Virtual navigation-guided radiofrequency ablation for recurrent hepatocellular carcinoma invisible on ultrasound after hepatic resection
2021-01-07QiYuZhoLiTingXieShuoChunChenXioXuTinAnJingShuSenZheng
Qi-Yu Zho , Li-Ting Xie , Shuo-Chun Chen , Xio Xu , Tin-An Jing , Shu-Sen Zheng
a Department of Ultrasound, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
b Division of Hepatobiliary Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
Keywords:Ultrasound Hepatocellular carcinoma Ablation techniques Nomogram
A B S T R A C T Background: No reports are available on the technical efficiency and therapeutic response of virtual navigation (VN)-guided radiofrequency ablation (RFA) for patients with recurrent hepatocellular carcinoma(HCC) after hepatic resection. The aim of this study was to investigate the overall technical performance and outcome of VN-guided RFA in recurrent HCC patients. In addition, a nomogram model was developed to predict the factors influencing the overall survival (OS).Methods: This was a prospective study on 76 recurrent HCC patients who underwent VN-guided RFA between June 2015 and February 2018. The technical feasibility, success, and efficiency, OS, local tumor progression, and complications were evaluated. A multivariate Cox regression analysis was conducted to predict the significant factors, and a nomogram including independent predictive factors was subsequently plotted to predict OS.Results: The technical feasibility, success, and efficiency rates of VN-guided RFA were 86.4%, 94.7%, and 97.4%, respectively. The cumulative OS rates at 1-, 2-, and 3-year were 88.1%, 79.7%, and 71.0%, respectively. The cumulative local tumor progression rates at 1-, 2-, and 3-year were 5.5%, 8.7%, and 14.0%,respectively. In addition, the minor and major complication rates were 5.3% and 3.9%, respectively. No intervention-related deaths occurred during the follow-up period. The C-index of the OS nomogram in this study was 0.737.Conclusions: VN-guided RFA is an effective therapeutic option in recurrent HCC patients and improves the long-term outcomes especially for the lesions that cannot be detected in the two-dimensional ultrasound. Besides, the nomogram may be a useful supporting tool in predicting OS to estimate the individual survival probability, optimize treatment options, and facilitate decision-making.
I ntroduction
Hepatocellular carcinoma (HCC) is the sixth most frequent malignancy worldwide, and its incidence is still increasing according to the latest global cancer statistics [1] . Hepatectomy and liver transplantation still represent the most effective therapies,although the follow-up revealed that local HCC recurrence is the most severe problem despite the therapy [ 2 , 3 ]. Several studies reported that the 5-year recurrence rate of HCC after hepatectomy was higher than 70% [ 4 , 5 ]. Repeated hepatectomy is usually used to treat recurrence, and the 5-year survival rate ranged from 35.3%to 49.8% [ 6 , 7 ]. Nevertheless, it can only be performed in a small number of patients because of hepatic insufficiency of the liver remnant or widespread recurrence [8] . Thus, it is of utmost importance to find an alternative approach to treat recurrent HCC after hepatic resection.
Percutaneous image-guided radiofrequency ablation (RFA) is the most relevant therapeutic approach for an unresectable tumor or as a bridge to liver transplantation, as recommended by the European Association for the Study of the Liver [9] and American Association for the Study of Liver Disease guideline [10] . RFA is minimally invasive, safe, and effective, and is widely performed in HCC patients [11] . Furthermore, RFA is specifically indicated in recurrent HCC because these recurrent lesions are generally discovered when they are still small, and RFA has minimal side effect on the deterioration of liver function [2] . However, RFA is not always working in all patients; indeed, 15% -45% of tumors are considered unfeasible because of the invisibility of tumors, the unavailability of electrode paths, and the damage of important structure [ 12 , 13 ].
Virtual navigation (VN) with conventional ultrasound (US) and CT/MRI images were recently demonstrated of being able to overcome these problems [14] . Previous studies showed that fusion navigation can improve the tumor visibility, help in designing ablation strategies, and provide an immediate result on the treatment response, thus enabling a successful percutaneous RFA with excellent treatment outcomes [15-18] . VN-guided approach is applied in different fields of medicine, achieving favorable therapeutic outcomes. Several studies investigated the safety and efficiency of VNguided approach applied on HCC. A recent work reported that contrast enhanced ultrasound (CEUS)/AutoSweep three-dimensional US fusion imaging is a helpful approach for RFA to guide and evaluate the therapeutic efficacy of HCC patients [19] . Song et al.showed that fusion imaging-guided RFA is a safe and efficient therapy for subcentimeter recurrent HCC patients and is feasible in approximately 2/3 of patients [20] . However, no report is available on the technical efficiency and therapeutic response of VN-guided RFA for recurrent HCC patients.
The nomogram is a graphic tool that accurately evaluates the probability of a clinical event in an individualized and evidencebased manner, widely developed for many tumor models [ 21 , 22 ].A previous study on non-small-cell lung cancer developed a clinical nomogram in a large sample size of 5261 patients, demonstrating that using this tool, clinicians could more accurately estimate the survival of individual patients who need a specific treatment strategy [23] . Another recent study showed that nomogram is effective and simple when used in colorectal cancer patients with liver metastasis who underwent thermal ablation [24] . However,no nomogram was developed to determine the significant factors influencing OS after RFA under the VN guidance in recurrent HCC patients.
The present study was to assess the technical feasibility and therapeutic response of VN-guided RFA in recurrent HCC patients after hepatic resection, and to develop a nomogram to predict the factors influencing OS after RFA.
Methods
Patients
HCC patients who were subjected to RFA in our hospital between June 2015 and February 2018 were included in this study.This prospective research was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine, and all patients provided written informed consent prior to be subjected to RFA.
The inclusion criteria were as follows: (1) Diagnosis of HCC based on pathology or typical CT/MRI images; (2) Patients with a history of HCC after hepatectomy; (3) Patients admitted to our department to be subjected to local ablation; (4) Patients who were inappropriate for, or refusing repeated liver resection; (5) Patients with valid CT/MRI images in DICOM format; (6) Only a single recurrent tumor ablation by RFA under the VN guidance because multiple recurrent tumors may affect the survival and tumor progression of patients; and (7) Tumors invisible by the twodimensional US.
The exclusion criteria were the following: (1) Patients with Child-Pugh class C cirrhosis; (2) The largest tumor diameter of more than 5 cm measured on hepatobiliary dynamic CT or MRI phase; (3) No CT/MRI results available at one month after RFA; and(4) Patient’s follow-up of less than four months.
CEUS acquisition
The CEUS examination was performed using a MyLab 90 US machine (Esoate, Genoa, Italy) equipped with the abdominal probe CA541 (1-8 MHz). Firstly, 2.4 mL SonoVue (Bracco, Milan, Italy)was intravenously injected. Then, saline solution (5 mL) was injected and the US probe was kept in a stable state. Three enhancement phases (arterial phase, portal venous phase and delayed phase) were recorded after asking each patient to hold their breath for 10-15 s during CEUS image acquisition.
Conventional US and real-time VN
Before the US examination, the operator reviewed the clinical information and the hepatic CT/MRI image of the patient. Next,the operator accurately detected the target tumor on the US and assessed whether the tumor was clear enough to perform an effective RFA. Subsequently, it was also necessary to identify a safe needle path, and assess the risk of damage to the adjacent organs,the associated liver disease, and the therapy history.
VN was performed according to the magnetic navigation system containing a magnetic field generator and two receivers. The detailed procedure was as follows: (1) The fusion navigation system was connected to the US scanner and the patient was placed on the operating bed; (2) The operator began to scan the patient’s abdomen; (3) The previously obtained CT/MRI data (DICOM format image) were transferred to the fusion system; (4) The operator selected the suitable CT/MRI image which showed the target tumor and the surrounding anatomical structures; (5) The manual plane match and point to point registration were performed using particular anatomic landmarks, such as the right portal vein,left portal vein, or the branches of the intrahepatic blood vessels; (6) Then the ultrasonic transducer was slowly moved to detect the ablation area on the traditional US along with CT/MRI images, and the anatomical signs surrounding the ablation area were synchronously recorded; (7) The size and location of the target tumor were determined by CEUS routinely. A suitable electrode needle was chosen, and VN-guided intervention was conducted. Finally, the images and videos were saved and the procedure was recorded. Fig. 1 shows the procedure of VN.
Percutaneous RFA procedure
The RFA procedure was performed on patient under local anesthesia combined with an intravenous injection of sober sedatives and analgesics. RFA was performed using the same system for the US examination under the VN guidance. A Cool-tip system (Covidien, Mansfield, MA, USA) was used, including an RF generator with a maximum power of 200 W and a 17 g internal cool straight electrode. The parameters of the RFA system were set according to the manufacturer’s protocol. The procedure ended when the echobubble displayed on the fusion CEUS completely covered the target lesion and had a sufficient safe boundary of at least 5 mm. After the ablation, the electrode was removed and the puncture path was cauterized to prevent tumor implantation and reduce the risk of bleeding.
The RFA therapeutic parameters, surgery duration, and complications were recorded. The complete ablation of the tumor and the occurrence of procedure-related complications were assessed by routine CEUS. If US-CT/MRI fusion imaging showed that the nonperfusion area on the CEUS image did not cover the target, the uncovered area was immediately ablated under the VN guidance to achieve a complete coverage.

Fig. 1. Schematic diagram of the procedure of the US-CT/MRI multimodal fusion imaging and virtual navigation system.
Follow-up and assessment of the therapeutic outcome
Immediately after the operation, fusion and two-dimensional ultrasound were used to evaluate whether the ablation area completely covered the tumor area and if it was larger than the safe boundary of 5 mm. If the ablation was not complete, a second ablation was performed after 10 min. MRI or CT and alphafetoprotein evaluation test were then repeated 1 month after the initial ablation, since they were used as criteria for the evaluation of the technical efficiency. US and CEUS follow-up were performed every 3 months, and CT/MRI follow-up was every 6 months. When liver recurrence or new hepatic tumors were discovered, a secondary RFA was performed on the basis of HCC treatment guidelines [25] . The technical feasibility, technical success, technical efficiency, OS, cumulative local tumor progression (LTP), and complications were evaluated. The HCC nodules were divided into the high-risk and low-risk location groups and the OS and LTP of the two groups were compared. The detailed definitions are listed in Table 1 [ 26 , 27 ].
Statistical analysis
Statistical analysis was performed using R software version 3.5.0 and SPSS version 23.0 (IBM Corp., Armonk, NY, USA). Continuous variables were described as median with range, and categorical variables were described as numbers and percentages. Cumulative incidences of LTP and OS were calculated using the Kaplan-Meier method, and the differences were compared with the log-rank test.
The significant factors for OS and LTP were determined by univariate and multivariate Cox regression analyses. The factors used in the nomogram were predicted by the multivariate Cox regression analysis and the nomogram was developed using R statistical software. C-index, the calibration curve, and the Kaplan-Meier curve were evaluated by the root mean square (RMS) package andsurvival package, while the decision curve analysis (DCA) curve was obtained by the “stdca.R” function. APvalue less than 0.05 was considered statistically significant.

Table 1 The definition of the clinical outcomes.
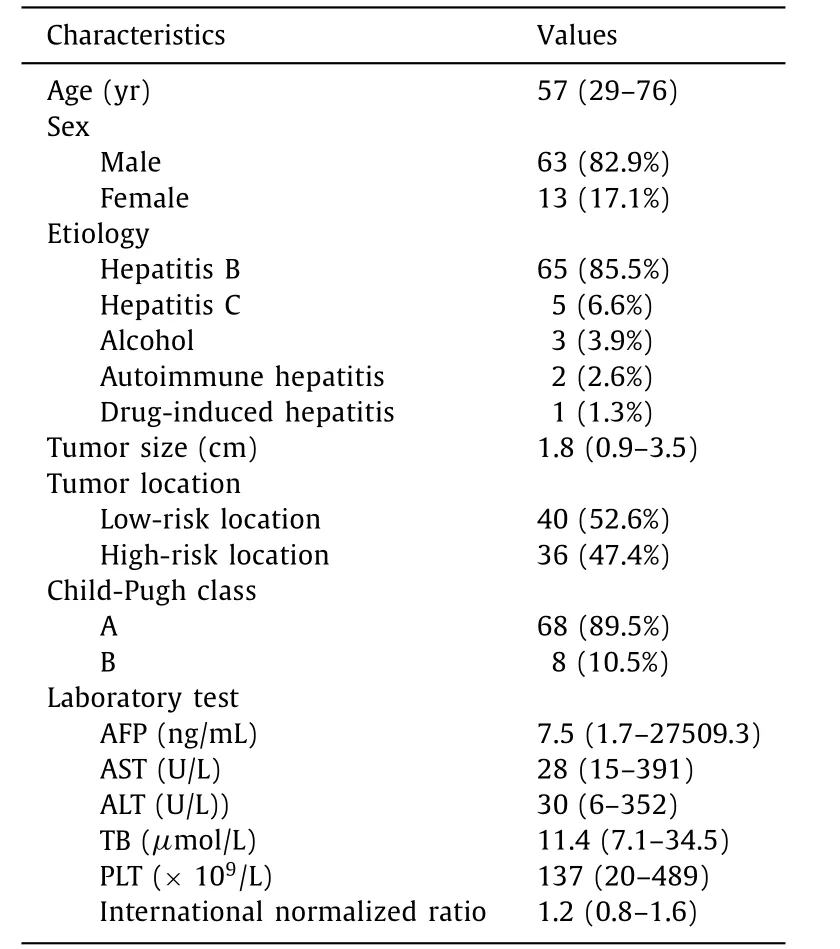
Table 2 Baseline characteristics of the patients and lesions ( n = 76).
Results
Patients and tumor profile
A total of 116 patients were initially included. Twenty-eight patients were excluded: CT/MRI was not performed at 1 month after ablation (n= 10), the maximum tumor diameter was more than 5 cm (n= 7), presence of Child-Pugh class C cirrhosis (n= 6), and a follow-up period of less than four months (n= 5). Therefore, 88 eligible patients were finally enrolled in this study.
The results of VN-guided RFA showed that 12 HCC patients were not feasible. The main reason for the infeasibility of RFA was the presence of inconspicuous target tumors without peritumoral anatomic landmarks during the US (n= 7). Another reason was the poor electrode path selected (n= 5). Thus, the remaining 76 lesions were feasible, and the technical feasibility rate was 86.4%(76/88). The basic characteristics and the clinical outcomes and therapeutic response are summarized in Tables 2 and 3 .
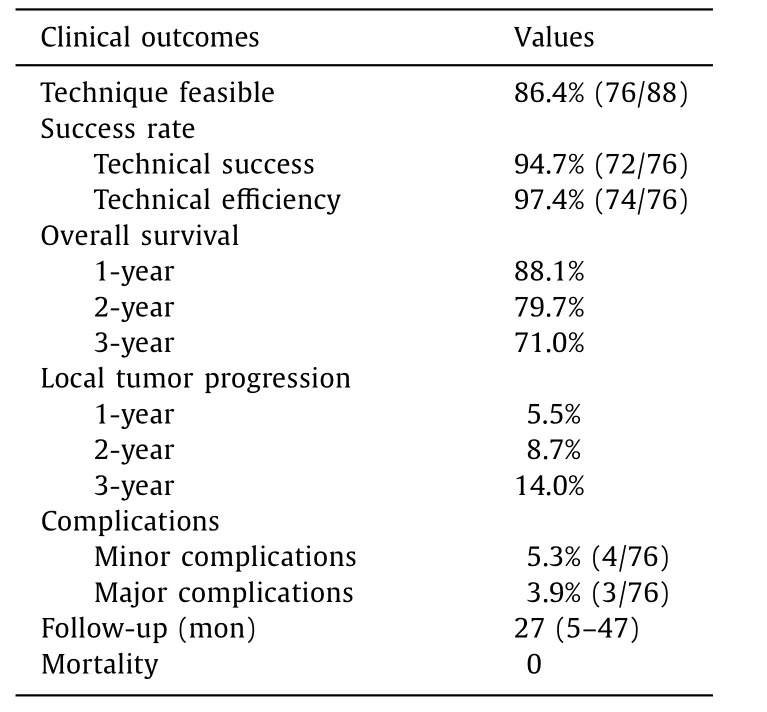
Table 3 The clinical outcomes and therapeutic response.
Therapeutic efficiency after RFA
Among the 76 target tumors, 72 were technically successful.The presence of residual tumors was verified by CEUS image immediately after RFA, and a second RFA treatment was conducted in four tumors after immediate postoperative evaluation. Thus, the technical success rate of the ablation was 94.7% (72/76). A successful VN-guided RFA in one recurrent HCC patient is shown in Fig. 2 . A number of 74/76 (97.4%) HCC patients received an effective treatment, while an incomplete ablation was observed in 2 patients (2.6%). One of these two patients with incomplete ablation was subjected to hepatectomy, while the other one was subjected to transarterial chemoembolization.
Follow-up and local tumor control
The median follow-up period was 27 months (range 5-47 months). The follow-up ended at the time of death. The estimated 1-, 2- and 3-year cumulative OS rates were 88.1%, 79.7%, and 71.0%,respectively ( Fig. 3 A). The subgroup stratification analysis of OS according to the tumor location of the nodules resulted in a significantly shorter OS in patients with high-risk location nodules compared to patients with low-risk location nodules ( Fig. 3 B).
The cumulative LTP rates at 1- and 2- and 3-year were 5.5%,8.7%, and 14.0%, respectively ( Fig. 3 C). The subgroup stratification analysis of LTP according to the tumor location revealed a significantly higher LTP in patients with high-risk location nodules compared to patients with low-risk location nodules ( Fig. 3 D).

Fig. 2. A 68-year-old male patient with a small hepatocellular carcinoma was subjected to US-MRI multimodal fusion imaging and virtual navigation-guided radiofrequency ablation. A and B : US-MRI imaging registration and fine adjustment were performed; C : US-MRI fusion imaging was used to locate the position; D - F : CEUS-MRI fusion imaging confirmed the hyper-enhanced nodule and set the puncture path. CEUS revealed a 13-mm hyper-enhanced nodule next to the left branch of the hepatic vein; G :The electrode was inserted under the guidance of US-MRI fusion imaging and radiofrequency ablation was started; H : One month later, the technique efficacy confirmed by CEUS.
No intervention-related deaths occurred during the follow-up period. Minor complications occurred in 4 cases (5.3%), including 1 case of fever, 1 case of mild bleeding, and 2 cases of abdominal pain. Major complications occurred in three of the 76 patients(3.9%), such as slight thermal damage of the diaphragm (n= 2)and colon (n= 1).
Independent predictors of OS and LTP
The results of the univariate and multivariate Cox analyses in predicting OS and LTP are summarized in Tables 4 and 5 . Liver cirrhosis, Child-Pugh class,α-fetoprotein, tumor size, and tumor location were predictors of OS according to the univariate regression analysis. Child-Pugh class, tumor size and tumor location were the significant predictors corrected with the development of OS according to the multivariate Cox regression analysis. Tumor size and tumor location were the significant factors associated with the LTP according to the multivariate Cox regression analysis.
Nomogram for OS
The OS nomogram was constructed using the predictive factors according to the results of the multivariate Cox regression model( Fig. 4 A). The nomogram revealed a favorable discrimination with a C-index of 0.737 for OS. The calibration curve of 1-, 2- and 3-year OS demonstrated a consistency between the nomogram-predicted OS probability and actual OS probability, suggesting a good calibration effect of the OS nomogram ( Fig. 4 B). The nomograms of the 1-,2- and 3-year DCA curves are shown in Fig. 4 C.

Fig. 3. Cumulative curves of OS and LTP after RFA. A : The 1-, 2- and 3- year cumulative OS after radiofrequency ablation; B : Comparison of OS in the high-risk location and low-risk location groups ( P < 0.001); C : The 1-, 2- and 3- year cumulative LTP rates after RFA; D : Comparison of LTP between the high-risk location and low-risk location groups ( P < 0.001). OS: overall survival; LTP: local tumor progression; RFA: radiofrequency ablation.
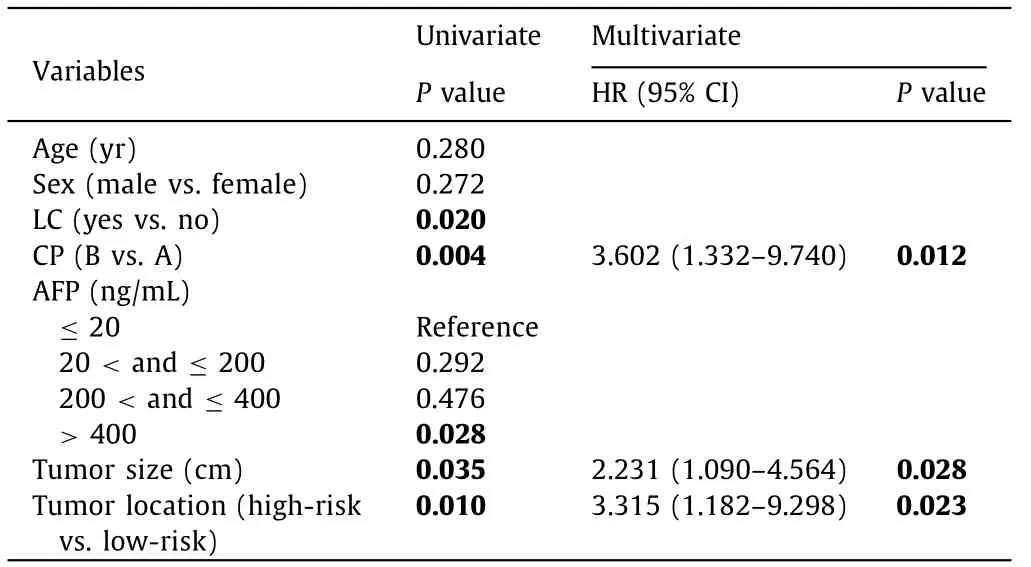
Table 4 Univariate and multivariate analysis of overall survival.
Discussion
This study evaluated the technical efficiency and outcome of real-time VN-guided RFA in the treatment of recurrent HCC patients. Our results demonstrated that RFA under the VN guidance was an efficient treatment option, resulting in a good therapeu-tic response in HCC patients. In addition, the constructed nomogram revealed favorable prediction with a good discrimination and calibration, being potentially helpful in facilitating clinical decision making. To our knowledge, this is the first attempt to perform a nomogram model to predict OS in VN-guided RFA for recurrent HCC patients.
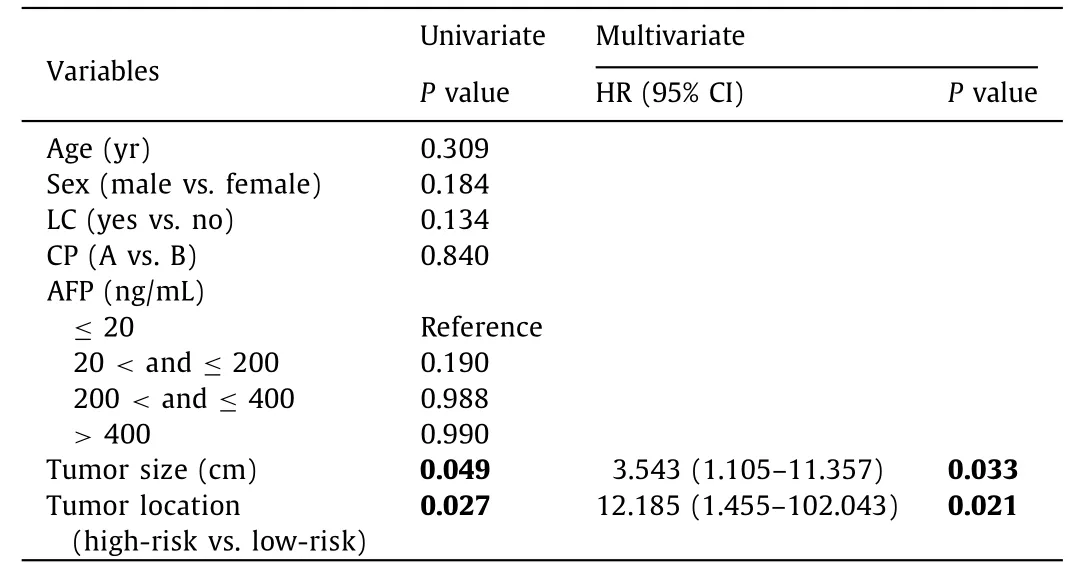
Table 5 Univariate and multivariate analysis of local tumor progression.
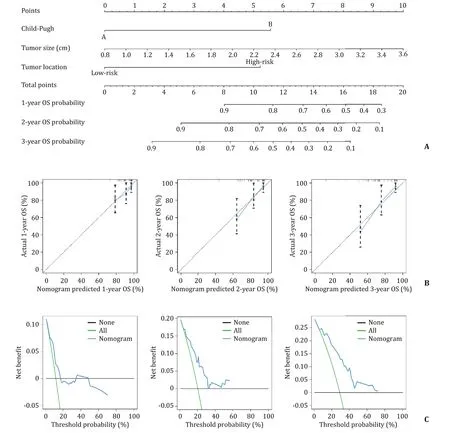
Fig. 4. Nomogram for the prediction of OS and its predictive performance. A : The OS associated nomograms for HCC patients. The nomogram summed the points identified on the scale for each predictor. The total points projected on the bottom scales indicate the probability of 1-, 2- and 3-year OS; B : Calibration curve of 1-, 2- and 3-year OS associated nomograms; C : Decision curve analysis of the nomogram for 1-, 2- and 3-year OS. OS: overall survival.
In our study, VN-guided RFA for recurrent HCC patients achieved a high technical success rate and technical efficiency. This high success rate might be due to the superiority of the VN system, since fusion imaging is able to precisely detect and locate the target invisible lesion, which is a great option to increase the feasibility of ablation in invisible tumor, or to enhance operator confidence during ablation [28-30] . Prospective literature [31] on RFA performed under the guidance of US-CT/MRI fusion imaging in the therapy against HCC revealed that the conspicuity of lesions and technical feasibility of the RFA were enhanced by the application of the fusion navigation technology. Another retrospective study [32] also demonstrated that fusion imaging technology can enhance the visibility of HCC, which was invisible when traditional US was used. A confident visualization of the target tumor and the precise placement of the probes are the most important pre-conditions for a safe and effective RFA; besides, the accuracy of thermal ablation is the key factor in a successful therapy [ 33 , 34 ].
The current practice guidelines for the management of HCC still suggest RFA as the standard therapy on the basis of a good technical success and therapeutic outcome. Our study revealed that the VN-guided RFA resulted in a good OS and a low level of complications. The advantages of a favorable outcome and increased safety of VN-guided RFA might be due to the easier planning of the electrode pathway and the better display of the anatomical structure. As a result, the potential inadequate ablation and adjacent structure damage due to ablation can be reduced [ 31 , 35 ].In addition, the fusion imaging technology helps monitoring the ablation process and reveals the suitability of the chosen ablation edge during the intervention. Therefore, the fusion navigation technique received much attention and was also introduced in hepatobiliary surgery and ultrasonic intervention to ensure the success of the treatment [33] . Unfortunately, our cumulative LTP seems to be slightly higher than that in previous studies [ 20 , 31 , 36 ], probably because of the characteristics of recurrent HCC after hepatectomy of our enrolled patients. Besides, the tumors in majority of the patients (52.6%) were in a high-risk location, such as close to the hepatic capsule and large intrahepatic vessels. Furthermore, realtime US images are mostly scanned under free-breathing, thus,they are affected by the hepatic deformation during breathing or during exercise [35] . However, it is difficult to compare our outcomes with those of other studies because we were the first to use VN-guided RFA in the treatment of recurrent HCC patients.
Our results showed that tumor size and tumor location were the remarkable factors in predicting OS and LTP. Previous reports showed that the factors predicting worse OS and LTP included large tumor size, inadequate ablative edge, tumor adjacent to the main blood vessels and ducts, sub-capsular tumor, infradiaphragmatic tumor, and history of a previous therapy [ 31 , 37-40 ].However, it has also been reported that the size, location or edge of nodules do not affect LTP rate [41] , which may be due to different inclusion criteria and different prediction models. To the best of our knowledge, this is the first attempt to develop a nomogram model to predict OS in recurrent HCC candidates for RFA after hepatic resection under the VN guidance. We demonstrated the great application potential of this model in the field of fusion imaging and VN. More importantly, the proposed nomogram showed a sufficient discrimination and accurate calibration. The nomogram is the visualization of a statistical model specifically performed for individual prediction. The optimized nomogram in the treatment of HCC patients after RFA offered a better predictive value compared with the traditional staging or scoring system [42] . Kao and coworkers created a nomogram based on the albumin-bilirubin grade to assess the outcomes of RFA in patients with early-stage HCC and found that the nomogram provides a personalized survival information in these patients [43] . We demonstrated that our nomogram, based on Child-Pugh class, tumor size, and tumor location, is feasible to predict OS in recurrent HCC patients treated with VN-guided RFA. However, this nomogram needs to be validated.
This study contains few limitations. First, although this was a prospective research, it was a single-center study with a relatively small sample size. Second, the fusion imaging was used in all patients without assessing routine US/CEUS-guided RFA alone,despite that we compared it with the results of previous studies. Third, only the US-CT/MRI fusion navigation device was evaluated, and the results should not be extended to other fusion imaging systems. Other modalities such as US-FDG PET, should be employed in further studies. A long-term, multicenter, prospective,and randomized cohort study should be performed in the future.
In conclusion, this prospective study revealed that VN-guided RFA is an efficient and useful therapeutic option for recurrent HCC patients, especially for the invisible lesions in the two-dimensional US. A nomogram is developed for the first time to predict the OS in recurrent HCC patients who were subjected to RFA under the VN guidance. This novel prognostic nomogram allows the estimation of the individual survival probability, the optimization of the treatment options, and helps doctors in making an appropriate therapeutic regimen.
Acknowledgements
None.
CRediT authorship contribution statement
Qi-Yu Zhao:Investigation, Writing - original draft.Li-Ting Xie:Investigation, Writing - original draft.Shuo-Chun Chen:Methodology, Data curation, Formal analysis.Xiao Xu :Supervision, Funding acquisition, Writing - review & editing.Tian-An Jiang :Supervision,Funding acquisition, Writing - review & editing.Shu-Sen Zheng:Conceptualization, Supervision.
Funding
The study was supported by grants from the National S&T Major Project of China (2017ZX10203205), the State Major Research Program of China ( 2018 YFC0114900 ), the National Natural Science Foundation of China ( 81971623 ), the Major Research Project of the National Natural Science Foundation of China( 91630311 ), the Natural Science Foundation of Zhejiang Province( SZ20 H180 0 02 ), and the Zhejiang Society Joint Foundation for Mathematical Medicine (LSY19 H180015).
Ethical approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine, and all participants provided written informed consent.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Non-operative management of pancreatic trauma in adults
- Torin2 overcomes sorafenib resistance via suppressing mTORC2-AKT-BAD pathway in hepatocellular carcinoma cells
- Efficacy and cost-effectiveness of antiviral regimens for entecavir-resistant hepatitis B: A systematic review and network meta-analysis
- Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management
- Hepatobiliary&Pancreatic Diseases International
- Safety and efficacy of an integrated endovascular treatment strategy for early hepatic artery occlusion after liver transplantation
