Speciation analysis of arsenic in coal and its combustion by-products in coal-fired power plants
2020-12-30HEKaiqiangSHIMengdanLIYanJIANGYanghongLIYuanpengYUANChungang
HE Kai-qiang, SHI Meng-dan, LI Yan, JIANG Yang-hong, LI Yuan-peng, YUAN Chun-gang,2,*
(1.Hebei Key Lab of Power Plant Flue Gas Multi-Pollutants Control, Department of Environmental Science & Engineering, North China Electric Power University, Baoding 071000, China; 2.MOE Key Laboratory of Resources and Environmental Systems Optimization, College of Environmental Science and Engineering, North China Electric Power University, Beijing 102206, China)
Abstract: The speciations of Arsenic(As)in coal will inevitably convert during the combustion process.The As speciations in coal and its by-products are closely related to human health and environmental safety which is urgent to be identified.However, there is a lack of pretreatment procedure and analysis method on the As species in coal-related products in power plants.In this study, the As species in coal, fly ash(FA), and gypsum were successfully determined by high performance liquid chromatography coupled with hydride generation atomic fluorescence spectrometry(HPLC-HG-AFS).The instrument parameters, extract reagents, and pretreatment methods(i.e.ultrasound and microwave-assisted)were optimized.The whole separation time of inorganic As was shorten to 7 min after optimization, with the detection limit of 1.8 and 4.6 ng/g for As(III)and As(V), respectively.The efficient As extract reagent was the mixture of 1.0 mol/L H3PO4 and 0.1 mol/L ascorbic acid solution.Microwave-assisted(2000 W, 80 ℃, 40 min)and ultrasound-assisted(40 kHz, 20 ℃, 40 min)schemes were the optimal extraction methods for coal/FA and gypsum samples, respectively.Under the proposed microwave and ultrasound extraction procedure, the recovery of As(III)and As(V)could reach to 95.8%/104.5% and 90.6%/89.7%, respectively.The dominant occurrence of As species in coal was As(V)with a small percentage of As(III), while As(V)was the only occurrence form observed in FA and gypsum.It is indicated that revealing the transformation of As(III)to As(V)is the key for gaseous As capture.The As species distribution investigation provides a scientific insight to the controlling of As emission from power plant.
Key words: arsenic; speciation; coal; combustion by-products; HPLC-HG-AFS
Coal is the dominant primary energy in China and is widely consumed in thermal power plants[1,2].Coal contains a variety of toxic pollutants and trace heavy metals.Most of the trace elements in coal undergo a series of complex transformations and eventually discharge into the environment along with fly ash, gypsum, etc[3-6].More attention has been paid to the migration and transformation of mercury from power plants in previous studies, but another trace element arsenic(As)is rarely studied[7].Arsenic is one of the most harmful and common carcinogens to human beings.Excessive exposure to As can result in severe harm in nervous, cardiovascular, and digestive systems[8].In addition to the concentration, As toxicity depends on the occurrence forms(speciations/species)in the combustion products.It is well known that inorganic As is more toxic than organic As, while the toxicity of As(III)is more than 50 times that of As(V).Therefore, the accurate extraction and determination of As speciations in coal-related substances from power plants are urgent.
At present, the main methods for As speciation analysis are high-performance liquid chromatography inductively coupled plasma mass spectrometry(HPLC-ICP-MS)and high-performance liquid chromatography hydride generation atomic fluorescence spectrometry(HPLC-HG-AFS)systems[9-13].The former has advantages of less interference, multiple elements online simultaneous detection, but the application is limited by the high maintenance cost and complex operation.The latter has high sensitivity, good reproducibility, low cost, and simple operation, which is widely used in the speciation analysis field.Sun et al[14]used HPLC-HG-AFS system for the first time to analyze the inorganic As speciation in coal samples.Xu et al[15]established a HPLC-HG-AFS procedure for the determination of As speciation in fly ash from municipal solid waste incineration.The result indicated that the percentage of As(III)was less than 2% to total inorganic As.Another study also demonstrated that the most abundant species of As in FA extracted by water was As(V).However, former researchers discovered that the sum concentration of various As speciations was much lower than the total As concentration in the sample.Different from the total amount digestion, the principle of speciation extraction is to keep the original As valences and maximally extract As.The development of efficient As species extracting procedure is still challenging.
Due to the diverse element compositions of coal, FA, and gypsum, it is essential to develop different extraction methods.Conventional As species extractions are conducted by hot-water bath, shaking, ultrasound, and microwave-assisted methods[16].Although hot-water bath is low-cost, the high temperature may lead to the conversion of As species during the extraction process.For shaking procedure, the whole operation time is generally up to a few hours or even tens of hours.By contrast, ultrasound and microwave-assisted methods have advantages of high efficiency, low labor cost, and fewer transformations.The ultrasound and microwave energy could significantly accelerate the As species separation through the cavitation and dipole rotation effect, respectively[3,17].
In this work, we optimized the instrument parameters of HPLC-HG-AFS system and compared the extraction efficiency of different solvents for As species.In addition, microwave and ultrasound-assisted extraction methods were applied to effectively separate As species from coal, FA, and gypsum samples.Satisfied results were obtained by the developed procedures.This work is of significance for indicating the occurrence form of As, evaluating the As pollution risk, and understanding its migration/enrichment rules in power plants.
1 Materials and methods
1.1 Samples preparation
Coal, FA, and gypsum samples were collected from six power plants located in Hebei Province, China.Each sample was freeze-dried for 48 h and sieved through a 200-mesh sieve, following stored in the dryer for standby before the experiment.
1.2 Reagents
Diammonium hydrogen phosphate((NH4)2HPO4), methanol(CH3OH), phosphate buffered saline(PBS, pH=7), ascorbic acid(VC), hydrofluoric acid(HF), nitric acid(HNO3), hydrogen peroxide(H2O2), hydrochloric acid(HCl), sodium hydroxide(NaOH), potassium borohydride(KBH4)and thiourea(CH4N2S)were purchased from Kermel Chemical Company(Tianjin, China).Ammonium dihydrogen phosphate(NH4H2PO4)and phosphoric acid(H3PO4)were obtained from Guangfu Chemical Company(Tianjin, China).All chemicals were of analytical grade or better.Arsenite(As(III)), monomethylated arsenic(MMA), dimethyl arsenic(DMA), and arsenate(As(V))standard solutions were prepared from the stock solution(National Institute of Metrology, China).A coal certified reference material(GBW11117)was used for quality control during the procedure.
1.3 As extraction and determination
1.3.1TotalAsanalysis
Each sample of 0.1 g was digested by the mixture of 4 mL HNO3, 1 mL HF, and 1 mL H2O2through the microwave digestion instrument.The digestion procedure was carried out at 3 MPa and 180 ℃ for 45 min.Blank experiments were conducted under the same procedure.Finally, As in suspension was diluted, passed through 0.45 μm filter membrane, pre-reduced by thiourea/ascorbic, and determined by an atomic fluorescence spectrometer(AFS-933, Titan Instruments Co., Ltd., Beijing, China).The certified value of As in standard coal material(GBW11117)was 51 μg/g and the recovery of As was 103.6% under the total digestion procedure.
1.3.2Asspeciationanalysis
Arsenic species in 0.5 g samples were extracted with 20 mL of 1 mol/L H3PO4and 0.1 mol/L VC mixed solution.The As species extractions of coal and FA samples were conducted by a microwave digestion instrument MWD-800(Metash Instruments Co., Ltd., Shanghai, China)at 2000 W, 80 ℃ for 40 min.Arsenic species in gypsum samples were extracted using an ultrasonic processor KQ-250B(Shumei Ultrasonic Instruments Co., Ltd, Kunshan, China)at the condition of 40 kHz, 20 ℃ for 40 min.The extraction efficiency was calculated by comparing the extracted As concentration to the total As concentration of the sample.Arsenic speciation was analyzed by a high performance liquid chromatography(SAP-10, Titan Instruments Co., Ltd.)coupled with atomic fluorescence spectrometry system.In this work, PRP-X100 was chosen as the analytical column relying on the efficient and stable separation performance of As speciation[14,15].The operating parameters of mobile phase type, mobile phase flow rate, and AFS instruments are displayed in Table 1.
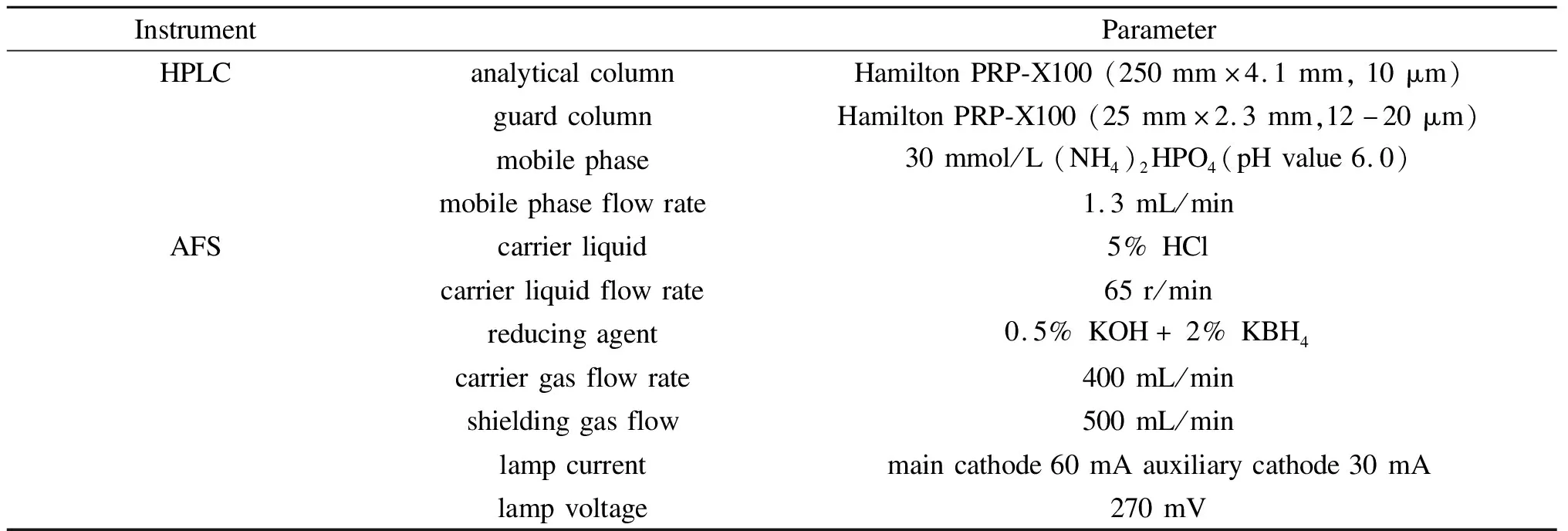
Table 1 HPLC-HG-AFS instrument conditions for As species determination
2 Results and discussion
2.1 As chromatographic separation
The aim of chromatographic parameters optimization is to shorten the separation time without affecting the valence state and to accurately analyze the concentration of separated elements.The main parameters include the flow rate, concentration, and pH value of the mobile phase.Because of the poor acid-base tolerance of the anion-exchange chromatographic column used in this study, pH gradients were not investigated.Therefore, flow rate and concentration of the mobile phase were optimized.
When the flow rate of mobile phase increased from 1 mL/min to 1.3 mL/min, the As analysis time considerably decreased from 17 min to 12 min.The chromatographic separation results of As after mobile phase flow rate optimization are shown in Figure 1.Notably, the column pressure reached 6.5 MPa when increasing the flow rate to 1.3 mL/min.Hence, the flow rate was not further increased in order to maintain the service life of the chromatographic column.

Figure 1 Chromatogram of As species after mobile phase flow rate optimization
It can be seen from Figure 1 that the MMA and DMA peaks were close to As(III)peak.Hence, further shorting the separation time may lead to peaks overlap.It is reported that there is no organic As in coal, nor in FA and gypsum after high-temperature combustion[14].Therefore, the influence of mobile phase concentration on the inorganic As separation was investigated.Experiment results indicated that increasing the mobile phase concentration could effectively shorten the chromatographic separation time of inorganic arsenic.As shown in Figure 2, the chromatographic separation of As(III)and As(V)was completed within 7 min when the mobile phase concentration was 30 mmol/L.The separation time of inorganic As in HPLC-HG-AFS system was close to that in literature[15].Considering the maintenance of the peak shapes of As(III)and As(V), the separation time was not further shortened.

Figure 2 Chromatogram of As species after mobile phase concentration optimization
2.2 As speciation extraction
2.2.1Optimizationofextractionreagent
The choice of extraction agent is a key factor during the process of As speciation analysis.It is necessary to select an efficient and non-destructive solvent for the As extraction.In this study, several common extracts including CH3OH-water(1∶1, volume ratio), 1%(volume ratio)HCl, 0.15 mol/L(NH4)2HPO4, 1 mol/L NH4H2PO4, PBS(pH=7), and 1.5 mol/L H3PO4+ 0.1 mol/L VC were used to extract As species[16,18,19].The solid/liquid ratio was 1∶40 during the extraction process.Due to the lack of certified standard material of As speciation, a coal sample with total As concentration of 9.35 μg/g was used as a reference material in this study.The extraction experiments were performed under ultrasonic treatment at 20 ℃, 40 kHz for 40 min.As Figure 3(a)shows, the As extraction concentration by H3PO4and VC solution was obviously higher than that of other extractants.Phosphorus and arsenic are homologous elements, so phosphorus can interfere with the chromatographic separation of pentavalent arsenic.Ascorbic acid(0.1 mol/L)solution could alleviate the influence of H3PO4solvent on arsenic speciation determination and prevent the As(III)oxidation[20].Hence, H3PO4and VC were suitable reagents to extract As species.Based on the above discussion, a gradient experiment of H3PO4concentration(0.5 mol/L, 1 mol/L, and 1.5 mol/L)was conducted to optimize the extract.It can be seen from Figure 3(b)that the concentration of extracted As reached the highest value when the H3PO4concentration was 1 mol/L.Therefore, 1 mol/L H3PO4and 0.1 mol/L VC were chosen as the As extractant in the following experiment.It is notable that when the concentration of H3PO4was up to 1 mol/L, the peak shape of As(V)chromatogram became wider and the peak height became lower.This is because the concentration of phosphate in the extract was clearly higher than that in the mobile phase.
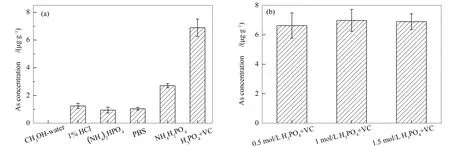
Figure 3 (a)As concentrations extracted by different reagents and(b)the effect of H3PO4 concentrations on the As extracting
2.2.2Effectofdifferentextractionmethods
After the optimization of extracting reagent, different extracting methods including microwave, ultrasonic, and traditional shaking were used to extract As species in coal, FA, and gypsum samples.The extraction of each method was repeated 5 times for each sample.Table 2 shows the extraction efficiencies of these samples using different extraction methods.
Microwave-assisted extraction(80 ℃, 2000 W for 40 min)exhibited the highest efficiency for coal and FA samples.The order of As extraction efficiency in coal was microwave > shaking > ultrasonic, while the order was microwave > ultrasonic > shaking for fly ash.In case of gypsum, ultrasound treatment(20 ℃, 40 kHz for 40 min)showed the best performance with the extraction efficiency of 57.6%.Longer extraction time(60 min)of ultrasound and microwave procedure was also investigated and has been proved to have little effect on the improvement of extraction efficiency.Compared with the conventional shaking, microwave and ultrasound extraction has excellent efficiency with simple operation.Their good performance is because the radiation energy directly reaches the inside of the solution.Then, microwave and ultrasound energy accelerate the As desorption process from mineral particulates by the ionic conduction/dipole rotation and cavitation effect[3,17].In conclusion, the application of microwave and ultrasound are reasonable and effective to extract As species in coal/FA and gypsum, respectively.
2.2.3Asstabilityduringextraction
Another concern is the stability of As speciation during the extraction process because individual As specie can easily convert to other species under the operating conditions.Due to the lack of standard materials for As species analysis, the stability and reliability of the proposed methods were verified by adding 10 μg/L of As(III)and As(V)standard solutions to the mixture of 1 mol/L H3PO4and 0.1 mol/L VC solution.Then the extraction was conducted under the corresponding microwave/ultrasound condition.Each method was repeated three times.Table 3 shows that the As(III)and As(V)recoveries are in the range of 89.7%-104.5%.No obvious transformation of As species was found, the as-mentioned methods are suitable for stable extraction of the As species.
2.3 Method validation
Under the optimal conditions, the linearities(r), limits of detection(LOD), and limits of quantification(LOQ)of As(III)and As(V)were accessed.The summary is shown in Table 4.It can be seen that the linearities were higher than 0.9979 in the range of 10-200 μg/L.The LOD was 1.8 and 4.6 ng/g for As(III)and As(V), whereas the LOQ was 6.3 and 14.7 ng/g.The methods are accurate and precise, which can be further used for As species analysis.
2.4 Arsenic speciation in coal and its by-products
The proposed microwave and ultrasound-assisted extraction coupled with HPLC-HG-AFS systems were used to investigate the As speciation in real samples from different power plants.
2.4.1Asspeciationincoal
There are many kinds of coals in China which contain various As contents.Arsenic species distribution determinates its transformation process from combustion to deposition[21].In this work, six coal samples collected from different power plants were analyzed.The total As concentrations in the coals ranged from 5.23 to 9.17 μg/g(Table 5).It can be seen from Figure 4 that the proposed microwave-assisted method extracted 30.6% to 95.2% of the total As in coal.
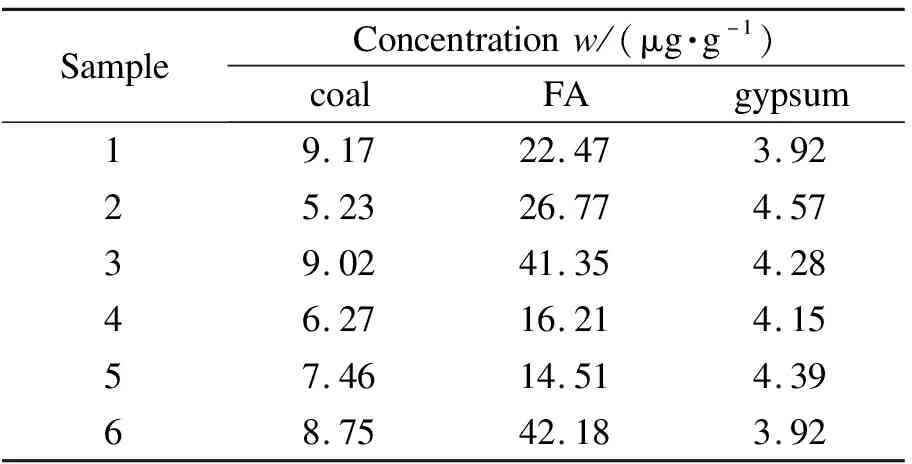
Table 5 The total As concentrations in coal, FA, and gypsum samples

Figure 4 Extraction efficiencies and species distributions of As in six coal samples
The extraction efficiency had a wide variation, which was mainly attributed to the different mineral compositions of coal samples.Because of the difference in geochemistry conditions, minerals exhibited disparate affinities to As.For example, arsenic bonded to Fe and Al compounds are more difficult to separate than that adsorbed on other mineral surface[3].Unfortunately, it is impractical to apply more competitive ions to further extract As species because it may affect the valence state of As during the extraction process.Similar phenomenon was demonstrated in As speciation analysis of soil samples[20].Speciations distribution results indicated that As in coal mainly existed in the form of As(V), which was considerably higher than As(III)concentration.Although As(V)percentage is high, As(III)is more toxic and its speciation transformation should be given more attention during the combustion process.
2.4.2Asspeciationinflyash
After combustion, most of As discharged from coal will be gathered in FA particulates[6].Whether FA is stored in landfills or used as building materials, adsorbed-As in FA has secondary exposure risks to the environment and human beings.Therefore, it is very important to study the As speciation in FA.As Table 5 shows, the total As concentration in different FA samples ranged from 14.51 to 42.18 μg/g, which was obviously higher than that in coal samples.This result evidenced the enrichment of As in FA.The extraction efficiencies of As species in FA samples by H3PO4-VC solution under microwave procedure were between 35.9%-94.2%(Figure 5).
Due to the diverse minerals of feed coal, composition of burning products(fly ash)was also quite different.Hence, the extract efficiency of As speciations in FA samples also fluctuated greatly as same as that of coal.It is worth noting that As(V)was the only species in FA samples studied in this work.
2.4.3Asspeciationingypsum
It is estimated that 6.64 million tons of flue gas desulfurization gypsum is produced from coal-fired power plants in China in 2016, and 80.6% of them were recycled[22].Due to low-cost and abundant, they are widely used to improve soil quality.However, As in gypsum may migrate to soil and plants, ultimately transfer to human body[23].Hence, six gypsum samples were analyzed to investigate the As speciation distribution.The total As concentration in gypsum was between 3.92-4.57 μg/g(Table 5).Gypsum samples had the lowest As content compared with that of coal and FA samples.As shown in Figure 6, the extraction efficiency under ultrasound treatment ranged from 24.4% to 59.2%.Arsenic species had relatively low extraction efficiency in gypsum.This is due to the fact that prior to the formation of desulfurizing gypsum, the desulfurizing slurry has separated part of the As in soluble state.Therefore, As had a more stable fraction in gypsum which was difficult to extract.The As species distribution in gypsum was consistent with that in FA, and only As(V)existed.More As removal methods should be conducted before using gypsum to improve soil quality.
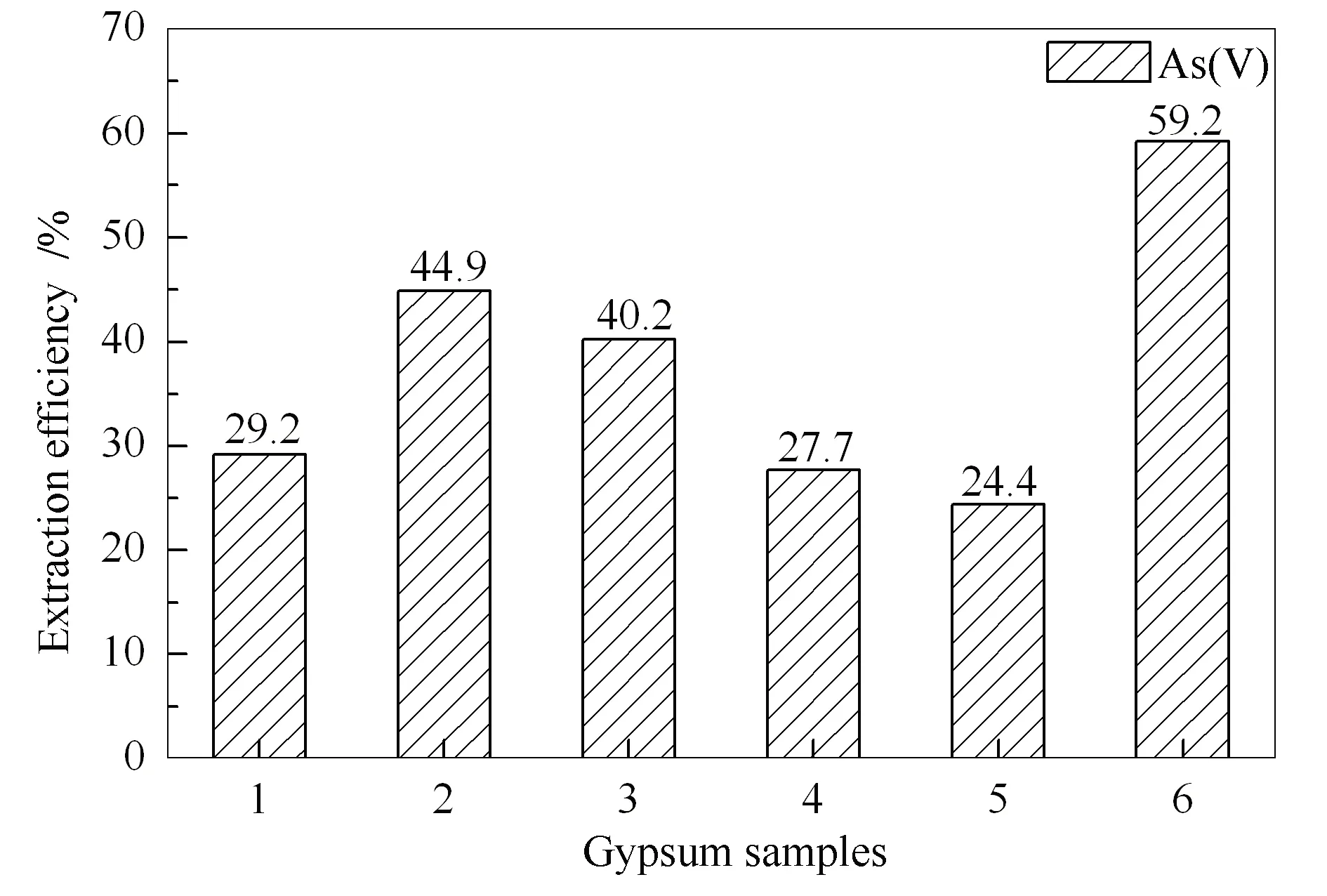
Figure 6 Extraction efficiencies and species distributions of As in six gypsum samples
2.5 As species transformation
In general, As will emit from coal during combustion process and form gaseous As2O3/As2O5in the presence of O2.As2O5has poor stability at high temperature(>700 ℃)and will further decompose to As2O3[24].Hence, As in trivalent state may be the main As specie in the flue gas.FA and gypsum have been regarded as good containers for gaseous As in power plants.Interestingly, the chromatography results indicated that As(V)was the only As speciation in FA and gypsum samples.The presence of As(V)demonstrated that the transformation from As(III)to As(V)on FA and gypsum particles occurred during combustion.Chemical adsorption of metal composition was the main way to capture gaseous As over FA and gypsum particulates rather than physical condensation.At present, more attention is focused on the As emission control from coal power plants due to high toxicity and easy mobility of gaseous As.The mainstream approaches are through physisorption or chemisorption by adsorbents.This work provides a significant insight to As capture in flue gas, that is, the transformation of As(III)to As(V)is the key point before adsorption.Hence, the enhancement of chemisorption is beneficial for As removal in power plant.
3 Conclusions
In this study, As speciations in coal and coal combustion by-products were analyzed by HPLC-HG-AFS system combining with microwave and ultrasound-assisted extraction procedures.Microwave and ultrasound-assisted extraction procedures were suitable ways to extract As species in coal/FA and gypsum, respectively.With the assistance of microwave and ultrasound, the total operation time was considerably shortened to 40 min compared to the conventional shaking extraction procedure, and satisfactory results could be obtained.This work provided detailed information on As species in coal, FA, and gypsum samples from power plants.As(V)was the only inorganic species in FA and gypsum, while As(III)and As(V)coexisted in coal samples.It was noted that the extraction efficiency was considerably affected by the composition of coal and its combustion by-products, resulting in a wide range of extract recoveries.The proposed schemes were promising methods to reveal the transformation of As speciation during the combustion process in coal-fired power plants.
杂志排行
燃料化学学报的其它文章
- Study on the environmental effects of heavy metals in coal gangue and coal combustion by ReCiPe2016 for life cycle impact assessment
- 复合聚并协同脱除燃煤颗粒物及颗粒态重金属的中试研究
- In-situ reaction between arsenic/selenium and minerals in fly ash at high temperature during blended coal combustion
- 典型钙/镁基吸附剂对二氧化硒吸附特性研究
- 燃煤烟气中As、Se、Pb的形态分布及S、Cl元素对其形态分布的影响
- 燃煤电厂砷、硒、铅的排放与控制技术研究进展
