1H, d,J= 8.2 Hz, H-3), 5.87 (1H, brs, H-5), 4.28 (1H, brs, H-7), 4.57 (1H, dd,J= 10.7, 1.4 Hz, H-8), 1.43 (1H, dd,J= 10.7, 9.5 Hz, H-9), 1.10 (1H, dd,J= 11.0, 9.5 Hz, H-11), 4.87 (1H, dd,J=11.0, 3.9 Hz, H-12), 2.92 (1H, dq,J=3.9, 7.3 Hz, H-13), 1.07 (3H, d,J= 7.6 Hz, H-16), 2.09 (3H, brs, H-17), 1.09 (3H, s, H-18), 0.82 (3H, s, H-19), 1.04 (3H, d,J= 7.3 Hz, H-20), 2.07(3H, s, OCH3), 6.91 (1H, qq,J=6.0, 1.2 Hz, H-3′), 1.81 (3H, dq,J=6.0, 1.6 Hz, H-4′) , 1.84 (3H, dq,J=1.6, 1.6 Hz, H-5′)。13C NMR (150 MHz, CDCl3) δ: 31.8 (C-1), 29.1 (C-2), 76.1(C-3), 74.3(C-4), 117.1 (C-5), 141.7 (C-6), 76.4 (C-7), 76.3 (C-8), 23.8 (C-9), 19.1 (C-10), 31.6 (C-11), 70.9 (C-12) , 43.2 (C-13), 207.9 (C-14), 72.8 (C-15), 16.3 (C-16), 17.5 (C-17), 29.2 (C-18), 16.3 (C-19), 13.4 (C-20), 170.7 (OCOCH3), 21.0 (COCH3),167.7 (C-1′), 138.2 (C-2′), 128.7 (C-3′), 12.0 (C-4′), 14.5 (C-5′)。以上数据与文献报道一致[7],确定为12-O-acetyl-8-O-tigloylingol 。
化合物6(47.5 mg):白色固体。HR-ESI-MS:分子量450,分子式C24H34O8;1H-NMR (600 MHz, CDCl3) δ: 2.82 (1H, dd,J= 15.0、9.3 Hz, H-1α), 1.66 (1H, d,J= 15.0, 1.9 Hz, H-1β), 2.58 (1H, m, H-2),5.21 (1H, d,J= 8.4 Hz, H-3), 5.75 (1H, s, H-5), 4.21 (1H, s, H-7), 3.46 (1H, dd,J= 10.3、1.9 Hz, H-8), 1.25 (1H, m, H-9), 1.06 (1H, m, H-11), 4.85 (1H, dd,J= 11.0、3.9 Hz, H-12), 2.93 (1H, m, H-13), 0.90 (3H, d,J= 7.4 Hz, H-16), 1.97 (3H, s, H-17), 1.09 (3H, s, H-18), 1.08 (3H, s, H-19), 1.04 (3H, d,J= 7.3 Hz, H-20), 2.10 (6H, s, C12OCOCH3, C3OCOCH3)。13C NMR (150 MHz, CDCl3) δ: 31.7 (C-1), 29.6 (C-2), 77.7(C-3), 71.3 (C-4), 116.6 (C-5), 141.7 (C-6), 78.2 (C-7), 71.5 (C-8), 27.5(C-9), 18.4 (C-10), 31.0 (C-11), 70.9 (C-12) , 43.1 (C-13), 207.7 (C-14), 73.9 (C-15), 17.0 (C-16), 17.9 (C-17), 29.4 (C-18), 16.5 (C-19), 13.4 (C-20), 20.8 (C3OCOCH3), 21.1 (C12OCOCH3), 170.9 (C3OCOCH3), 17.07 (C12OCOCH3)。以上数据与文献报道一致[8],确定为3,12-di-O-acetylingol。
4 结 论
维药“排日非云”在当地民间用于治疗白内障,但尚未见有关其清除白内障的药理和化学研究报道。本实验前期通过硒性白内障大鼠动物模型筛选出多脂大戟树脂治疗白内障的活性部位,通过进一步分离得到6个单体化合物, 其中羊毛甾醇及其类似物eburicol 为主要成分。研究表明,羊毛甾醇可以降低兔白内障晶状体白内障严重程度、提高透明度,并在体内试验中减轻了狗白内障严重程度[9]。据此,初步推断维药“排日非云”用于治疗白内障可能与所含大量的羊毛甾醇及类似物相关。
