In-situ reaction between arsenic/selenium and minerals in fly ash at high temperature during blended coal combustion
2020-12-30HANJunLIANGYangshuoZHAOBoXIONGZijiangQINLinboCHENWangsheng
HAN Jun, LIANG Yang-shuo, ZHAO Bo,2,*, XIONG Zi-jiang,2, QIN Lin-bo, CHEN Wang-sheng
(1.Hubei Key Laboratory for Efficient Utilization and Agglomeration of Metallurgic Mineral Resources, Wuhan University of Science and Technology, Wuhan 430081, China; 2.Industrial Safety Engineering Technology Research Center of Hubei Province, Wuhan University of Science and Technology, Wuhan 430081, China)
Abstract: Blended coal combustion technology was extensively used in coal-fired power plants in China.In order to investigate the in-situ reaction between trace elements and minerals in fly ash during blended coal combustion, a bituminous(HLH), anthracite(ZW)and the blended coal of these two parent coals were combusted in a drop tube furnace at 1150 ℃.The ash gathered at high temperature segment(HTA)and low temperature segment(LTA)of the furnace were analyzed, respectively.The results indicated that the retention rates of arsenic in HTA were lower than that in LTA, which suggested that arsenic would be re-absorbed by ash during cooling down of flue gas.For HTA the retention rates of arsenic in ash of ZW, Z3H1, Z1H1, Z1H3, HLH were 60.31%, 26.85%, 13.29%, 20.23% and 36.11%, respectively.The arsenic was more difficult to be captured by HTA of blended coal than that of parent coal.As for selenium, the retention rates in HTA of five coal samples were 24.68%, 23.60%, 20.58%, 15.19% and 38.13%, which had the same retention law as arsenic.The results of X-ray diffraction(XRD)demonstrated that the mineral morphology was changed obviously during blended coal combustion.Unlike parent coal, mullite appeared in HTA of blended coal, and peak of mullite was enhanced with proportion of ZW increased in blended coal.It was consistent with the trend of retention of As and Se in HTA.It illustrated that change of mineral species and in-situ reaction between minerals and trace elements significantly affected emission of arsenic and selenium during blended coal combustion.
Key words: blended coal; arsenic; selenium; mineral; in-situ reaction
Trace elements(TEs)have been attracted much more attention due to its toxicity to environment and human healthy[1].One of the major anthropogenic sources of TEs is coal-fired power plants[2-4].Among the trace elements, arsenic and selenium, especially selenium, are volatility and commonly easily released out of the stack.It was difficult to decline release of the two trace elements in the present air pollution control device[5,6].However, Chinese government announced strict emission standards of trace elements in the Emission Standard of Air Pollutants for Thermal Power Plants[7].The average concentrations of arsenic per year were stipulated no more than 0.006 μg/m3[8].In order to develop effective methods to control the atmospheric arsenic and selenium emission, it was important to understand transformation of these two TEs in coal combustion.
Existing researches indicated that volatilization behavior of trace elements was significantly affected by many factors during coal combustion, such as coal ranks, the species of trace elements in coal, minerals, combustion temperature and so on[9].As for arsenic, ash composition greatly contributed to its retention in the solid phase[10].Calcium ortho-arsenate and alumino-silicate slag were the main arsenate in fly ash[11].Various arsenic species were mainly formed in flue gas at the downstream of a boiler as temperature decreased.At this stage, the arsenic species was related to alkalinity of the fly ash.Arsenic was found to be combined with sulfate, oxyhydroxide or iron oxide in high acid ash while Ca3(AsO4)2was extensively detected in highly alkaline ash[12].It illustrated that minerals in parent coal played a critical role in arsenic species distribution during coal combustion and cooling down process of flue gas.As for selenium, it was more volatile than arsenic, and the dominant species were Se and SeO2[13].The reaction between selenium and fly ash after combustion suggested that iron could react with selenium above 1200 ℃ and calcium began to react with selenium below 800 ℃[14].The retention of Se was contributed by formation of non-volatilized compounds between minerals in fly ash and selenium[15].Fe2O3was the best mineral for retention of Se, with the highest efficiency of 69.8%[15].CaO and MgO were also positive for retention of Se, but SiO2was benefit for release of gaseous Se[15].Chemical reaction between selenium oxides and ash composition was the main reason for retention of Se, and abundant reactive sites on surface of Fe-based minerals significantly promoted the retention process[16,17].It indicated that the ash components were greatly affected transformation behavior of Se during coal combustion.
Above research of arsenic and selenium distribution were obtained from the ash cooled down from high temperature(LTA), and this process may affect the trace elements species.Few studies concerned distribution of trace elements in ash gathered at high temperature(HTA)which was critical to revealin-situreaction between trace elements and minerals in fly ash during coal combustion.
Moreover, due to the complexity of coal types and universality range of source which was conflicted with the designed coal type for coal-fired power plant, blended coal combustion technology has been widely used in coal-fired power plants in China.Recently, concerns of co-combustion were combustion efficiency, slagging or fouling, and pollution emission, such as NOx, SO2and particulate matter[18,19].It has been clarified that distribution of minerals in blended coal combustion was absolutely different from that in single coal combustion.In ash of single ZD coal combustion, the primary crystallization was Na2CaAl4O8, while feldspar region was the main form in the co-combustion ash of ZD and WD[20].In addition, during anthracite and bituminous coal co-combustion, it was found that only a few of As, Pb and Cd in fine particles was volatilized as gaseous phase and others were almost all existed in bottom ash and fly ash in a 2.5 MWthpilot circulating fluidized bed[21].Trace elements were more likely to release from anthracite combustion than bituminous combustion[21].It indicated that change of minerals distribution in blended coal combustion might cause different retention rate of trace elements in ash.However, little data is available about effect of minerals on trace elements distribution during blended coal combustion.
To clearly clarifyin-situreaction between trace elements and minerals at high temperature, distribution of trace elements and evolution of minerals in HTA during blended coal combustion were investigated in this work.
1 Experimental
1.1 Coal samples
A bituminous(HLH)and anthracite(ZW), air-dried and sieved to 76-200 μm, were tested.The blended coal samples used in the investigation were composed of HLH and ZW, which were Z3H1(75% ZW blended with 25% HLH), Z1H1(50% ZW blended with 50% HLH)and Z1H3(25% ZW blended with 75% HLH).Table 1 listed ultimate/proximate analysis of the coal samples and total arsenic content.Ash composition of the samples was analyzed by XRF and the result is shown in Table 2.
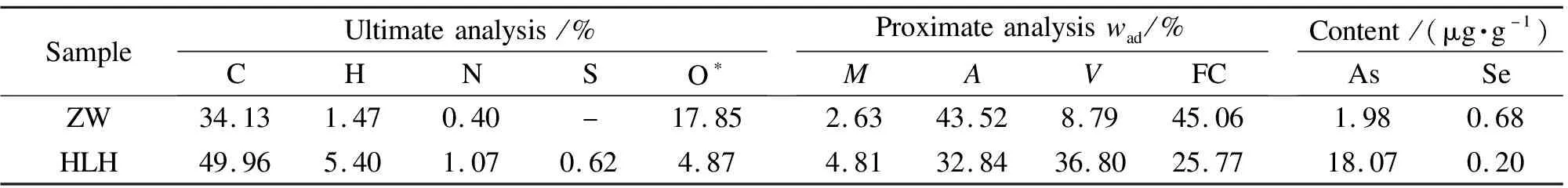
Table 1 Ultimate/proximate analysis of the samples
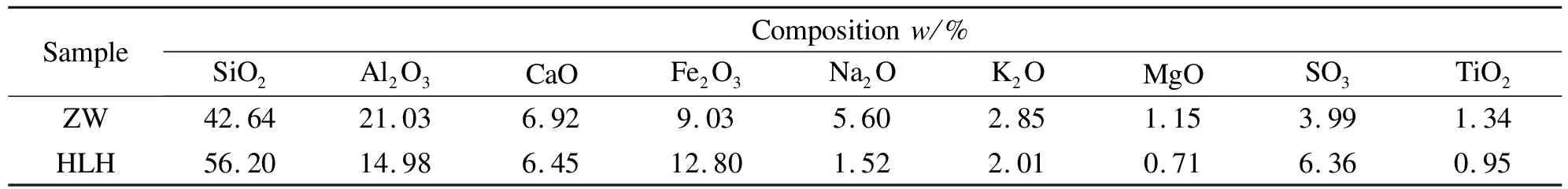
Table 2 Ash composition of the samples
1.2 Experimental conditions
As shown in Figure 1, all coal samples were combusted in a drop tube furnace(DTF)and the details of the equipment was described in work of Han et al[22].A difference was installed in the furnace, which fly ash was collected by a heat-resisting network with mesh size of 1000 on top of the isokinetic sampler reaching into the furnace.Thus, temperature of the ash collected in the furnace was same as that of coal combustion, which ensured thein-situreaction between trace elements and minerals in ash during coal combustion.As contrast, in order to corroborate re-adsorption phenomenon of minerals in fly ash during cooling down of flue gas, all fly ash at outlet of the furnace were also collected and analyzed.All coal samples were combusted in 79% N2/21% O2atmosphere at 1150 ℃, with a feeding rate of 0.2 g/min.The total flow rate in the experiments was 6 L/min, and the flow rates of the primary air and secondary air were 4 and 2 L/min, respectively.

Figure 1 Schematic diagram of the DTF used for the combustion tests
1.3 Analytical method
The contents of arsenic and selenium in coal samples were determined according to the Chinese standard GB/T 3058—2008 and GB/T 16415—2008[23,24].Coal samples were first mixed with Aldrin(66.6% MgO blended with 33.3% Na2CO3)and burned at 800 ℃, and then the burned products were dissolved in hydrochloric acid.Finally, an atomic fluorescence spectrometer(AFS-2202E, Beijing)was used to detect concentration of arsenic and selenium in the solution.The bottom ash samples were placed into a polytetrafluoroethylene(PTFE)vessels and were digested with a mixture of acids(HNO3∶HF = 3∶1)in the microwave digestion system.The solution was then sent for AFS analysis.Each test was repeated three times to ensure the reproducibility.All results were expressed by the retention rates which were calculated according to formulae(1).
(1)
Where,Rwas retention rate of arsenic or selenium in ash under the coal base,m0was arsenic or selenium content in coal,m1was arsenic or selenium content in ash after coal combustion,Awas ash content of the coal sample.
In order to obtain the speciation of arsenic in raw coal, the sequential chemical extraction method was used to extract arsenic which referred to the work of Zou et al[25].Four forms of arsenic including exchangeable, sulfide-bound, organic-bound and residual were divided.The schematic of sequential chemical extraction analysis is presented in Table 3.
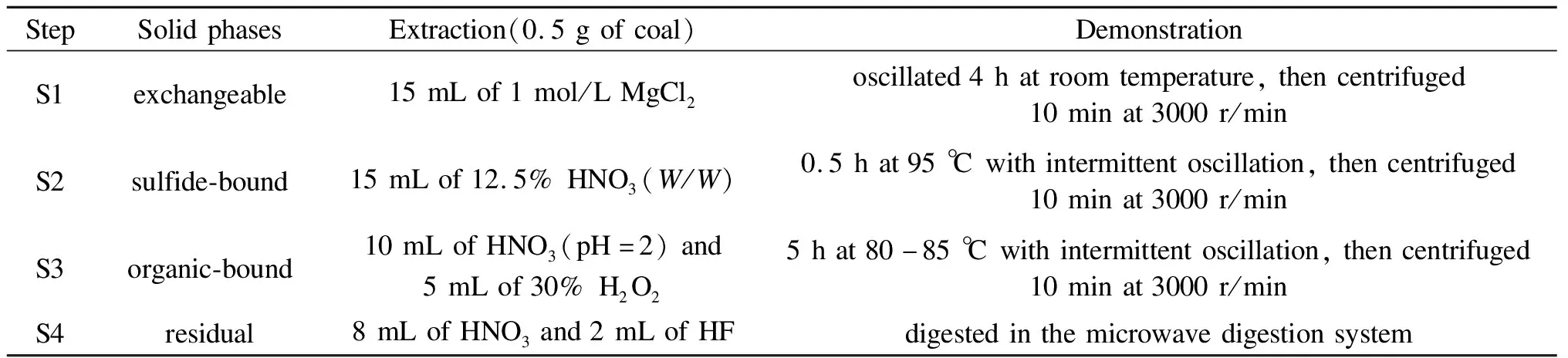
Table 3 Sequential chemical extraction method
2 Results and discussion
2.1 Speciation of arsenic in coal samples
As shown in Figure 2, the results of sequential extraction suggested that the main form of arsenic in ZW was residual which accounted for 67.43%, and the proportion of other forms of arsenic including exchangeable, sulfide-bound and organic-bound, were 1.38%, 26.63%, 4.56%, respectively.For the speciation of arsenic in HLH, the proportion of exchangeable, sulfide-bound, organic-bound and residual were 0.57%, 70.92%, 16.29% and 12.22%, respectively.Zou et al[25]indicated that sulfide-bound and organic-bound arsenic were more likely to gasify and volatilize from the coal particle during combustion, however, residual was a stable form and hard to decompose.It indicated that the arsenic in HLH might be more easily volatilized than that in ZW during coal combustion.
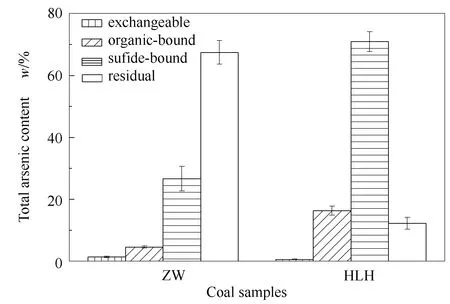
Figure 2 Results of sequential extraction of arsenic
2.2 Retention of arsenic in HTA and LTA
As shown in Figure 3, retention rates of arsenic in LTA of ZW, Z3H1, Z1H1, Z1H3, HLH were 63.19%, 30.41%, 23.12%, 24.14% and 42.17%, respectively.While, arsenic retention rates in HTA of the five coal samples were 60.31%, 26.85%, 13.29%, 20.23% and 36.11%, respectively.The difference in retention rates of arsenic between LTA and HTA ranged from-0.09% to 9.83%.It was found that retention rates of arsenic in HTA were lower than those in LTA.It was because LTA was collected at the low temperature section at the outlet of drop tube furnace, where the flue gas was cooled down.Gas phase arsenic could condense and be adsorbed by the ash and react with minerals subsequently.Thus, clarifying the relationship between minerals and retention rate of HTA can revealin-situreaction of minerals and arsenic in the ash.
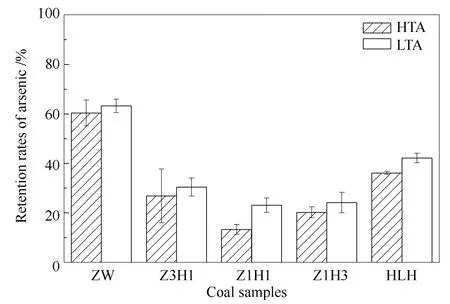
Figure 3 Retention rates of arsenic in HTA and LTA at 1150 ℃
For arsenic, retention rate of HTA from ZW was 60.31%, while the value was 36.11% in HTA from HLH.Additionally, arsenic was more preferable to release as gaseous during the three blended coal combustion, especially Z1H1, just 13.29% of arsenic was retained in HTA.It suggested that arsenic in blended coal was more easily released than that in parent coal during combustion.Besides, the residual proportion of arsenic in HTA of ZW was higher than that in HLH.It was clarified that the content of residual arsenic was positive for retention of arsenic in HTA.It suggested that the arsenic speciation in parent coal might play a critical role in distribution of arsenic during coal combustion.Above 1100 ℃, nearly all of the organic-bound and sulfur-bound arsenic were decomposed and mostly released as gas phase[26].Higher proportion of organic-bound and sulfur-bound arsenic caused the lower retention rates of arsenic in ash.Thus, most of arsenic in HLH was released to the gas phase.However, for ZW most arsenic was retained in the ash.
The results in Figure 3 suggested that most of the arsenic were released during the blended coal combustion.Because of the difference of coal quality between the parent coal, the release of arsenic in blended coal was higher than the weighted value of the parent coal during combustion of lignite mixed with bituminous[26].With the mass ratio of HLH increasing, the volatile matter of blended coal increased because of the high volatile matter of HLH.During coal combustion, lot of new pores were formed in coal particle with the releasing and burning of volatile matter.More oxygen diffused to the char through the microporous channel, and enhanced burnout characteristics of the blended coal.Liu et al[27]proved that exchangeable and organic-bound arsenic were released with the release of volatile matter, however, oxygenolysis of sulfide-bound arsenic regularly with the char combustion.With mass ratio of HLH in the blended coal increasing, the combustion characteristics were improved, and facilitated release of the sulfide-bound arsenic of ZW.

Figure 4 XRD patterns of HTA during blended coal combustion at 1150 ℃
Besides, for HLH residual arsenic accounted for 12.22% in the total arsenic, however, retention rate of arsenic in HTA of HLH was 36.11%.It indicated that the gas phase arsenic could be re-captured by minerals in the HTA.Several studies have found that arsenic in coal would release and reallocate into fly ash, bottom ash, and gaseous state during coal combustion[9,28].Some reports pointed out that heavy metal elements could be captured by silicate in the ash because of the reticular structure of silicate melted[29,30].Yang et al[31]indicated that the rapid melting of ash components or formation of amorphous aluminosilicates was an approach to facilitate arsenic stabilization in ash matrix.Moreover, mineral elements in the ash could react with arsenic to form arsenate, such as calcium-and iron-arsenate[32-34].The possible reactions involved in the process were as follows:
As2O3(g)+3CaO(s)+O2(g)→Ca3(AsO4)2(s)
(2)
As2O3(g)+Fe2O3(s)+O2(g)→2FeAsO4(s)
(3)
In order to clarify effect of mineral morphology changed in the ash on arsenic retention, XRD analysis of HTA was conducted.Figure 4 illustrated that the mineral composition in HTA of parent coal was slightly different.It revealed that the major mineral phases were quartz, anorthite and hercynite in the ash of HLH.However, besides quartz, anorthite, hercynite and nepheline, there was a lot of hematite in HTA of ZW.Iskhakov et al[35]indicated that minerals in coal could be divided into internal and external minerals, and the former were mineral particle that closely wrapped in organic carbon, while the later existed as independent parent particle.For external minerals, they could go through a series of changes during coal combustion, including vaporize, break or transform into new substances.However, different external minerals generally could not react with each other[36].Iron ore contained in ZW may be mainly external minerals.It was difficult to contact with inner minerals including SiO2and Al2O3, which led to the iron ore transformed into Fe2O3during coal combustion.Because of the high arsenic retention activity of Fe2O3, a large amount of Fe2O3in HTA of ZW could react with arsenic to form FeAsO4, which reduced content of arsenic in the flue gas.It was consistent with the higher retention rate of arsenic in HTA of ZW in Figure 3.Different from the parent coal, XRD peaks correspond to mullite could be observed obviously in HTA of blended coal.It may be caused by redistribution of mineral elements in ash after mixing of different kinds of coal.With proportion of ZW increased in the blended coal, peak of mullite was enhanced.Moreover, content of volatile in HLH was quite high, addition of HLH promoted the combustion characterizes of ZW combusting more easily[37].With the char of ZW burned quietly, the inner minerals of ZW constantly sloughed off.Existing researches have proved that a large of ash particles with the diameter of 1-10 μm would be generated during coal combustion[38].Helble et al[39]found that a coal particle would produce 200-500 ash particles with the diameter of 1 μm because of fracture of the char.Large ash particles at high temperature were constantly making random Brownian motion, in contact with each other and polymerized[40].As a large number of mullite was generated during the blended coal combustion, the minerals containing iron ore and calcium ore were fully wrapped and reacted.This reduced probability of contact and collision with gas phase arsenic, thus inhibiting re-adsorption of arsenic by minerals.Besides, Song et al[41]deduced that the number of lattice oxygen in CaO was the main factor to effect retention of arsenic, and diminution of effective lattice oxygen on the surface would lead to a lower capture capacity of CaO.Wu et al[42]indicated that calcium or iron bearing minerals would react with refractory minerals such as mullite and quartz, to form low melting point minerals(anorthite, fayalite, hercynite), which were shown as follows:
(4)
(5)
During co-combustion the presence of a large amount of mullite would fully react with calcium, iron and occupy the active sites for the reaction of those minerals with As, hence retention rates of HTA of blended coal were lower than that of parent coal.
2.3 Retention of selenium
In Figure 5, retention rates of selenium in LTA of ZW, Z3H1, Z1H1, Z1H3, HLH were 37.45%, 29.85%, 33.72%, 21.21% and 45.26%, respectively.While, selenium retention rates in HTA of the five coal samples were 24.68%, 23.60%, 20.58%, 15.19% and 38.13%, respectively.LTA could capture more selenium than HTA, and the difference of their retention rates was 6.02%-13.14%.It manifested that selenium could also be re-absorbed by minerals in ash during flue gas cooling down.However, retention rates of selenium in HTA of all coal samples were relatively low at 1150 ℃.The reason was that the content of selenium in parent coal was much less than arsenic, whereas it was more volatile for selenium during coal combustion[43].The retention rate of selenium in HTA of HLH was 38.13%,while that of ZW was just 24.68%.Moreover, retention rates of selenium in HTA of blended coal were slightly lower than the value of parent coal, especially Z1H3 with 15.19%.
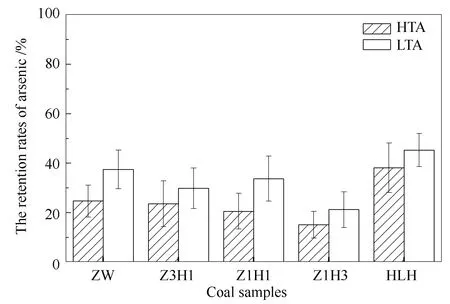
Figure 5 Retention rates of selenium in HTA and LTA at 1150 ℃
Selenium was almost released as gas phase at high temperature during coal combustion.Contreras et al[44]found that SeO2(g)was the main selenium compound released into the gas phase at 850 ℃.Han et al[45]clarified that SeO2(g)was the main form of selenium at 500-1200 ℃ regardless of the proportion of blended coal.Senior et al[34,46]considered that selenium would completely vaporize in the flame zone to form SeO2, and most of selenium in coal would release during coal combustion.On the other hand, it could be re-captured by the minerals in ash[47].It was reported that SeO2in the gas phase could react with calcium and iron to form selenide[48].Fan et al[49]found that SeO2could be absorbed and react on the surface of CaO through density functional theory(DFT)calculations and adsorption experiments.Querol et al[50]considered that gaseous selenium might be chemically absorbed by iron oxides and translate into Fe-Se compounds.The possible reactions involved in the process were as follows:
SeO2(g)+CaO(s)→CaSeO3(s)
(6)
SeO2(g)+Fe2O3(s)→Fe2(SeO4)3(s)
(7)
The formation of selenide, including CaSeO3and Fe2(SeO4)3, affected distribution of selenium in the solid and gas phases.During coal combustion, calcium/iron in ash was favorable for selenium vapor to migration to the solid phase.However, Li et al[51]found that SeO2adsorption efficiency of CaO decreased above 780 ℃.Because the reaction product between calcium and selenium was not stable, which may be the main reason for the low retention of selenium in HTA of blended coal.Besides, the XRD results revealed that there were a lot of mullite in HTA of blended coal.Mullite would react with calcium and iron in the ash.Thus, just like arsenic, the presence of a large amount of mullite would occupy the active site for SeO2adsorption.On the other hand, it would also reduce probability of Ca and Fe to contact with SeO2, thereby inhibited retention of selenium during co-combustion.
3 Conclusions
The retention of trace element in HTA and LTA during combustion of HLH bituminous, ZW anthracite and the blended coal was investigated.Arsenic would be re-absorbed by the ash during flue gas cooling down.Gathering ash from high temperature segment of inside furnace was more reliable to reveal distribution of arsenic during coal combustion.The retention rate of arsenic in HTA of ZW and HLH were 60.31% and 36.11%, however, the retention of arsenic in HTA of blended coal was lower than the weighted value of the parent coal, the value were only 26.85%, 13.29% and 20.23%.The addition of high volatile coal enhanced combustion characteristics of blended coal and increased release of trace elements.For selenium, retention rate of selenium in HTA of ZW and HLH were 24.68% and 38.13%; however, the value in HTA of blended coal were 23.6%, 20.58% and 15.19%, which was consistent with the results of the retention rule of arsenic.Besides, XRD patterns showed that there were a lot of mullite in HTA of blended coal, which was the main reason for decreasing retention rate of arsenic and selenium in HTA of blended coal.The presence of a large amount of mullite in HTA could react with calcium ore and iron ore, which occupied the active sites for arsenic or selenium adsorption.
Acknowledgement
In the process of learning the use of thermodynamic software, we sincerely thank all teachers and students for their great help.Finally, thanks to the reviewers for their attention and guidance.
杂志排行
燃料化学学报的其它文章
- Study on the environmental effects of heavy metals in coal gangue and coal combustion by ReCiPe2016 for life cycle impact assessment
- 复合聚并协同脱除燃煤颗粒物及颗粒态重金属的中试研究
- 典型钙/镁基吸附剂对二氧化硒吸附特性研究
- Speciation analysis of arsenic in coal and its combustion by-products in coal-fired power plants
- 燃煤烟气中As、Se、Pb的形态分布及S、Cl元素对其形态分布的影响
- 燃煤电厂砷、硒、铅的排放与控制技术研究进展
