Safety and efficacy of an integrated endovascular treatment strategy for early hepatic artery occlusion after liver transplantation
2021-01-07HnKaiZhuLiZhuanChnChnZhaoDanZhuoYiWanWuZhaGuoHonCaoShuSnZhn
Hn-Kai Zhu , Li Zhuan , Chn-Z Chn , Zhao-Dan Y , Zhuo-Yi Wan ,Wu Zha n , Guo-Hon Cao , Shu-Sn Zhn d, ,*
a Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou 310 0 03, China
b NHC Key Laboratory of Combined Multi-organ Transplantation, Hangzhou 310 0 03, China
c Key Laboratory of the Diagnosis and Treatment of Organ Transplantation, Research Unit of Collaborative Diagnosis and Treatment for Hepatobiliary and Pancreatic Cancer, CAMS, Hangzhou 310 0 03, China
d Key Laboratory of Organ Transplantation, Zhejiang Provincial Research Center for Diagnosis and Treatment of Hepatobiliary Diseases, Hangzhou 310 0 03,China
e Department of Hepatobiliary and Pancreatic Surgery, Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine, Hangzhou 310022, China
f Department of Intensive Care Unit, Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine, Hangzhou 310022, China
g Department of Radiology, Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine, Hangzhou 310022, China
Keywords:Liver transplantation Hepatic artery occlusion Hepatic artery thrombosis Hepatic artery stenosis Endovascular treatment
A B S T R A C T Background: Hepatic artery occlusion (HAO) after liver transplantation (LT) is typically comprised of hepatic artery thrombosis (HAT) and stenosis (HAS), both of which are severe complications that coexist and interdependent. This study aimed to evaluate an integrated endovascular treatment (EVT) strategy for the resolution of early HAO and identify the risk factors associated with early HAO as well as the procedural challenge encountered in the treatment strategy.Methods: Consecutive orthotopic LT recipients ( n = 366) who underwent transplantation between June 2017 and December 2018 were retrospectively investigated. EVT was performed using an integrated strategy that involved thrombolytic therapy, shunt artery embolization plus vasodilator therapy, percutaneous transluminal angioplasty, and/or stent placement. Simple EVT was defined as the clinical resolution of HAO by one round of EVT with thrombolytic therapy and/or shunt artery embolization plus vasodilator therapy. Otherwise, it was defined as complex EVT.Results: Twenty-six patients (median age 52 years) underwent EVT for early HAO that occurred within 30 days post-LT. The median interval from LT to EVT was 7 (6-16) days. Revascularization time (OR = 1.027;95% CI: 1.005-1.050; P = 0.018) and the need for conduit (OR = 3.558; 95% CI: 1.241-10.203, P = 0.018)were independent predictors for early HAO. HAT was diagnosed in eight patients, and four out of those presented with concomitant HAS. We achieved 100% technical success and recanalization by performing simple EVT in 19 patients (3 HAT + /HAS- and 16 HAT-/HAS + ) and by performing complex EVT in seven patients (1 HAT + /HAS-, 4 HAT + /HAS + , and 2 HAT-/HAS + ), without major complications. The primary assisted patency rates at 1, 6, and 12 months were all 100%. The cumulative overall survival rates at 1, 6,and 12 months were 88.5%, 88.5%, and 80.8%, respectively. Autologous transfusion < 600 mL (94.74% vs.42.86%, P = 0.010) and interrupted suture for hepatic artery anastomosis (78.95% vs. 14.29%, P = 0.005)were more prevalent in simple EVT.Conclusions: The integrated EVT strategy was a feasible approach providing effective resolution with excellent safety for early HAO after LT. Appropriate autologous transfusion and interrupted suture technique helped simplify EVT.
Introduction
Hepatic artery contributes to approximately 25% flow of the dual blood supply, of which significant elevation has been observed intra- and postoperatively in liver transplantation (LT) [ 1 , 2 ]. Adequate hepatic perfusion is of critical importance to ensure graft and recipient survival [ 3 , 4 ]. Hepatic artery occlusion (HAO) consists of totally occluded hepatic artery (i.e. complete occlusion by severe stenosis or thrombosis) and significantly partially occluded hepatic artery (i.e. partial stenosis or thrombosis) [5-7] , and is a devastating complication occasionally leading to ischemic biliary injury, sepsis, graft failure, or even mortality [ 5 , 8 ]. The incidence of hepatic artery thrombosis (HAT) and stenosis (HAS) were reported to be 5% -7% and 5% -11%, respectively [9-12] . Moreover, up to 65% of untreated HAS progresses into subsequent HAT within 6 months, implicating that HAT and HAS composed a broader ischemic spectrum [ 13 , 14 ]. Thus, under the coexistence, interdependence and shared goal to improve the hepatic perfusion between HAT and HAS, the therapeutic strategy is considered as an integrity.
It is generally agreed that endovascular or surgical therapeutic intervention is urgently required for HAO patients, and even retransplantation may be appropriate in cases of irreversible graft failure [15-17] . In recent years, endovascular treatment (EVT) has emerged as the standard procedure that is preferred over traditional surgical hepatic artery revision or urgent retransplantation for revascularization as techniques involved in EVT are reportedly minimally invasive and are associated with lower rates of morbidity and mortality and similar long-term overall survival, compared with traditional intervention methods [ 18 , 19 ]. Previous studies reported a preference for catheter-directed thrombolysis, percutaneous transluminal angioplasty (PTA), and stent placement to treat HAT and HAS [ 16 , 20 ]. However, some transplant centers tended to deploy open surgery, to delay EVT and to avoid potential severe complications, such as target vessel dissection and anastomosis rupture, in early period after LT [ 10 , 21 ]. At our center, we implemented an integrated EVT strategy for the resolution of early HAO by adopting thrombolysis, shunt artery embolization plus vasodilator therapy, PTA, and/or stent placement, as appropriate. This study aimed to evaluate the safety and efficacy of the integrated EVT strategy and identify the risk factors associated with early HAO and the EVT procedural challenge.
Methods
Study design
The study comprised of consecutive patients who received orthotopic LT at the Shulan (Hangzhou) Hospital, China from June 2017 to December 2018. All the data including demographic, pre-,peri- and post-transplant parameters of donors and recipients were retrieved from a prospectively collected database and retrospectively analyzed. We defined early HAO as occurring within 30 days after LT. This study was approved by the Institutional Review Board and the requirement for written informed consent was waived due to the retrospective nature of the study.
The protocol for interrogating the artery patency after LT was daily Doppler ultrasonography (US) in the first 10-day period, then twice a week before discharge, and every 3 months on follow-up,or every time readmitted to the hospital for any reason related to the graft. Computed tomography angiography (CTA) was regularly obtained between post-transplant day 7-10, before discharge, and urgently under ultrasonic abnormalities. If CTA resulted in highly suspicious arterial complications, patients were transferred to digital subtraction angiography (DSA) and subsequent EVT if indicated.The HAO diagnosis was confirmed by DSA finding of ≥50% luminal diameter narrowing or thrombi.
Based on the Reporting Standards of the Society for Vascular Surgery and the American Association for Vascular Surgery [22] ,primary patency was defined as the hepatic artery patency after the first round of EVT during follow-up. Primary assisted patency was defined as the hepatic artery patency following repeat EVT in case of a hepatic artery reocclusion. Patients were followed up until death, lost to follow-up, or last follow-up. The study ended on December 31, 2019.
Liver transplantation details
The transplant procedure was a modified piggyback technique with whole liver allografts. The manner of arterial reconstruction was based on the graft’s vascular anatomy, the recipient’s arterial inflow, and quality. Back-table reconstruction of any graft accessory artery and transplant arterial anastomosis were accomplished with interrupted 7-0 or 8-0 polypropylene sutures under 3.5 surgical loupes. An interposition conduit using the donor iliac artery was indicated if there was a gap between the candidate sites of the graft and recipient arterial system. Multiple arterial anastomoses were defined as more than one anastomosis to create the continuity. Intraoperative hepatic vasculature inflows and outflows were measured by Doppler US immediately after all the vascular reconstruction. A duct-to-duct anastomosis or Roux-en-Y hepaticojejunostomy was performed for biliary reconstruction.
Interventional techniques
EVT techniques in this study included shunt artery embolization(splenic artery, gastroduodenal artery and/or left gastric artery)plus vasodilator therapy, thrombolytic therapy, PTA, and stent placement.
All procedures were performed by two interventional radiologists (Ye ZD and Cao GH) with more than 3 years of experience. A 5-F Yashiro catheter (Terumo, Tokyo, Japan) was used for selective hepatic artery catheterization via a right femoral artery approach and angiography to identify HAO. A 3-F coaxial microcatheter (Terumo) was carefully advanced over a microwire passing through the occlusion to evaluate the hepatic arterial branches.
When thrombosis was identified, the coaxial microcatheter was carefully inserted into the thrombi over the microwire. Urokinase 10 0 0 0 0 IU was dissolved in 50 mL normal saline and injected through the microcatheter; this was repeated until the thrombus dissolved completely. If the thrombolysis was unsatisfactory,the microcatheter would be advanced to the residual thrombus and retained to administer continuously pumped urokinase infusion in the intensive care unit at a rate of 10 0 0 0 0 IU/h (upper limit dosage 1 0 0 0 0 0 0 IU) over 6-12 h. The rate and dosage were adjusted according to the complete blood count, coagulation test findings, and the type of drainage fluids. After complete thrombolysis, the accompanying stenosis was managed as the isolated stenosis.
In stenosis cases, shunt artery embolization with coils plus vasodilator therapy with nicorandil were firstly performed in the superselective visceral arteries to dilate the hepatic artery. Embolization in the proximal splenic artery was preferable than the distal splenic, left gastric, or gastroduodenal arteries. Nicorandil 12 mg was diluted to 24 mL with normal saline and injected through the microcatheter in three boluses with a 5-min interval ( Fig. 1 ). PTA and/or stenting was performed to restore the hepatic artery patency if stenosis persisted ( Fig. 2 ). Recanalization success was defined as DSA estimation of<30% residual stenosis of the treated hepatic artery.
The catheter sheath was retained for 1-2 days. If the follow-up hepatic arteriography showed unresolved occlusion, EVT was repeated. Surgical revision or retransplantation would be attempted after an endovascular recanalization failure.
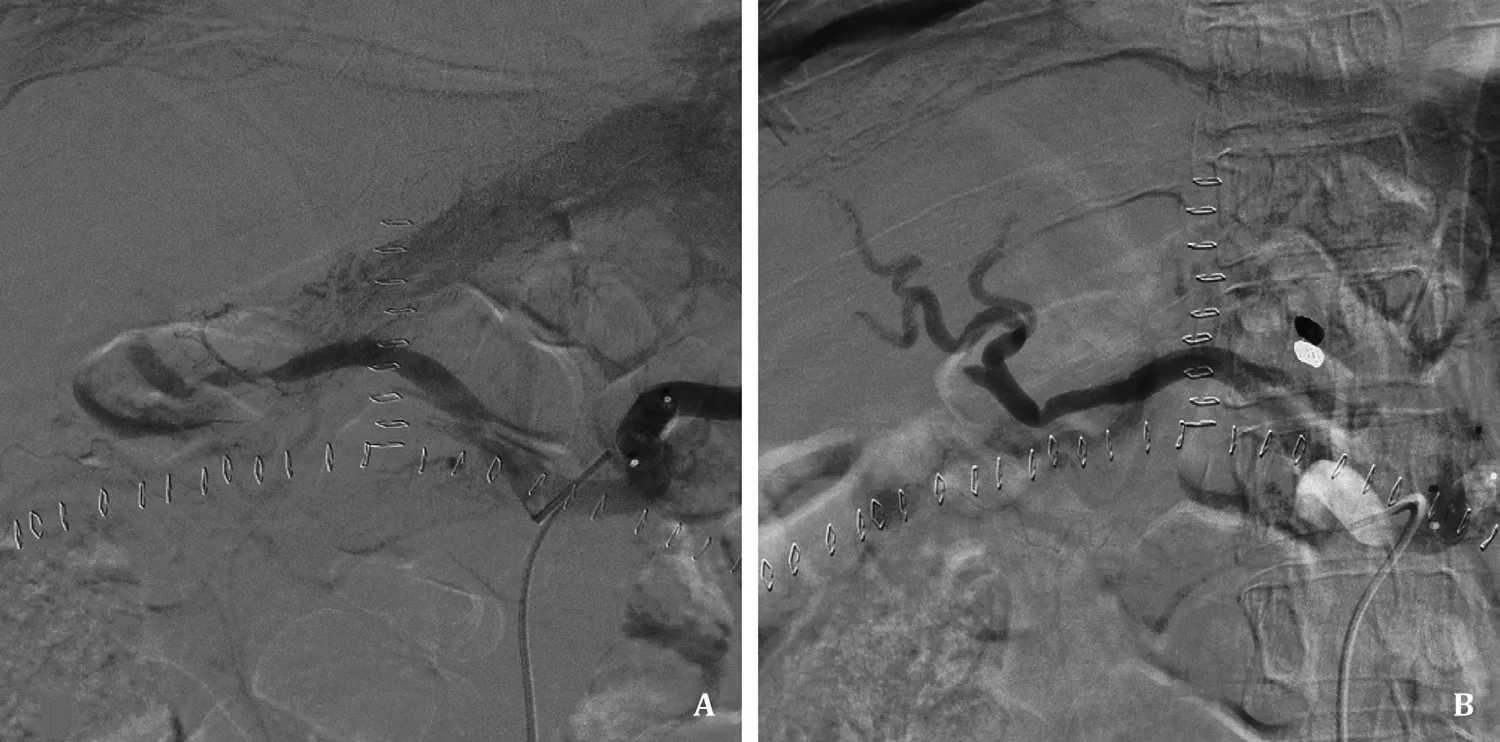
Fig. 1. Simple endovascular treatment of hepatic artery occlusion. A: The stenotic segment of hepatic artery was not improved after embolizing shunt artery; B: Left gastric artery embolization and vasodilator (nicorandil) therapy were performed leading to successful revascularization.
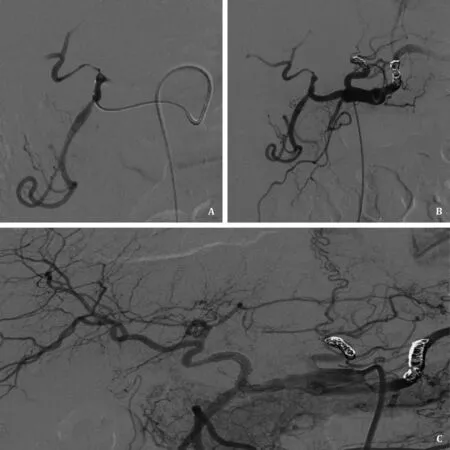
Fig. 2. Complex endovascular treatment of hepatic artery occlusion. A: The distal segment of hepatic artery was stenotic and the intrahepatic artery branches were hardly shown; B: Shunt artery embolization and vasodilator therapy were performed but the improvement was limited; C: Hepatic artery patency was achieved after stent placement.
Definition
Simple EVT (sEVT) was defined as the clinical resolution of the hepatic artery occlusion by a single round of EVT with thrombolytic therapy and/or shunt artery embolization plus vasodilator therapy. Complex EVT (cEVT) was defined as (i) PTA and/or stent placement performed; or (ii) hepatic artery occlusion requiring two or more rounds of EVT by any techniques; or (iii) surgical revision or retransplantation performed following the failure of an EVT technique.
After the intervention
After recanalization, 4100 U low-molecular-weight heparin was administered subcutaneously for anticoagulation every 12-24 h for 1 week. Subsequently, antiplatelet therapy using aspirin or clopidogrel would be administrated for 3-6 months. Patients who underwent stent placement were recommended life-long antiplatelet therapy. Imaging workup, liver function test, and coagulation test were monitored closely before discharge and as part of routine follow-up after discharge. If reocclusion was suspected on the basis of Doppler US findings, along with clinical manifestations and laboratory findings during the follow-up, CTA would be performed immediately.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 8.0.0 for MacOS (GraphPad Software, San Diego, CA, USA).Data were expressed as median (interquartile range, IQR) or number with proportion (%). Receiver operating characteristic curve analysis was performed to identify the ideal cutoff point for continuous variables. Differences in values derived from categorical data were compared using the Fisher’s exact test. Student’st-test or Mann-WhitneyUtest was used for continuous variables according to the normality of the distribution. Factors in the univariate analysis (P<0.1) were then considered for inclusion to the backward stepwise logistic regression analysis to estimate the odds ratio (OR), 95% confidence interval (95% CI) and identify independent predictors. APvalue<0.05 was considered statistically significant.
Results
Patients, graft, and treatment information
A total of 366 adult patients with a median age of 52 years, including 300 males and 66 females, underwent orthotopic LT during the research period. Of those, 26 consecutive patients received 34 rounds of EVT for determined early HAO at a median 7 (IQR 6-16)days after LT. Eight patients were treated for HAT and four of those(50.0%) presented concomitant HAS, while the others (n= 18)were diagnosed as HAS. The incidence of early HAO, HAT, and HAS were 7.1%, 2.2%, and 6.0%, respectively. Table 1 showed the baseline demographic and perioperative characteristics in the whole cohort and factors associated with early HAO. The most prominent indication for LT was hepatic malignancy, followed by decompensated liver cirrhosis from hepatitis B virus and alcohol. A total of 74.04% patients were free of pre-transplant transcatheter arterial(chemo)embolization. Liver grafts from donation after brain-death and donation after circulatory death (DCD) were accepted. DCD amounted to 58.20% of all transplanted grafts. Nineteen (73.08%)out of 26 early HAO patients received DCD grafts. Of the remaining 340 patients without early HAO, 194 (57.06%) received DCD grafts.According to the univariate and multivariate analyses, the revascularization time (OR = 1.027; 95% CI: 1.005-1.050;P= 0.018) and the need for conduit (OR = 3.558; 95% CI: 1.241-10.203,P= 0.018)were independent early HAO predictors ( Table 1 ).
Endovascular treatment details
As shown in Fig. 3 , all 26 patients underwent EVT complying with the strategy and were divided into the sEVT group and cEVT group. Thrombolytic therapy was attempted in all eight HAT patients to restore artery patency. Four HAT + /HAS- were resolved by either single-round (n= 3) or repeat (n= 1) thrombolytic therapy. Moreover, four HAT + /HAS + were treated with repeat shunt artery embolization plus vasodilator therapy (n= 1), PTA (n= 2)and stent placement (n= 1). HAT-/HAS + (n= 18) was resolved by single-round of shunt artery embolization plus vasodilator therapy in 16 patients, by repeat embolization plus vasodilator therapy in one and by additional PTA plus stenting in one.
Risk factors associated with the EVT procedural challenge
The pre- and post-LT parameters were compared to evaluate the risk factors affecting the difficulty of the technique between the sEVT and cEVT groups ( Table 2 ). Interestingly, only two intraoperative parameters were associated with the EVT procedural challenge. Univariate analysis identified that autologous transfusion (<600 mL) (94.74% vs. 42.86%,P= 0.010) and interrupted suture for arterial anastomosis (78.95% vs. 14.29%,P= 0.005) were more frequent in the sEVT group ( Table 2 ). Distribution of intraoperative splenic/gastroduodenal artery ligation and conduit reconstruction did not differ between the two groups.
Patency and survival after endovascular treatment
Technique success and recanalization were achieved in all 26 patients to restore artery patency. Six of the 26 patients received 2-4 rounds of treatment before discharge. The median followup period was 17.9 months (IQR 13.1-22.5). One patient received stenting because of restenosis at 3 months during the follow-up.The primary patency rates at 1, 6, and 12 months were 76.9%,72.7%, and 72.7%, respectively ( Fig. 4 A). Furthermore, the primary assisted patency rates at 1, 6, and 12 months were all 100%. During the follow-up period, two patients died of severe sepsis, one died of pneumocystis carinii pneumonia, one died of graft-versushost disease, and two died of hepatocellular carcinoma recurrence.The cumulative overall survival rates at 1, 6, and 12 months were 88.5%, 88.5% and 80.8%, respectively ( Fig. 4 B).
Complications and prognosis
No major complications such as target vessel dissection, hepatic artery rupture, or pseudoaneurysm formation occurred. Partial splenic infarction was found in nine (47.37%) patients in the sEVT group and three (42.86%) in the cEVT group ( Table 3 ). Among those patients, only one developed symptomatic infarction (fever and left abdominal pain) and splenic necrosis, which were relieved with nonsteroidal anti-inflammatory drugs. The overall incidence of biliary complications was 38.46% (10/26). In the sEVT group, two cases of bile leak, five of biliary anastomotic stricture, and one of biliary nonanastomotic stricture were developed in six patients. All six patients received biliary drainage and biliary stent under endoscopic retrograde cholangiopancreatography or percutaneous transhepatic cholangial drainage. One of them underwent surgical revision after a failed endoscopic retrograde cholangiopancreatography. In the cEVT group, two cases of bile leak, two of biliary anas-tomotic stricture, and one of biliary nonanastomotic stricture developed in four patients. Surgical biliary revision was performed in one patient with extensive bile duct necrosis after failure of endoscopic intervention. During the follow-up, this patient received retransplantation at post-EVT day 745 because of portal vein thrombosis and graft failure. Other three patients underwent 1, 2, and 6 times of endoscopic intervention for biliary complications. Biloma was not observed in all 26 patients during the follow-up. Sepsis was induced by severe bile leak in one sEVT patient and by graft necrosis in one cEVT patient, both leading to mortalities.

Table 1 Baseline demographic and perioperative characteristics of all recipients and analysis of risk factors associated with early HAO.
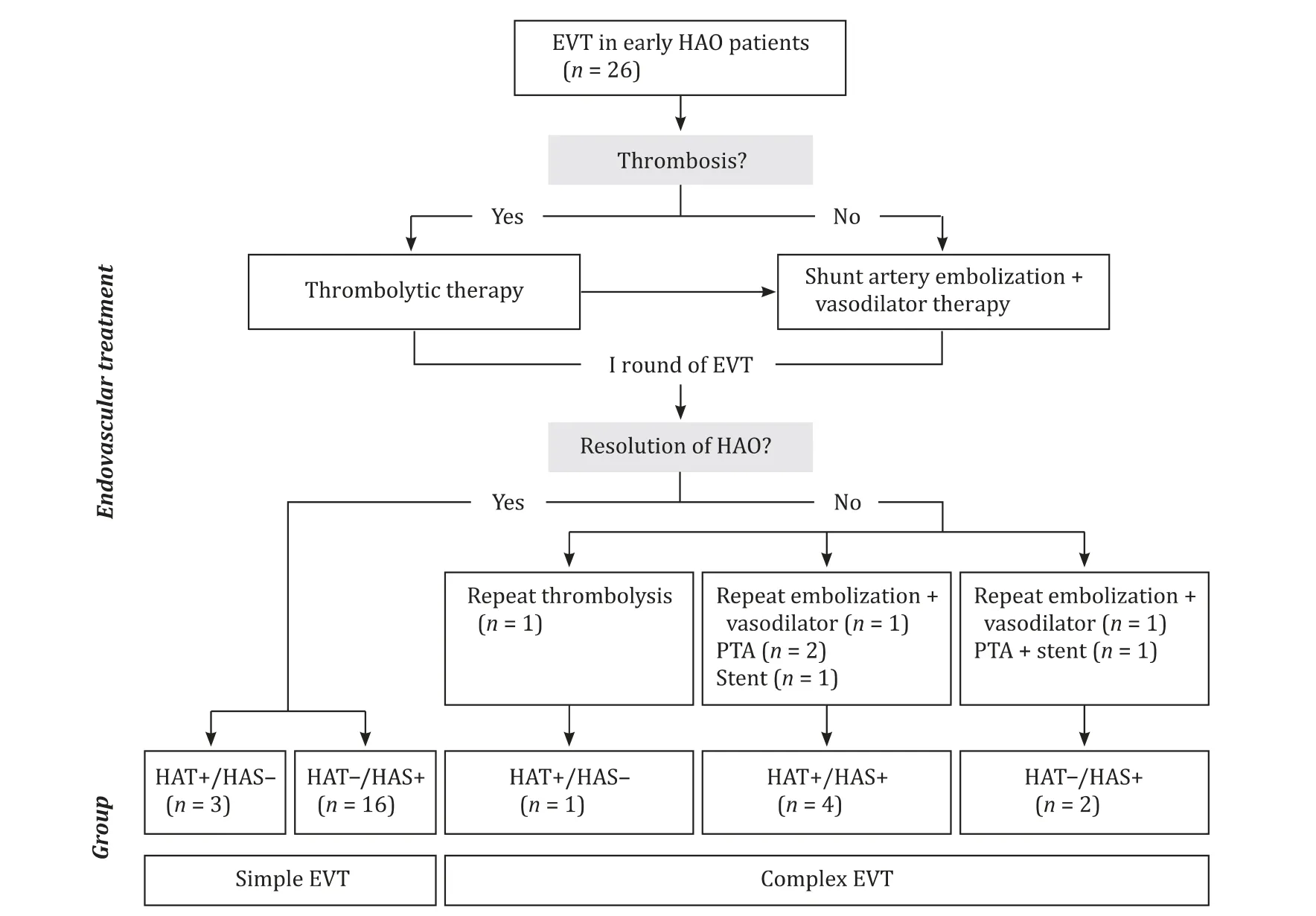
Fig. 3. Flowchart of the integrated endovascular treatment strategy for early hepatic artery occlusion after liver transplantation.
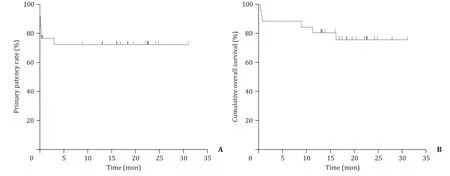
Fig. 4. Patency and survival after endovascular treatment. A: The primary patency rates at 1, 6, and 12 months were 76.9%, 72.7%, and 72.7%, respectively; B: The cumulative overall survival rates at 1, 6, and 12 months were 88.5%, 88.5%, and 80.8%, respectively.
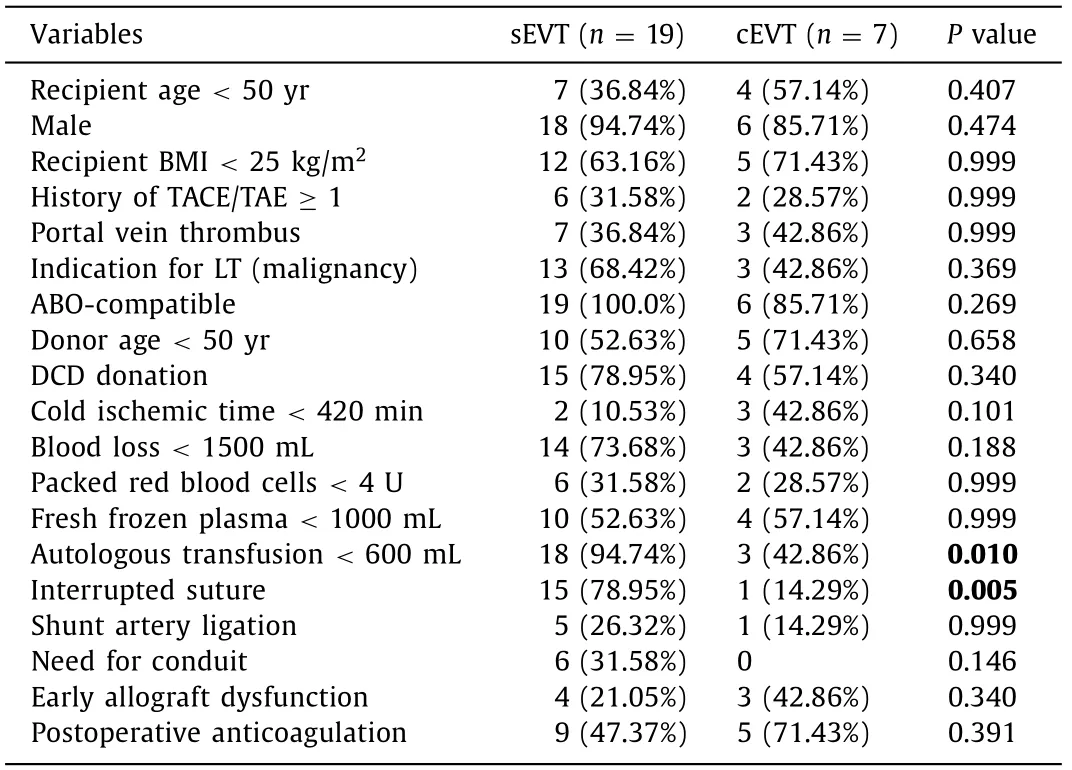
Table 2 Risk factors associated with the EVT procedural challenge for early HAO.
Discussion
Early HAO has long been recognized as an important cause of morbidity and mortality [ 8 , 23-25 ]. Early HAO usually keeps silent until the graft is damaged resulting in extensive hepatic parenchyma necrosis or severe ischemic cholangiopathy. Moreover,HAO remained lacking in the consensus on the criteria for surveillance, treatment protocol, and reporting standard. Kim et al. [ 6 , 7 ]proposed a diagnostic protocol for early HAO based on imaging workup and clinical practice; therefore, promoting early intervention. Under the perspective of the broader graft ischemic spectrum,the shared therapeutic goal of HAT and HAS is to relieve occlusion and improve hepatic perfusion regardless of any recanalization procedures. Thus, the integrated EVT strategy proposed in this study is pathophysiologically and clinically appropriate. In our cohort, the HAT and HAS rates were 2.2% and 6%, respectively; incidences were comparable to a previous report [5] and those conditions were endovascularly resolved following the strategy without major complications. Though some research suggested that conservative management of asymptomatic HAT or HAS is safe, it is unclear when or how the collateral blood supply to the graft and bile duct could compensate the HAO. Timely and proper managementis still crucial to a favorable outcome especially for early HAO patients.
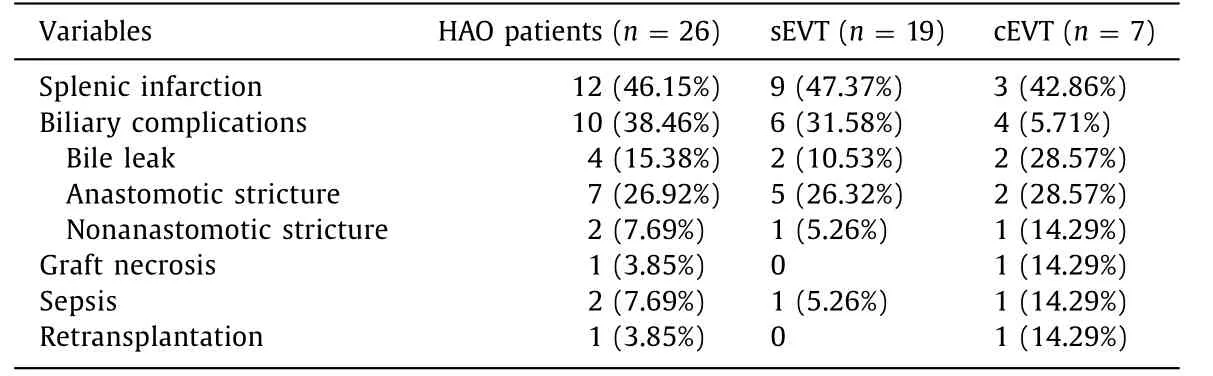
Table 3 Complications and prognosis after EVT for early HAO in two groups.
As high as 85% of HAO cases used to be treated by traditional surgical revascularization, including embolectomy, stenosis site resection, reanastomosis, arterial conduits graft, interposition vein graft, or vein patch angioplasty [23] . The development of new devices and techniques has enabled more endovascular approaches to salvage hypoperfused graft. PTA, stenting, thrombolysis, and experimental mechanical thrombectomy have shown encouraging results with 82% -100% initial technique success rate and 68% -97%patency [ 16 , 19 , 21 , 26 ].
After the first two cases of successful catheter-directed thrombolytic therapy for HAT was reported by Hidalgo et al. [27] ,several studies have reported varying recanalization rates(68% -91%) [17 , 20] . This study reported eight HAT cases wherein thrombolytic therapy was 100% successful. Clear imaging of hepatic artery after thrombolysis, especially the stoma site, enabled thorough evaluation and effective HAO management. In half of the cases, thrombosis was concomitant with stenosis (HAT + /HAS + )and patients underwent additional procedures as did the HAT-/HAS + patients. Shunt artery embolization was most frequently performed in the proximal splenic artery to alter the arterial blood flow distribution while less frequently in enlarged left gastric artery or gastroduodenal artery. Nicorandil is a vasodilator that mainly attributed to the nitric oxide donor and the ATP-sensitive potassium channel opener properties. Moreover, it reportedly exhibits dual action including coronary and peripheral vasodilatation and protective effect through ischemic preconditioning [28] .A previous study in porcine models suggested that nicorandil increases blood flow to the liver and decreases hepatic ischemiareperfusion injury [29] . Nicorandil not only dilates stenotic artery but also improves hepatic microvascular perfusion. Combination of shunt artery embolization and vasodilator therapy directly increases hepatic perfusion by redistributing artery flow to the drug-dilated hepatic artery and indirectly through hepatic arterial buffer response reducing concomitant portal hyperperfusion. Effective shunt artery embolization and vasodilator therapy obviated the need of PTA, stenting and life-long antiplatelet agents, or otherwise at least safely prolonged the time window for inevitable PTA and/or stenting. Our study classified EVT into the simple and complex groups. Multiple rounds of treatment, PTA, and stent placement were regarded as cEVT components as it is a technically challenging procedure and is associated with a higher risk of developing major complications in less than 30 days after LT. Indeed, following the aforementioned strategy, 6 out of 26 patients receiving 2-4 rounds of EVT before discharge led to a relatively inferior primary patency rate (1 month, 76.9%). However,primary assisted patency, overall survival, and incidence of biliary complication of our study were comparable to those reported in previous studies [ 23 , 25 ]. From our perspective, the integrated EVT strategy is a promising therapeutic approach for HAO. It provides the benefits of graft perfusion improvement while limiting potential complications.
Complications of EVT are reported in 0 -19% of the patients [9 , 16 , 19 , 21 , 26] , and include target vessel dissection,rupture, pseudoaneurysm, and sequential thrombosis. The endovascular salvage technique for major arterial complications highly depend on the experience of the surgeon and immediate availability of necessary equipment. For example, covered stents are generally difficult to obtain (the Jomed Coronary Covered Stent)or are oversized (the Viabahn). Moreover, patients remain under extremely high risk of subsequent HAT after salvage endovascular procedure and need much closer surveillance. Goldsmith et al.achieved successful salvage rate as high as 75% [9] . However,the incidence of subsequent HAT rose from 1.4% to 50%. In some institutes, intervention for early critical HAO was delayed to avoid the risk of anastomosis rupture and bleeding [21] . Conservative management with anticoagulant or antiplatelet agents that neither improve hepatic perfusion nor relieve occlusion may increase the incidence of thrombosis, exacerbate biliary complications, and decrease survival. In our center, low-molecular-weight heparin was carefully administrated in high risk patients as prophylaxis instead of treatment for HAO patients.
Both surgical and nonsurgical risk factors contributing to HAO have been widely investigated, but what facilitated the intervention remains unclear. In our analysis, autologous transfusion<600 mL and interrupted suture of the hepatic artery were more prevalent in the sEVT group. Autologous transfusion with cell salvage collected blood plays an important role in managing intraoperative hemodynamics [ 30 , 31 ]. Akamatsu et al. [32] reported that the decrease in both procoagulants and anticoagulants in LT recipients results in a vulnerable hemostatic balance and predisposes recipients to both hemorrhagic and thrombotic events. Generally accompanied by massive hemorrhage, as well as additional perioperative blood component transfusion, low responsiveness to EVT is believed to be associated with impaired coagulation status caused by excessive autologous transfusion. It is recommended to adjust autologous transfusion according to blood coagulation tests findings, the blood clot formation during surgery, and thromboelastometry findings [ 30 , 31 ]. We look forward to further evidence on blood component usage and hemostasis management criteria in this population, which could help achieve a good, safe,and low thrombogenic profile. In order to achieve a low incidence of hepatic artery related complication, several transplant centers adopt microsurgical interrupted sutures for arterial anastomosis especially in living donor liver transplant and small caliber hepatic artery [33] . In this study, interrupted sutures were used in a significantly high proportion of sEVT cases. Occluded arteries with interrupted sutures were more responsive to vasodilator and shunt artery embolization, which simplify EVT. Although speculative, it was believed that interrupted suture provides the anastomosis enough capacity to expand in the absence of restriction between knots. This suggested that interrupted sutures may be the favored option for arteries with small caliber or grafts with high risk of HAO.
The present study has some limitations. As a small sample size, single-center study with a relatively short follow-up duration,our findings have limited generalizability. Longer follow-up of patients is required to determine long-term effectiveness. In addition,we did not enroll a control group of HAO patients who received conservative management, so the superiority of early intervention could not be determined.
In conclusion, the risk factors for developing early HAO include prolonged revascularization time and the need for conduit during transplantation. The integrated EVT strategy adopting thrombolysis, shunt artery embolization plus vasodilator therapy, PTA,and/or stent placement for the treatment of early HAO was an effective approach for hepatic artery patency restoration without major complications. Appropriate autologous transfusion and interrupted suture technique help simplify EVT.
Acknowledgments
None.
CRediT authorship contribution statement
Heng-Kai Zhu:Conceptualization, Data curation, Formal analysis, Investigation, Writing - original draft.Li Zhuang:Conceptualization, Formal analysis, Funding acquisition, Writing - original draft.Cheng-Ze Chen:Data curation, Investigation.Zhao-Dan Ye:Data curation, Investigation.Zhuo-Yi Wang:Data curation, Investigation.Wu Zhang:Funding acquisition, Writing - review & editing.Guo-Hong Cao:Conceptualization, Supervision, Writing - review &editing.Shu-Sen Zheng:Conceptualization, Supervision, Writing -review & editing.
Funding
This study was supported by grants from the National Major Science and Technology Projects of China ( 2017ZX10203201 and 2018ZX10301201 ) and Project of Medical and Health Technology Program in Zhejiang Province ( 2016KYA073 ).
Ethical approval
This study was approved by the Institutional Review Board of Shulan (Hangzhou) Hospital. Due to the retrospective nature of the study, written informed consent was waived.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Non-operative management of pancreatic trauma in adults
- Torin2 overcomes sorafenib resistance via suppressing mTORC2-AKT-BAD pathway in hepatocellular carcinoma cells
- Efficacy and cost-effectiveness of antiviral regimens for entecavir-resistant hepatitis B: A systematic review and network meta-analysis
- Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management
- Hepatobiliary&Pancreatic Diseases International
- Virtual navigation-guided radiofrequency ablation for recurrent hepatocellular carcinoma invisible on ultrasound after hepatic resection
