Role of phosphorylated Smad3 signal components in intraductal papillary mucinous neoplasm of pancreas ✩
2021-01-07YuihiHoriTsuksIkeurTkshiYmguhiKtsunoriYoshidKoihiMtsuzkiMitsukiIshidSoheiStoiKzuihiOkzki
Yuihi Hori , Tsuks Ikeur , Tkshi Ymguhi , Ktsunori Yoshid , Koihi Mtsuzki ,Mitsuki Ishid , Sohei Stoi , Kzuihi Okzki , *
a Department of Gastroenterology and Hepatology, Kansai Medical University, 2-5-1 Shinmachi, Hirakata, Osaka 5731191, Japan
b Department of Pathology, Kansai Medical University, 2-5-1 Shinmachi, Hirakata, Osaka 5731191, Japan
c Department of Surgery, Kansai Medical University, 2-5-1 Shinmachi, Hirakata, Osaka 5731191, Japan
Keywords:Intraductal papillary mucinous neoplasms of the pancreas Immunohistochemistry Phosphorylated Smad Carcinogenesis Prognosis
A B S T R A C T Background: Malignant intraductal papillary mucinous neoplasm (IPMN) has poor prognosis. The carcinogenesis of IPMN is not clear. The aim of this study was to clarify transitions in phosphorylated Smad3 signaling during IPMN carcinogenesis.Methods: By using immunohistochemistry, we examined the expression of pSmad3C and pSmad3L from 51 IPMN surgical specimens resected at our institution between 2010 and 2013. We also examined the expression of Ki-67, c-Myc and p-JNK.Results: The median immunostaining index of pSmad3C was 79.2% in low-grade dysplasia, 74.9% in highgrade dysplasia, and 42.0% in invasive carcinoma ( P < 0.01), whereas that of pSmad3L was 3.4%, 4.3%,and 42.4%, respectively ( P < 0.01). There was a negative relationship between the expression of pSmad3C and c-Myc ( P < 0.001, r = -0.615) and a positive relationship between the expression of pSmad3L and c-Myc ( P < 0.001, r = 0.696). Negative relationship between the expression of pSmad3C and Ki-67( P < 0.01, r = -0.610) and positive relationship between the expression of pSmad3L and Ki-67 ( P < 0.01,r = 0.731) were confirmed. p-JNK-positive cells were frequently observed among pSmad3L-positive cancer cells. The median of pSmad3L/pSmad3C ratio in the non-recurrence group and the recurrence group were 0.58 (range, 0.05-0.93), 3.83 (range, 0.85-5.96), respectively ( P = 0.02). The median immunostaining index of c-Myc in the non-recurrence group and the recurrence group were 2.91 (range, 0-36.9)and 82.1 (range, 46.2-97.1), respectively ( P = 0.02). The median immunostaining index of Ki-67 in the non-recurrence group and the recurrence group were 12.9 (range 5.7-30.8) and 90.9 (range 52.9-98.5),respectively ( P = 0.02).Conclusions: pSmad3L was upregulated in malignant IPMN. pSmad3L/pSmad3C ratio may be a useful prognostic factor in IPMN.
Introduction
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is the precursor lesion of pancreatic cancer, characterized by intraductal papillary growth and mucin secretion [ 1 , 2 ]. Unlike pancreatic ductal adenocarcinoma (PDAC), which is characterized by a progressive pattern from pancreatic intraepithelial neoplasia(PanIN) to invasive ductal carcinoma [1] , the IPMN carcinogenesis proceeds in an adenoma-carcinoma sequence that evolves from adenoma (low-grade dysplasia) to non-invasive cancer(high-grade dysplasia), and eventually to invasive cancer (invasive carcinoma) [ 3 , 4 ]. IPMN is a slowly growing cancer; however, once it progresses to invasive carcinoma, the prognosis is poor, and the five-year survival is approximately 40% -60% [5] . Therefore,in clinical practice, it is important to make an accurate decision on the timing of surgical resection for high-grade dysplasia and invasive carcinoma and to avoid excessive and unnecessary surgery for low-grade dysplasia.
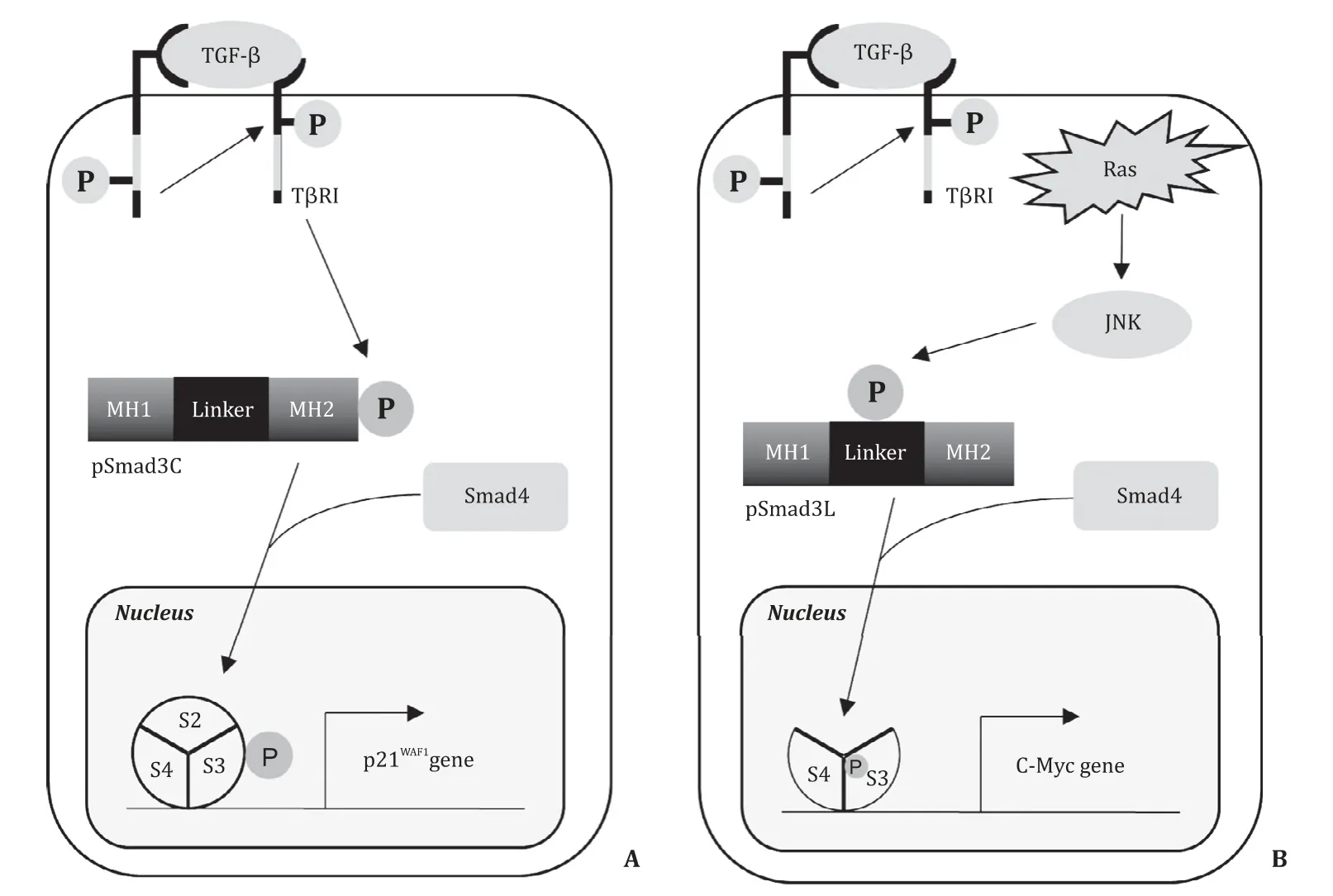
Fig. 1. The pathways of phosphorylated Smad3 signals in tumors. A : pSmad3C suppresses the cell growth via activation of p21 WAF1 gene; B : pSmad3L stimulates tumor proliferation via activation of c-Myc gene. TGF- β: transforming growth factor beta; T βRI: TGF- β type I receptor; MH1: mad homology 1; MH2: mad homology 2; Linker:linker lesion; JNK: c-Jun NH2-terminal kinase.
Transforming growth factor beta (TGF-β) is a multifunctional cytokine that regulates cell growth and differentiation, matrix production, and apoptosis. The TGF-βsignal is transmitted mainly through Smads, although a non-Smad transmission pathway is also known. Initially, TGF-βbinds to the type II receptor on cell membrane and recruits the type I receptor (TβRI). The activated TβRI catalytically phosphorylates the COOH-terminal serine residues of receptor-activated Smads (R-Smads), such as Smad2 and Smad3 [6] . The Smad protein is composed of Mad homology 1 (MH1), the intermediate linker, and Mad homology 2 (MH2)domains [7] . The linker domain of Smad3 is phosphorylated by the mitogen-activated protein kinase (MAPK) family members,including c-Jun NH2-terminal kinase (JNK) [7-11] . Thus, TβRI and JNK phosphorylate Smad3 in a different pathway. Three types of phospho-isoforms of Smad3 have been identified, namely COOH-terminally phosphorylated Smad3 (pSmad3C), linkerphosphorylated Smad3 (pSmad3L), and Smad3 phosphorylated at both the sites (pSmad3L/C). The phosphorylated Smad3 binds to Smad4, and is then translocated to the nucleus, where it regulates the expression of target genes, both by direct DNA binding and through interaction with other transcription factors, coactivators,and corepressors [ 7 , 9 ].
Smad-dependent signaling results in biphasic function through two distinct pathways: a cytostatic TGF-βsignal is transduced via pSmad3C and a pro-tumorigenic TGF-βsignal is transduced via pSmad3L ( Fig. 1 ). In normal epithelial cells, TGF-βmediated pSmad3C signaling represents a major growth inhibitory signal [12] . In cancer cells, on the other hand, JNK-mediated pSmad3L signaling interferes with pSmad3C signaling and promotes tumor development and growth by upregulation of c-Myc expression [ 7 , 9 , 12 ]. Although activated JNK induces the transition from a tumor-suppressive pSmad3C pathway to an oncogenic pSmad3L pathway during liver and colorectal carcinogenesis in humans [13] ,there are no published studies on pSmad3 signaling in IPMN.
The aim of the present study was to test a hypothesis that transition from a tumor-suppressive pSmad3C pathway to an oncogenic pSmad3L pathway occurs in the progress of IPMN from lowgrade to high-grade dysplasia, and eventually to invasive carcinoma. Moreover, we also determined whether the assessment of pSmad3C and pSmad3L in resected IPMN specimens would help in predicting the postoperative prognosis of patients with malignant IPMN.
Methods
Patients and tissues
This study was approved by the Ethics Committee of Kansai Medical University (No. H151047).
A retrospective analysis of data from 51 post-operative patients with IPMN registered in our institution from 2010 to 2013 was performed. Of the 51 patients, 38 underwent surgical resection because of presence of predictors for malignancy, such as enhancing solid component within cyst, main pancreatic duct>10 mm in size, and mural nodule, according to the international consensus guidelines for the management of IPMN [ 14 , 15 ]. The remaining 13 patients without predictors for malignancy had undergone surgical resection because of dilated main pancreatic duct (5-9 mm in diameter) or enlarging size of cyst during follow-up. Samples of normal pancreas were collected from the pancreatic tissue adjacent to the resected serous cyst neoplasms for use as controls. The following clinicopathological characteristics of patients were assessed:sex, age at surgery, image findings (main pancreatic duct diameter, existence of mural nodule, cyst diameter), laboratory data (carcinoembryonic antigen value, carbohydrate antigen 19-9 value),prognosis after surgery, and histological findings of the resected specimen.
Histological analysis
IPMNs were histologically categorized into IPMN with lowgrade dysplasia, IPMN with high-grade dysplasia, and invasive carcinoma according to the World Health Organization Classification of Tumors of the Digestive System [16] .
Domain-specific antibodies against phosphorylated Smad3
Two polyclonal anti-phospho-Smad3 sera, namely pSmad3L(Ser208/213) and pSmad3C (Ser423/425), were raised against the phosphorylated linker and COOH-terminal regions of Smad3 by immunizing rabbits with synthetic peptides. The antisera were affinity-purified using phosphorylated peptides as described below: one antiserum to the phosphorylated linker region of Smad3(αpSmad3L [Ser 208/213]) was raised against the synthetic peptide (amino acids 200-215), in which serine residues at positions 208 and 213 were phosphorylated. Another antiserum to the phosphorylated C-terminal region of Smad3 [αpSmad3C (Ser 423/425)] was raised against the synthetic peptide (amino acids 416-425), in which serine residues at positions 423 and 425 were phosphorylated. We previously confirmed that anti-pSmad3L antibody (Ab) and anti-pSmad3C Ab selectively recognized the phosphorylated linker region and phosphorylated C-terminal region,respectively [11] .
Immunohistochemistry
Immunohistochemical analysis using the two polyclonal antiphospho-Smad3 sera described above was performed as described previously [17] . Tissues were fixed by formalin within 3 h of resection, then fixed with 10% formalin solution for 24 h. Briefly,formalin-fixed and paraffin-embedded specimens were prepared,and 3-μm thick sections were cut. The sections were deparaffinized and rehydrated using xylene and a graded descending series of alcohol before staining. Nonenzymatic antigen retrieval was performed by heating the sections to 121 °C in 0.01 mol/L sodium citrate buffer (pH 6.0) for 15 min. After cooling, the sections were rinsed in Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated in a mixture of methanol and 3% H 2 O2for 30 min to quench the endogenous peroxidase activity. Subsequently, the slides were incubated overnight at room temperature with each Ab. Thereafter, the sections were rinsed in TBST, incubated with peroxidase-labeled polymer conjugated to goat anti-mouse or antirabbit immunoglobulin (DAKO) for 1 h at room temperature, and subsequently rinsed again in TBST. Finally, the sections were developed with 3,3 ′ -diaminobenzidine tetrahydrochloride (Vector Laboratories, Burlingame, CA), counterstained with hematoxylin (Merck,Darmstadt, Germany), and mounted under coverslips.
Primary antibodies used in this study included rabbit polyclonal anti-Smad3C (Ser 423/425 phosphorylated) Ab (0.5μg/mL;Immuno-Biological Laboratories Co., Gunma, Japan), rabbit polyclonal anti-Smad3L (Ser 208/213 phosphorylated) Ab (2μg/mL;Immuno-Biological Laboratories Co.), mouse monoclonal antic-Myc Ab (10μg/mL; Santa Cruz Biotechnology, Santa Cruz,CA, USA), mouse monoclonal anti-Ki-67 Ab (0.8μg/mL, DAKO,Glostrup, Denmark), anti-p-JNK Ab (G-7, 1:50; Santa Cruz Biotechnology.), and anti-Smad4 Ab (B-8, 1:100; Santa Cruz Biotechnology). The affinity-purified rabbit polyclonal anti-pSmad3L(2μg/mL) and anti-pSmad3C (0.5μg/mL) Abs were described above.
Evaluation of immunostaining for pSmad3C, pSmad3L, and Ki-67
The cells that were immunoreactive for pSmad3C, pSmad3L, c-Myc, and Ki-67 in IPMN were counted in three randomly selected fields of view under high magnification ( × 400). The average percentage of immunoreactive cells in the three areas was defined as the immunostaining index.

Table 1 Demographics of patients in the present study ( n = 51).
Statistical analysis
All statistical analyses were carried out using JMP statistical software (version 13; SAS Institute, Cary, NC, USA) and the results were expressed as median and range. Comparisons of categorical variables were conducted using the Fisher exact tests. Comparisons between the two groups and among the three groups were made using Mann-WhitneyUtest and Nonparametric Wilcoxon test, respectively.Pvalues less than 0.05 were considered statistically significant.
Results
Clinicopathological features of patients with IPMN
The clinicopathological features of patients enrolled in the current study are shown in Table 1. Of the 51 patients, 28 (54.9%)were diagnosed with low-grade dysplasia, 8 (15.7%) with highgrade dysplasia, and 15 (29.4%) with invasive carcinoma derived from IPMN. Based on the morphological type, three (5.9%) patients were classified into the main duct type, 17 (33.3%) were classified into the mixed type, and 31 (60.8%) were classified into the branch duct type of IPMN. Of the 51 patients, 5 received postoperative chemotherapy. Five (9.8%) patients had recurrence of carcinoma, and the median time to recurrence was 6.8 months (range 6.0-32.8 months).
Immunohistochemistry for Smad4
Expression of Smad4 was immnohistochemically confirmed in all cases (images not shown).
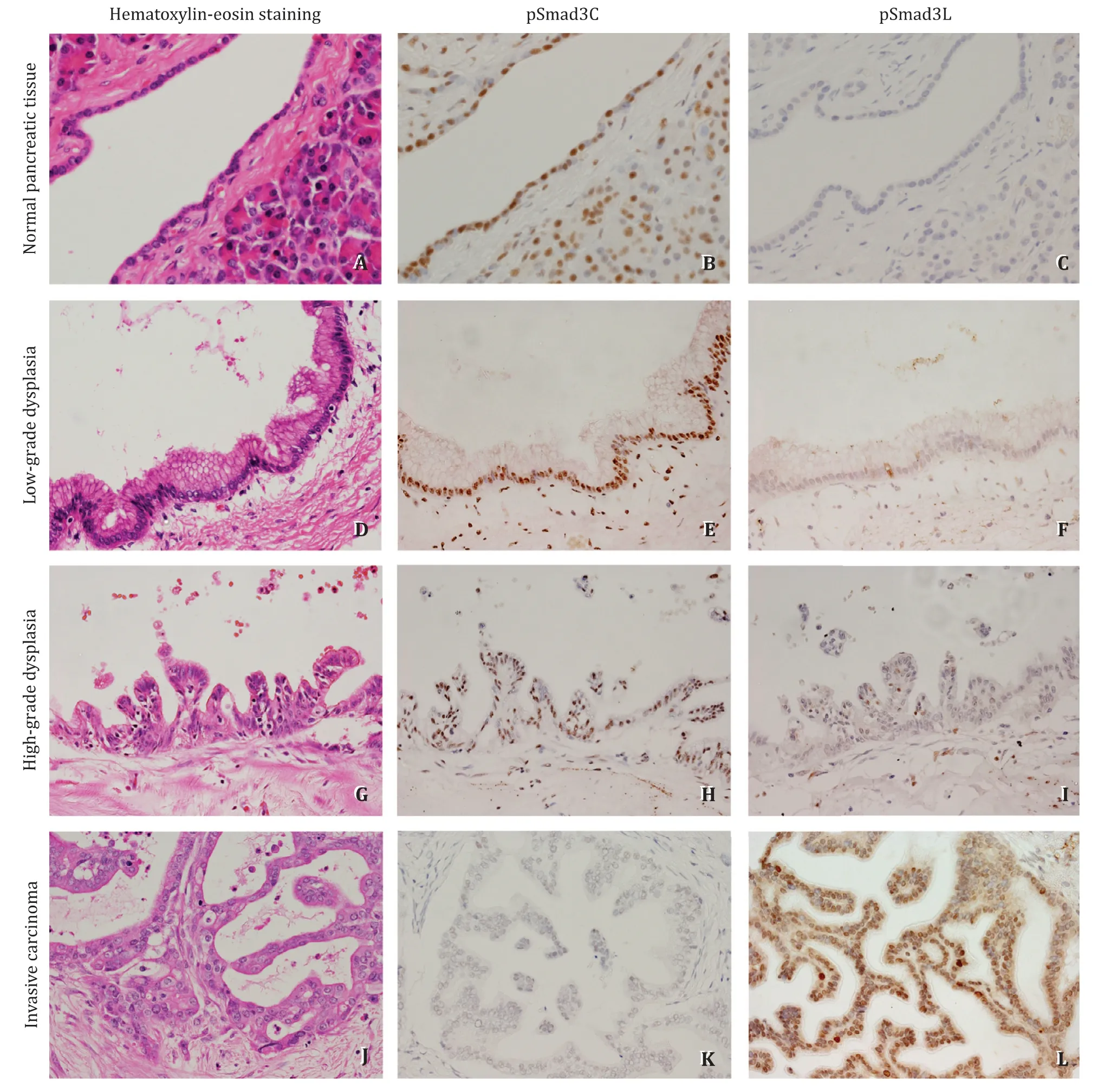
Fig. 2. Immunohistochemical staining of pSmad3L and pSmad3C in normal pancreatic tissue, IPMN with low- and high-grade dysplasia and invasive carcinoma. pSmad3C positive cells were gradually decreased following normal to invasive carcinoma transformation; pSmad3L positive cells were gradually increased following normal to invasive carcinoma transformation (original magnification × 400).
Immunohistochemistry for pSmad3L and pSmad3C in normal pancreatic tissues and IPMNs
In the normal pancreatic tissue, pSmad3C was frequently detected in the nucleus of pancreatic duct epithelial cells, acinar cells, and islets cells ( Fig. 2 B), whereas few pSmad3L-positive cells were observed in the specimen ( Fig. 2 C). The images of immunohistochemical staining for pSmad3L and pSmad3C in low-grade dysplasia, high-grade dysplasia, and invasive carcinoma are also shown in Fig. 2 . In IPMN with low-grade dysplasia, staining of pSmad3C was confirmed in the nucleus of numerous tumor cells( Fig. 2 E), whereas there were very few pSamd3L-positive tumor cells ( Fig. 2 F). In IPMN with high-grade dysplasia, less number of tumor cells were immunopositive for pSmad3C ( Fig. 2 H), whereas the immunopositivity of pSmad3L was increased compared to that in low-grade dysplasia ( Fig. 2 I). Furthermore, in invasive carcinoma, the expression of pSmad3C in tumor cells became much less( Fig. 2 K), whereas that of pSmad3L was enhanced ( Fig. 2 L).
The median total number of cells in the immunohistochemically evaluated area was 223.5 (165.5-304.5). As IPMN progressed from low-grade dysplasia to high-grade dysplasia, and subsequently to invasive carcinoma, the median immunostaining index of pSmad3C was decreased (79.2% in low-grade dysplasia, 74.9% in high-grade dysplasia, and 42.0% in invasive carcinoma) ( Fig. 3 A). Moreover,the median immunostaining index of pSmad3L increased with the progression of IPMN (3.4% in low-grade dysplasia, 4.3% in highgrade dysplasia, and 42.4% in invasive carcinoma) ( Fig. 3 B). There were statistically significant differences in the immunostaining index of pSmad3C and pSmad3L among low-grade dysplasia, highgrade dysplasia, and invasive carcinoma groups (P<0.01). The median of pSmad3L/pSmad3C ratio (the immunostaining index of pSmad3L divided by that of pSmad3C) in low-grade dysplasia, highgrade dysplasia, and invasive carcinoma were 0.04, 0.06, and 0.95,respectively, and the ratio significantly increased with the progression of IPMN (P<0.01, Fig. 3 C).

Fig. 3. Comparison of immunostaining index of pSmad3C and pSmad3L among low- and high-grade dysplasia, and invasive carcinoma. *: P < 0.01.
Distributive association of pSmad3L and c-Myc in invasive carcinoma derived from IPMN
Because pSmad3L signaling accelerates tumor growth and invasion in concordance with the up-regulation of c-Myc, we investigated the relationship between the distribution of pSmad3L and c-Myc in invasive carcinoma. The nuclei of numerous carcinoma cells exhibited immunoreactivity for c-Myc. In an adjacent serial section,immunoreactivity for pSmad3L was observed in the nuclei of cells in the cancerous area showing c-Myc positivity ( Fig. 4 ). Expression of c-Myc was rarely seen in low-grade dysplasia. The number of c-Myc positive cells increased in high-grade dysplasia, and c-Myc was more abundantly expressed in invasive carcinoma compared to that in low- and high-grade dysplasia ( Fig. 4 A-C). There was a significantly negative relationship between the expression of pSmad3C and c-Myc (P<0.001,r= -0.615; Fig. 4 D) and a significant positive relationship between the expression of pSmad3L and c-Myc (P<0.001,r= 0.696; Fig. 4 E).
Correlative evaluation of immunostaining index between pSmad3C and Ki-67 and between pSmad3L and Ki-67
The distribution of Ki-67-positive cells in IPMN is shown in Fig. 5 . Expression of Ki-67 was rarely seen in low-grade dysplasia( Fig. 5 A, D). The number of Ki-67-positive cells increased in highgrade dysplasia ( Fig. 5 B, E), and Ki-67 was more abundantly expressed in invasive carcinoma compared to that in low- and highgrade dysplasia ( Fig. 5 C, F). There was a significant negative relationship between the expression of pSmad3C and Ki-67 (P<0.01,r= -0.610) and a significant positive relationship between the expression of pSmad3L and Ki-67 (P<0.01,r= 0.731; Fig. 5 G, H).
Distributive association of pSmad3L and phosphorylated JNK (p-JNK)in invasive carcinoma
To investigate the upstream signal of pSmad3L, distributive association of pSmad3L and p-JNK was immunohistochemically evaluated. Significant expression of p-JNK was observed in the cytoplasm and nuclei of invasive carcinoma, and significant expression of pSmad3L was expressed in the nucleus in an adjacent serial section ( Fig. 6 ).
Comparison of pSmad3 signaling between the recurrence group and the non-recurrence group of IPMN with high-grade dysplasia/invasive carcinoma
To study the correlation between signal alteration of pSmad3 and recurrence of carcinoma, patients with high-grade dysplasia and invasive carcinoma were divided into two groups, namely recurrence group (n= 5) and non-recurrence group (n= 18). The pSmad3L/pSmad3C ratio between the two groups was compared. The median follow-up period of 23 patients with high-grade dysplasia and invasive carcinoma was 62 (38-75) days. The median pSmad3L/pSmad3C ratio in the non-recurrence group and the recurrence group were 0.58 (range, 0.05-0.93), 3.83 (range, 0.85-5.96)respectively (P= 0.02, Fig. 7 ). We also compared the immunostaining index of Ki-67 and that of c-Myc between the recurrence group and the non-recurrence group. The median immunostaining index of c-Myc in the non-recurrence group and the recurrence group were 2.91 (range, 0-36.9) and 82.1 (range, 46.2-97.1), respectively(P= 0.02). The median immunostaining index of Ki-67 in the nonrecurrence group and the recurrence group were 12.9 (range 5.7-30.8) and 90.9 (range 52.9-98.5), respectively (P= 0.02).
Discussion
The carcinogenesis of IPMN results from the accumulation of various molecular genetic abnormalities. An understanding of the molecular mechanisms can lead to the development of biomarkers for early detection of the cancer and devising of treatment strategies using agents for molecular targets. In the present study,a marked expression of cancer-related c-Myc was observed in pSmad3L-positive cells in invasive carcinoma, while pSmad3L and c-Myc-positive cells were less often seen in low-grade and highgrade dysplasia. Moreover, the expression of pSmad3L and Ki-67 exhibited a positive correlation. Similar to the previous studies on oncogenic process in liver and colorectal cancer [13] , these results suggest that pSmad3L can promote the proliferation of tumor cells through c-Myc in IPMN.
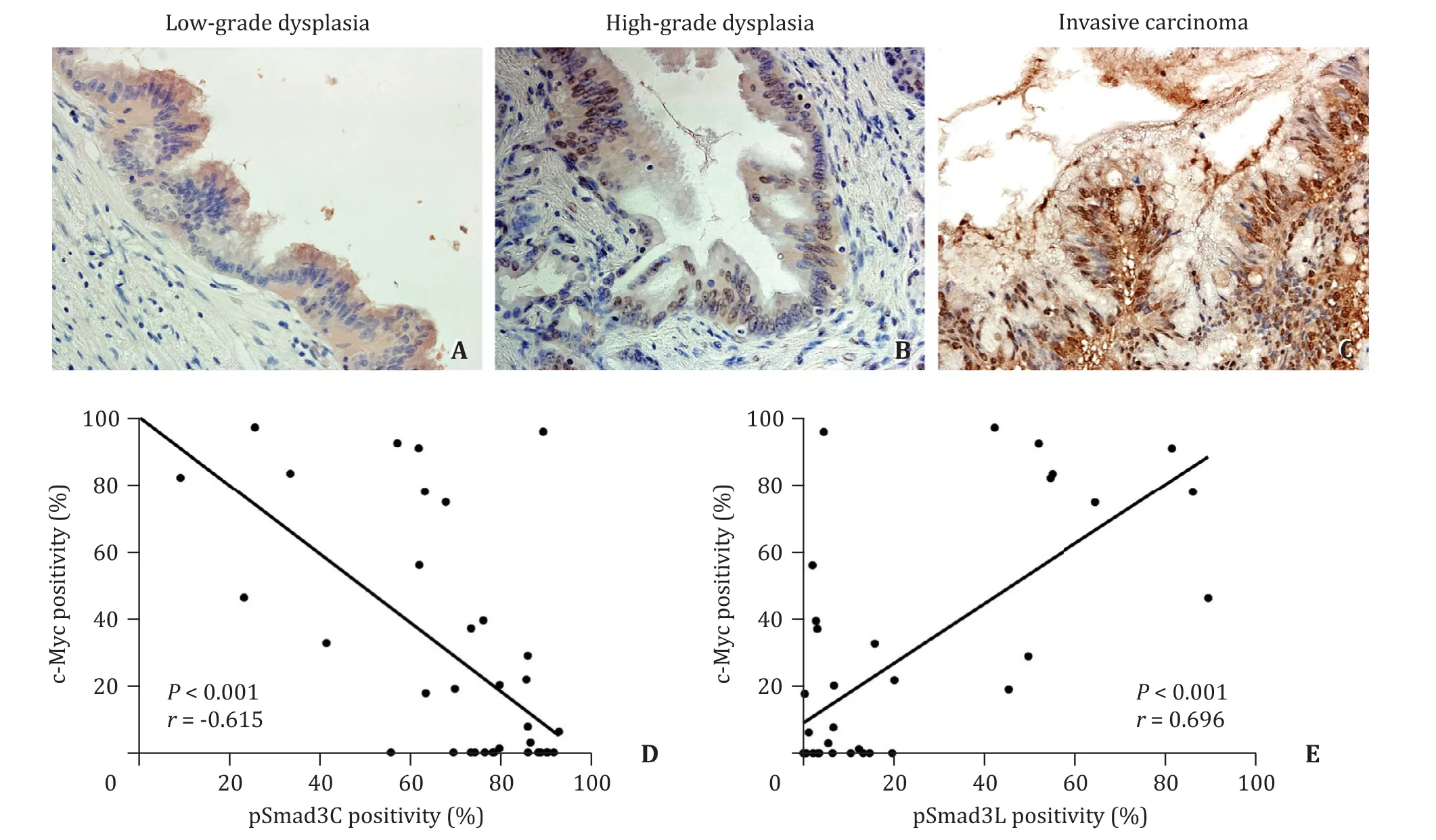
Fig. 4. c-Myc expression in different malignant grades (original magnification × 400, A - C ). There was a significantly negative relationship between the expression of pSmad3C and c-Myc ( D ) and a significantly positive relationship between the expression of pSmad3L and c-Myc ( E ).
Tumorigenic growth appears to be essentially a cellautonomous phenomenon that involves a shift from the tumorsuppressive pSmad3C pathway to the oncogenic pSmad3L pathway induced by alterations in the genome of cancer cells, such as in the Ras oncogene [12] . Previously, Sekimoto et al. demonstrated that Ras-activated JNK/pSmad3L signaling was able to provide oncogenic potential to the epithelial cells by up-regulation of c-Myc, resulting in strongly enhanced tumor growth and invasion [18] . In IPMN, mutation of Kras, the most frequently mutated Ras gene, was observed in 56%-81% of the cases, and its frequency was reported to increase with the progression of tumor [ 16 , 19 , 20 ]. Therefore, Kras-induced JNK/pSmad3L signaling may be one of the promoters of IPMN carcinogenesis.
The oncogenic pSmad3L signal and the tumor-suppressive pSmad3C signal have opposite functions. When phosphorylation of the linker region of Smad3 is specifically blocked by inhibiting JNK activation or by substituting the phosphorylation site of the linker region with an unphosphorylated amino acid, the pSmad signaling reverts from the oncogenic pSmad3L pathway to the tumor-suppressive pSmad3C pathway [ 12 , 21 ]. The reversibility from pSmad3L to pSmad3C pathway has been demonstrated not only in cultured cells, but also in animal experiments. As a result of administration of a JNK inhibitor to rats, hepatic carcinogenesis was significantly suppressed. In that case, alteration from the oncogenic pSmad3L/c-Myc signaling pathway to tumorsuppressive pSmad3C/p21WAF1signaling pathway was observed in hepatocytes of rat liver tissue upon administration of a JNK inhibitor. These results theoretically support the molecular target therapy of cancer. Considering the results obtained in the present study, biomolecular therapy targeting the JNK/pSmad3L signaling might offer an effective treatment for carcinoma derived from IPMN.
Currently, the Fukuoka criteria for the management of IPMN have been widely accepted. Although its sensitivity for prediction of high-grade dysplasia and invasive carcinoma in branch duct IPMN appears to be relatively high, the specificity still remains unsatisfactory [22] . Based on the results of the present study, preoperative molecular analysis of pSmad3C and pSmad3L in pancreatic juice obtained through endoscopic retrograde cholangiopancreatography (ERCP) or cystic fluid obtained using endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) may provide additional information on decision for surgery to improve the accuracy of the Fukuoka criteria.
Regarding Smad3 phosphorylation for predicting the risk of HCC, chronic hepatitis HBV/HCV patients, with high levels of pSmad3L and low pSmad3C in liver tissues, had a higher risk of developing HCC than those with low pSmad3L expression and high pSmad3C expression [23] . Thus, the grading of hepatocytic pSmad3C/3 L levels in HBV/HCV-inflected livers has been shown to be a clinically useful biomarker for predicting the risk of later development of HCC. In the present study, it has been demonstrated that the pSmad3L/pSmad3C ratio in the recurrence group was significantly higher than that in the non-recurrence group in resected pancreas in patients with high-grade dysplasia IPMN and invasive carcinoma. Regarding Ki-67 and c-Myc as well, significant differences were observed between the recurrence groups and nonrecurrence group. These results are considered reasonable because c-Myc and Ki-67 are downstream signals of pSmad3L. These data suggest that the immunohistochemical evaluation of pSmad3L and pSmad3C may be a potentially useful biomarker for prediction of postoperative recurrence in the patients with high-grade dysplasia and invasive carcinoma.
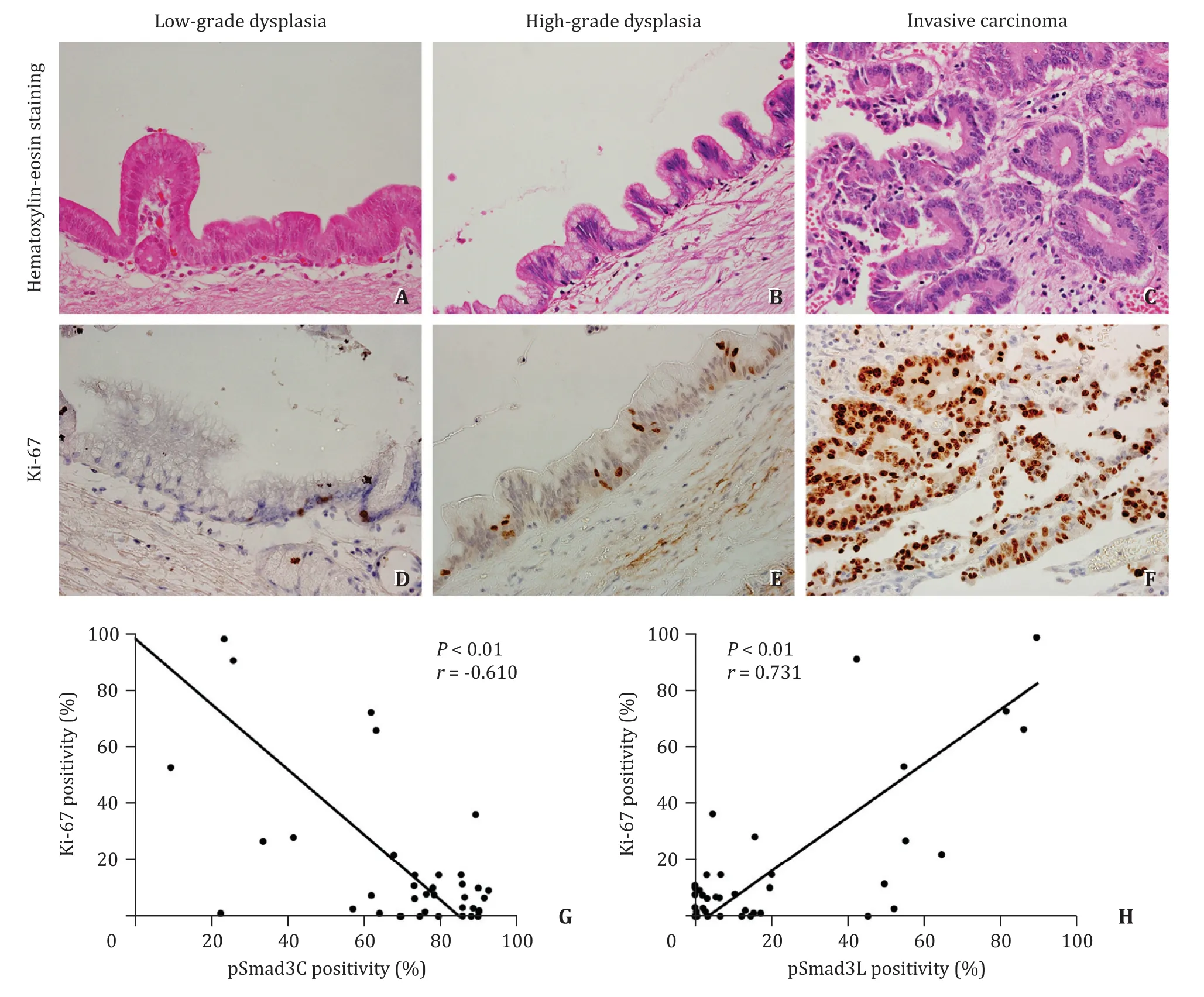
Fig. 5. Ki-67 staining and its correlation to phosphorylated Smad3. Hematoxylin and eosin staining of IPMN in different malignant grades (original magnification × 400,A-C ). HE staining pictures and Ki-67 staining pictures are serial sections. Ki-67-positive cells were increased as IPMN progressed from low-grade dysplasia, to high-grade dysplasia and subsequently to invasive carcinoma (original magnification × 400, D - F ). A significant negative relationship between the presence of pSmad3C and Ki-67 ( G )and a significant positive relationship between the presence of pSmad3L and Ki-67 were observed ( H ).
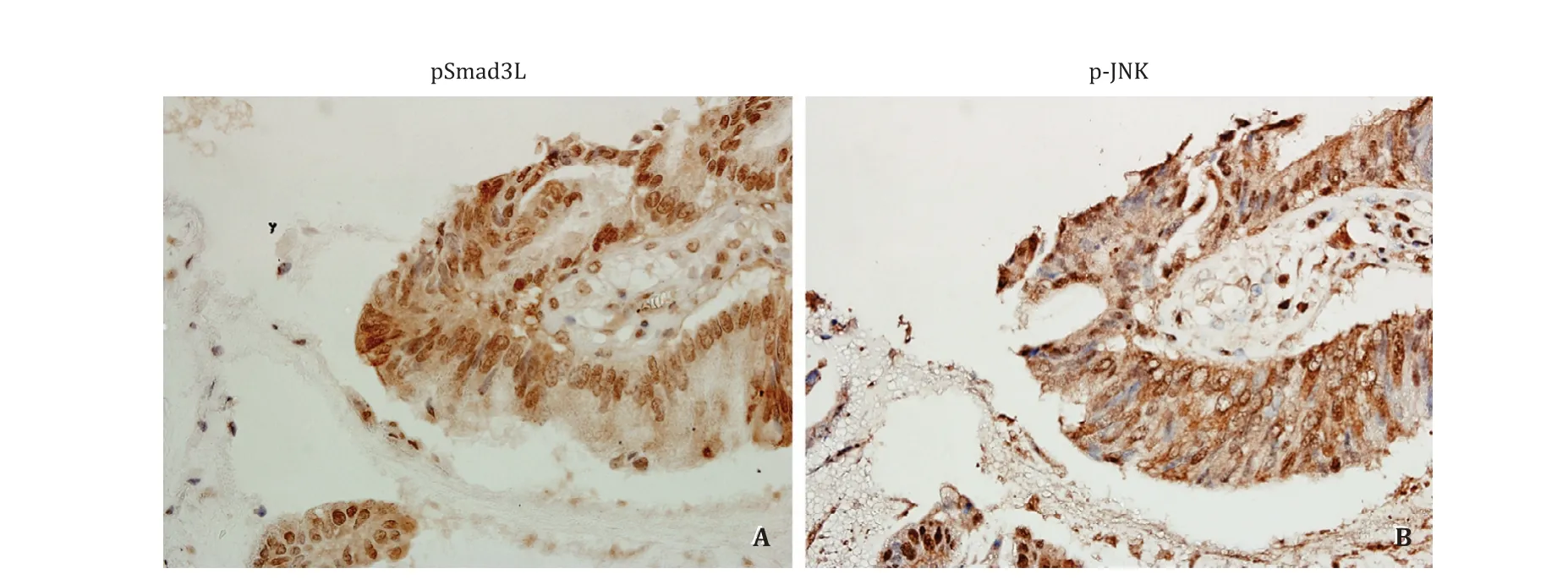
Fig. 6. Immunostaining of pSmad3L and p-JNK in cell nuclei and cytoplasm of invasive carcinoma (original magnification × 400). A : Significant expression of pSmad3L is expressed in the nucleus of invasive carcinoma; B : In an adjacent serial section, significant expression of p-JNK was observed in the cytoplasm and nuclei.
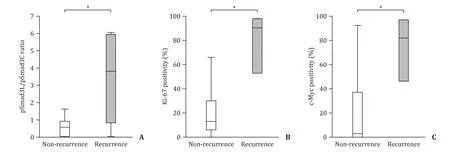
Fig. 7. Comparison of pSmad3L/pSmad3C immunostaining positivity ratio, immunostaining index of Ki-67 and c-Myc in the tumor cells between the recurrence group and the non-recurrence group of IPMN with high-grade dysplasia/invasive carcinoma. A : pSmad3L/pSmad3C ratio in the recurrence group is shown to be significantly higher than that in the non-recurrence group; B : There was a significant difference in immunostaining index of Ki-67 between the recurrence group and the non-recurrence group;C : In immunostaining index of c-Myc, a significant difference was observed between the recurrence group and the non-recurrence group. *: P < 0.05.
In conclusion, the present study reveals that phosphorylated Smad3 signaling in the pancreas of patients with IPMN is converted from pSmad3C signaling to pSmad3L signaling as the tumor progresses during the oncogenic process, suggesting that such a signal alteration of pSmad3 might contribute to carcinogenesis in IPMN. In addition, the pSmad3L/pSmad3C ratio in post-operative patients with high-grade dysplasia and invasive carcinoma may be a novel biomarker for predicting the recurrence. Further large-scale study may be needed to offer more reliable evidence on prediction of carcinogenesis and prognosis in IPMN by focusing on intracellular signaling alteration of pSmad3.
Acknowledgments
None.
CRediT authorship contribution statement
Yuichi Hori:Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing -original draft.Tsukasa Ikeura:Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation,Writing - review & editing.Takashi Yamaguchi:Conceptualization,Validation.Katsunori Yoshida:Conceptualization, Project administration, Validation.Koichi Matsuzaki:Project administration.Mitsuaki Ishida:Project administration.Sohei Satoi:Project administration.Kazuichi Okazaki:Conceptualization, Funding acquisition,Project administration, Resources, Supervision, Writing - review &editing.
Funding
This study was partially supported by grants from the Research Program from the Japan Medical Research and Development(AMED), and Grants-in-Aid from the Ministry of Education, Culture,Sports, Science and Technology of Japan.
Ethical approval
This retrospective study was approved by the Ethics Committee of the Kansai Medical University (No. H151047).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Non-operative management of pancreatic trauma in adults
- Serum non-high-density lipoprotein cholesterol level is increased in Chinese patients with nonalcoholic fatty liver disease
- Increased CMTM4 mRNA expression predicts a poor prognosis in patients with hepatocellular carcinoma
- Folfirinox chemotherapy prolongs stent patency in patients with malignant biliary obstruction due to unresectable pancreatic cancer
- Diagnostic accuracy of administrative database for bile duct cancer by ICD-10 code in a tertiary institute in Korea
- Long noncoding RNA HAND2-AS1 re duce d the viability of hepatocellular carcinoma via targeting microRNA-300/SOCS5 axis
