Influenc of graphene oxide(GO)on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting
2020-12-18JunXiToMingChunZhoYingChoZhoDengFengYinLongLiuChengdeGoCijunShuiAndrejAtrens
Jun-Xi To,Ming-Chun Zho,Ying-Cho Zho,Deng-Feng Yin,∗,Long Liu,Chengde Go,Cijun Shui,Andrej Atrens
a School of Materials Science and Engineering,Central South University,Changsha 410083,China
b College of Mechanical and Electrical Engineering,Central South University,Changsha 410083,China
cDivision of Materials,The University of Queensland,Brisbane,Qld 4072,Australia
Received 29 July 2019;received in revised form 13 October 2019;accepted 23 October 2019 Available online 8 July 2020
Abstract Different graphene oxide(GO)contents were chosen as the addition to prepare ZK30-xGO composites by selective laser melting(SLM).The microstructure and biodegradation of the SLMed ZK30-xGO composites were investigated.The results indicated that(i)SLM effectively produced a small grain size,(ii)the incorporation of GO into ZK30 caused a further decrease in grain size,and(iii)GO has a strong effect on the formation of the MgZn2 precipitates.The SLMed ZK30-0.6GO had the lowest biodegradation rate,which is attributed to the fact that the effect of the increased grain refinemen and decreased amount of the MgZn2 precipitates counteracted the effect of the increased GO content on the biodegradation rate.Furthermore,the SLMed ZK30-xGO composites had good cytocompatibility.This work provided a novel approach to the composition design and fabrication of novel biodegradable GO reinforced Mg-based biomedical implants.© 2020 Published by Elsevier B.V.on behalf of Chongqing University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Mg alloy;ZK30-xGO composite;Selective laser melting(SLM);Biodegradation;Cytocompatibility.
1.Introduction
Mg is a new revolutionary biodegradable metal for biomedical implants due to its good biodegradation and biocompatibility in physiological environments[1-6].However,the low strength of pure Mg means that pure Mg cannot meet the mechanical property requirements of biomedical implants.Mg alloys do have adequate mechanical properties[7-12]so that Mg Alloys have been a hot research topic for biomedical metallic implants.In particular,ZK30(Mg-3Zn-0.5Zr)has received considerable attention because Zn is essential for the human body,and Zr has good biocompatibility[13,14].
Graphene oxide(GO)is a new eye-catching reinforcement.There has been considerable interest in embedding GO into a metal matrix to improve mechanical properties[15,16].GO is graphene functionalized by oxygen containing groups[17].GO possesses excellent mechanical properties and biocompatibility[18,19],which makes it an ideal reinforcement for a biodegradable Mg-matrix composite.Thus,it would be desirable to prepare ZK30-GO composites by embedding GO into ZK30,in which the GO acts as a reinforcement,in order to produce a Mg-based biomaterial of sufficien strength.However,it is difficul to produce GO-reinforced ZK30-GO composites with good homogeneity by traditional casting technology,because GO has a layer structure in nanoscale thickness and may easily be aggregated[20,21].It is necessary to develop an easy method to produce GO-reinforced ZK30-GO composites with uniform homogeneity.

Fig.1.SEM morphologies of(a)ZK30 powder and(b)GO powder;and X-ray maps of GO for(c)C and(d)O.(e)XRD result of GO powder.
Selective laser melting(SLM)is a new,advanced,laserprocessing technology,which can rapidly melt the mixed powders of ZK30 and GO,and rapidly solidify the melt pool[22,23],thereby avoiding the agglomeration of GO,to produce a homogenous ZK30-GO microstructure with fin grains.Furthermore,the fin grain microstructure of Mg alloys is expected to have good mechanical properties and a low corrosion rate[24].SLM has successfully produced a variety of Mg alloys,iron alloys,ceramics and polymer resins[22-25].For example,Xu et al.used SLM to produce ZK30-xCu alloys with remarkably small grains,low biodegradation rates and high hardness[24].Zhao et al.prepared homogeneous Fe-xGO composites[25].However,little research has been performed on SLMed GO-reinforced Mg-based materials,especially SLMed ZK30-GO composites.In SLMed ZK30-GO composites,the content and distribution of GO are expected to be the critical factors affecting their microstructure and biodegradation.There is currently essentially no knowledge on the relationship between the GO content and(i)the microstructure and(ii)the biodegradation rate.This work prepared ZK30-xGO composites with different GO contents by SLM.The microstructure and biodegradation of the SLMed ZK30-xGO composites were investigated.
2.Experiment
ZK30 powders with a composition of 96.34% purity Mg containing(by mass),3.16% Zn,0.48% Zr,0.002% Al,0.002% Cu,0.01% Fe,0.0068% Mn,0.0036% Ni,0.01% Si,were purchased from Tangshan Weihao Materials Co.,Ltd.GO powders of 99% purity were purchased from Chengdu Organic Chemicals Co.Ltd.,Chinese Academy of Sciences,China,respectively.ZK30 powders contained the spherical particles,with an average particle size of 50μm as shown in Fig.1a.The GO powders consisted of nano-platelets with a few nanometers in thicknesses and a few to dozens of microns in extent as shown in Fig.1b.The X-ray maps of GO for C and O are exhibited in Fig.1c and d,respectively.The XRD pattern of GO is shown in Fig.1e,which presents high purity.

Fig.2.Schematic illustration of powder dispersion and SLM process.
The GO and ZK30 powders were separately added into ethanol,and subjected to ultrasonic vibration for 240min as schematically depicted in Fig.2.The ZK30 slurry was mixed with the GO slurry and subjected to ultrasonic vibration for60min.The resultant ZK30-xGO slurry(x=0,0.3,0.6,0.9 wt%,denoted as ZK30,ZK30-0.3 GO,ZK30-0.6 GO,ZK30-0.9 GO,respectively)was f ltered,vacuum dried at 40 °C for 300min,mechanically milled under SF6(1vol.%)and CO2(balance)atmosphere for 180min,and coated onto a steel plate.A self-regulating SLM system with a fibe laser was used to fabricate the ZK30-xGO composites.The fibe laser had an output power of 500W and a wavelength of 1064nm.The laser beam had a minimum focused spot diameter of 50μm.More details were presented elsewhere[26].The SLMed ZK30-xGO composites were produced by a laser power of 70W,a spot diameter of 200μm,a scanning spacing of 80μm,and a scanning speed of 500mm/min.The sintering process was repeated multiple times until the sintered specimen reached an appreciable thickness.The sintering process was performed in a chamber fille with high-purity argon.

Table 1Ion concentrations of SBF and human blood plasma according to ISO standard(10−3 mol/L).
The SLMed ZK30-xGO specimens were etched using two solutions:(i)ethanol(25mL),acetic acid(5mL),picric acid(1.5g)and distilled water(10mL);and(ii)4vol.% nitric acid ethanol solution,aiming to better visualize grain boundaries or intermetallic second phases,respectively.The microstructures were characterized using optical microscopy(OM),a scanning electron microscope(SEM)with an energy dispersive spectrometer(EDS),and X-ray diffraction(XRD)with Cu Kαradiation at 40 KV and 30mA using a scan rate of 0.02°/s in 2θrange of 5-80° Vickers hardness was measured using a load of 2.942N applied for 15s.
Potentiodynamic polarization tests were performed at 37±0.5 °C in a simulated body flui(SBF)of composition as given in Table 1 using a three-electrode cell and a electrochemical workstation,consisting of the sample as the working electrode(exposed surface area of 1 cm2in contact with electrolyte),a platinum foil as the counter electrode and a saturated calomel electrode(SCE)as the reference electrode.Potential was scanned between−1.6 and−1.2V(with a scanning rate of 0.5mV/s)versus open circuit potentials(OCP)which were determined after the OCP was stable.The biodegradation rate,Pi(mm y−1),was evaluated from the corrosion current density,icorr(current density)(mA cm−2),using the conversion[27,28]:

Immersion tests were performed in the SBF at 37±0.5 °C for 72h.Before all the immersion tests,samples with a 1 cm2surface and a thickness of 3mm were prepared by mechanical grinding successively to 1200 grit SiC paper,polishing to 0.5μm diamond,washing with distilled water,and drying with warm fl wing air.The ratio of surface area/solution volume was 1.25 cm2/mL according to ISO10993[29].The pH value of the immersion solution was measured by a digital pH meter for every 12h.For weight lose test,the initial weight of specimens was recorded before immersing,the SBF solution was refreshed every 24h.After immersion tests,the specimens were cleaned by chromic acid solution(300g/L Cr2O3+10g/L AgNO3)to remove the surface corrosion products,and then were weighted.The biodegradation rate,Pw(mm y−1),was evaluated from the weight loss rate,ΔW(mg cm−2d−1)using the conversion[27,28]:

Corrosion morphologies and topographic maps after the immersion test were characterized using SEM and a 3D measuring laser microscope,respectively.
Human osteosarcoma MG-63 cells were used to study the in vitro cell response of the ZK30-xGO composites.MG-63 cells were incubated in Dulbecco's modifie Eagle's medium(DMEM)(Gibco,Grand Island,USA)supplemented with 10% fetal bovine serum(FBS)(Gibco,GrandIsland,USA),100U mL−1penicillin,and 100mg mL−1streptomycin(BI,Kibbutz Beit-Haemek,Israel).Extracts were collected for SLMed ZK30-xGO samples separately soaked in DMEM with a surface area to extraction volume ratio of 1.25 cm2mL−1.For cell viability,the MG-63 cells were incubated in the 100%extracts for 3 days,the cells were digested,and seeded upon glass slide in 6-well plates(5×103 cells per 100mL).Afterwards,the cells upon the glass slide were stained with Calcein-AM and Ethidium homodimer at 25°C for 30min and visualized using a fluorescenc microscope.
3.Results
3.1.Microstructure and microhardness
Fig.3 shows optical micrographs of the SLMed ZK30-xGO etched by different solutions.Fig.3a through 3d show that(i)the grains were equiaxed in shape,(ii)the average grain size of the SLMed ZK30 was∼20μm,and was much smaller than cast ZK30(∼150μm)[30],and(iii)the average grain size of the SLMed ZK30-xGO decreased with increasing GO content.Fig.3e through 3h show that(i)the numerous second phase particles were well distributed throughout the substrate,(ii)the amount of the second phase particles decreased and became more discontinuous with increasing GO content,and(iii)when the GO content reached 0.9wt.%,the second phase particles became discrete dots.
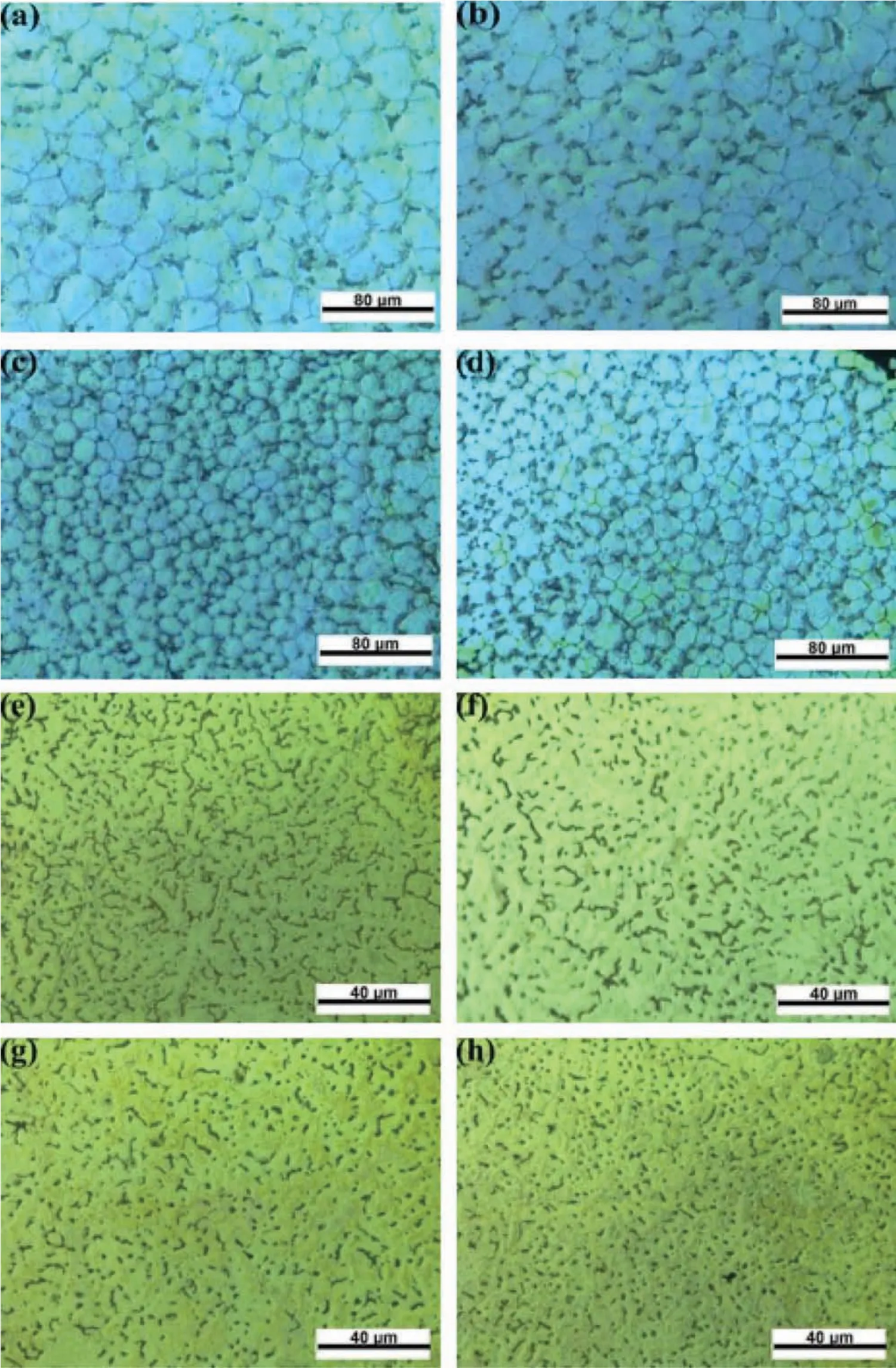
Fig.3.Optical microstructures of SLMed ZK30-xGO composites:(a)to(d)etched by solution(i)(ethanol(25mL),acetic acid(5mL),picric acid(1.5g)and distilled water(10mL))(a)x=0wt.%,(b)x=0.3wt%,(c)x=0.6wt%,(d)x=0.9wt%;(e)to(f)etched by solution(ii)(4vol.% nitric acid ethanol solution)x=(e)0wt%,(f)0.3wt%,(g)0.6wt%,(h)0.9wt%.
Fig.4 shows SEM micrographs of the SLMed ZK30-xGO at a higher magnification As the GO content increased from 0 to 0.9wt.%,the intermetallic second phase particles changed from a continuous white network to discrete dots.The amount of the precipitates evidently decreased with increasing GO content.The composition of the interphases at point A in Fig.4a are presented in Fig.5a using the EDS spectra.The X-ray maps for Mg and Zn in Fig.4a are presented in Fig.5b(blue)and 5c(green),respectively.These EDS results indicated the contents of the Mg and Zn in the interphases of the SLMed ZK30.MgZn2is the only intermetallic phase in Mg-Zn-Zr alloys and is easily precipitate at the grain boundaries[29].Therefore,these second phase particles in the SLMed ZK30 should be MgZn2.Similar EDS results for the interphases in ZK30-0.6GO are presented in Fig.5d for the composition of the interphases for point B in Fig.4c,and Fig.5e and f present the X-ray maps for Mg and Zn in Fig.4c.This indicates that the incorporation of GO into ZK30 caused a significan change in the distribution and amount of the precipitates,but the precipitates were still MgZn2.

Fig.4.SEM micrographs of SLMed ZK30-xGO:(a)x=0wt%,(b)x=0.3wt%,(c)x=0.6wt%,(d)x=0.9wt%.

Fig.5.(a)EDS results of point A,and X-ray map of(b)Mg and(c)Zn in Fig.4a for SLMed ZK30;(d)EDS results of point B,and X-ray map of(e)Mg and(f)Zn in Fig.4c for SLMed ZK30-0.6GO.

Fig.6.XRD patterns of ZK30-xGO composites.

Table 2Intensity ratios of the major XRD peaks of MgZn2 toα-Mg phase.
The above indicated that(i)SLM produced a small grain size,(ii)the incorporation of GO into ZK30 caused a further decrease in grain size,and(iii)the GO had a strong effect on the formation of the precipitates.The small grain size produced by SLM was attributed to the rapid melting and the rapid solidificatio of the melt pool[22,23].The additional grain refinemen by GO addition was attributed to GO providing additional nucleation sites,and pinning grain boundary and dislocations movement[16,30].The mechanism by which the GO affected the formation of the precipitates is described in the discussion section.
The powder X-ray diffraction patterns of SLMed ZK30-xGO are presented in Fig.6.There were peaks at 2-theta of 32°,34° and 37°,corresponding to the prism(1 0 0),basal(0 0 2)and pyramidal(1 0 1)planes of HCP Mg crystal,respectively.There were only peaks corresponding to Mg and MgZn2phases.There were no other intermetallic compounds.This indicates that the samples did not oxidize during SLM.Although peaks corresponding to GO should present at 2-theta of∼12° of the(0 0 2)plane,the XRD patterns had no GO peaks due to the relatively low mass fraction of GO.The melting temperature of the graphene was 3200 °C[31],higher than that of magnesium alloy(∼650 °C),which was conducive to GO survival during rapid heating under SLM conditions.The intensity ratios of major peaks of MgZn2(0 0 4 plane)toα-Mg(101 plane)phase were calculated from a magnifie view of the XRD patterns from 40 to 44°,and were listed in Table 2.The relative intensity ratios decreased with increasing GO content,which indicated that the GO particles regulated the precipitation of MgZn2.
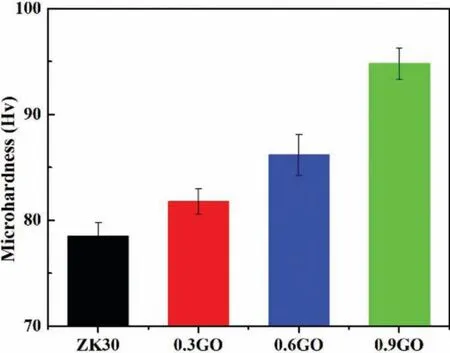
Fig.7.Microhardness of SLMed ZK30-xGO.
Fig.7 presents the Vickers hardness values measured on the polished surface of SLMed ZK30-xGO.The Vickers hardness of the SLMed ZK30 was∼80 HV,whilst a typical Vickers hardness of cast Mg alloys is∼70 HV[32].This indicated that the hardness of ZK30 was significantl increased by SLM,which was attributed to grain refinemen due to the rapid solidificatio of the SLM process.The incorporation of GO into ZK30 further increased hardness.The Vickers hardness increased with increasing the GO content.The maximum Vickers hardness was∼95 HV at 0.9wt% GO.This indeed verifie that GO was an effective reinforcement for Mg,and hardening could be obtained by the incorporation of GO into ZK30 via SLM.
3.2.Biodegradation
Fig.8a shows the potentiodynamic polarization curves for SLMed ZK30-xGO tested at 37±0.5 °C in SBF solution.The incorporation of GO into ZK30 by SLM resulted in a change in the corrosion potential(Ecorr)and the corrosion current density(icorr).TheEcorrof the SLMed ZK30-xGO became somewhat more negative with increasing GO content until 0.6wt.%GO,thereafter became more positive at 0.9wt%GO.Theicorrvalues evaluated from the polarization potential curves by Tafel extrapolation using the linear cathodic branch are shown in Table 3.Theicorrof the SLMed ZK30-xGO was the lowest at 0.6wt%GO.Fig.8b shows the pH variation during immersion for SLMed ZK30-xGO tested at 37±0.5 °C in SBF solution for 72h.The pH value increased rapidly in the initial 24h,and thereafter increased slowly with longer immersion time,which is attributed to some protectiveness of the corrosion layer.The pH value of the solution corresponding to SLMed ZK30,SLMed ZK30-0.3GO,SLMed ZK60-0.6GO and SLMed ZK30-0.9GO reached 9.0±0.1,9.2±0.1,8.6±0.1 and 9.7±0.1,respectively,at the end of the immersion test.Fig.8c presents the weight loss rate(mg cm−2d−1)of SLMed ZK30-xGO immersed in SBF for 72h.There was a direct correlation of the pH data and the weight loss data,that is,the weight loss rate increased somewhat with increasing GO content except 0.6wt% GO(i.e.SLMed ZK30-0.6GO).
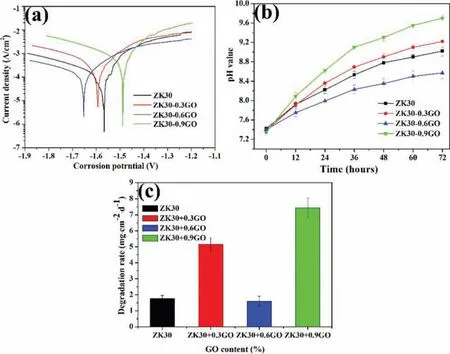
Fig.8.(a)Polarization potential curves,(b)pH variation cures,and(c)the weight loss rate for SLMed ZK30-xGO.
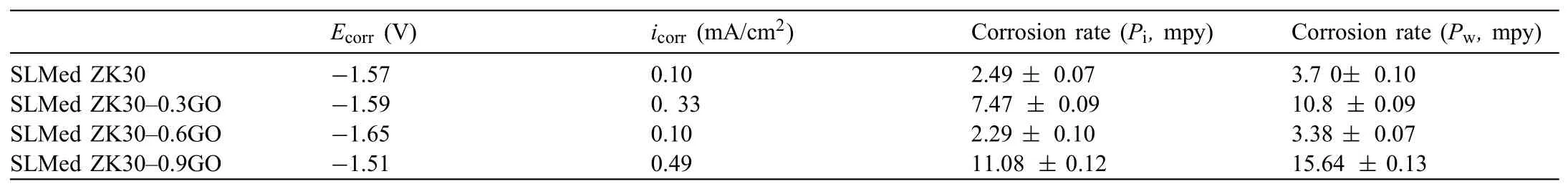
Table 3Degradation data of SLMed ZK30-xGO.
For easy comparison,the degradation rates are presented in Table 3.The degradation rate determined by the weight loss rate was significantl higher,as is often observed[24,27],but had the same trends as the corrosion rate evaluated from the polarization curves,and was consistent with the results of pH measurements.
Fig.9 shows surface morphologies of SLMed ZK30-xGO after immersion in SBF for 48h.The corroded surfaces of all samples presented various pits with different sizes and depths.Compared with SLMed ZK30-0.3GO and ZK30-0.9GO,the corroded surfaces of SLMed ZK30 and ZK30-0.6GO were relatively intact and compact,having more narrow and shallow pits in the Mg matrix.Many pits were located in the Mg matrix near the precipitates,attributed to galvanic corrosion between the second phase and the matrix[33-35].SLMed ZK30-0.3GO and ZK30-0.9GO had larger pits.Especially SLMed ZK30-0.9 GO had serious corrosion and large,deep corrosion pits.There were cracks in the corrosion layer on SLMed ZK30-0.3GO and ZK30-0.9GO,attributed to the shrinkage during dehydration of the thick corrosion products[36,37].
After washing with distilled water,the corresponding topographic surface maps of the SLMed ZK30-xGO are presented in Fig.10,in which different corrosion depths correspond to different colors.The corrosion topographic maps were consistent with the corrosion morphologies.The pitting corrosion occurred with an average depth of 60μm for SLMed ZK30(Fig.10a).When GO content was 0.3wt%,large depressed area in Fig.10b indicated that corrosion was deeper.In contrast,the evenly distributed depressed area in the ZK30-0.6GO indicated that the surface was only slightly corroded(Fig.10c).In the ZK30-0.9GO,the purple area indicated the corrosion pits were deep and large,and large area surfaces were corroded(Fig.10d).
3.3.Cytocompatibility
Acceptable cytocompatibility is essential and an in vitro cytotoxicity test is necessary for biomedical implants.Fig.11 shows the results of the fluorescenc staining microscopy of the MG63 cells cultured after 1 and 3 days,respectively.The green cells were living cells and the red cells were dead cells.There were no dead cells.The cells after culturing on all the samples exhibited well-spread morphology having no significan difference between them.The cells were round and fusiform in shape after 1 day,suggesting normal cell growth.Cultured for 3 days,all these cells increased in number.

Fig.9.Corroded surfaces for SLMed ZK30-xGO,(a)x=0wt%,(b)x=0.3wt%,(c)x=0.6wt%,and(d)x=0.9wt%.
Fig.12 shows the CCK-8 assay results of MG63 cells cultured for 3 days on the SLMed ZK30-xGO samples using 10%,50%,100% extracts,respectively.All the samples showed similar optic density in the 100% extract.There was increased viability for diluted extracts to 50%and 10%.There were the same trends as for 100%extracts.These results indicated that the SLMed ZK30-xGO had good cytocompatibility.
4.Discussion
4.1.Microstructure characteristics
GO is a good reinforcement for a biodegradable Mg-matrix composite to achieve higher strength.It is a challenge to produce GO-reinforced metal matrix composite with good homogeneity,because GO has a layer structure with nanoscale thickness.A conventional preparation technique such as the direct incorporation of GO into the melt could easily lead to the GO floatin and aggregating.
Distribution of the GO was analyzed by SEM observation combined with EDS analysis as shown in Fig.13.The corresponding X-ray maps for the elements C and O are presented in Figs.13b and c,in which red and yellow represent C and O,respectively.Red dots were dispersed throughout the whole matrix,indicating that carbon had a uniform distribution.Oxygen had a distribution similar to carbon.These results indicated the homogeneity of GO in the composite produced by the easy SLM fabrication.GO,mainly composed of C and O,was homogeneously distributed in the matrix,which was attributed to the rapid solidificatio during the laser processing,because the GO particles did not have enough time to accumulate in the melt pools.This easy SLM technology avoided the frequent problem of GO agglomeration caused by a long processing time.

Fig.10.Topographic maps for corroded SLMed ZK30-xGO,(a)x=0wt%,(b)x=0.3wt%,(c)x=0.6wt%,and(d)x=0.9wt%.

Fig.11.Fluorescent images of MG-63 cells cultured in 100% extracts for 1 and 3days:(a)(e)SLMed ZK30,(b)(f)SLMed ZK30-0.3GO,(c)(g)SLMed ZK30-0.6GO,(d)(h)SLMed ZK30-0.9GO.

Fig.12.Cell viability in different extracts for 3 days.
In addition to the uniform distribution of GO,another noticeable microstructure characteristic of SLMed ZK30-xGO is that the MgZn2precipitates became fewer and more smaller with increasing the content of GO as shown in Fig.4.This is related to the increase of solid solubility of Zn and Zr in Mg when GO was added during SLM.The rapid solidifica tion and rapid cooling process of SLM introduced a large number of crystal defects[38],and the presence of GO had a strong impact on the stress field of the dislocations.The unmovable GO particles bent and twisted the surrounding dislocations,and increased the effective length of the Burgers'vector,and thus strengthened the stress fiel of dislocations.The stress field of dislocations raised the free energy of the elemental atoms.Thus,tensile stresses,as typical for high dislocation densities,reduced the chemical potential and led to an enhanced solubility.The solubility enhancementx/x0[39],is given by:
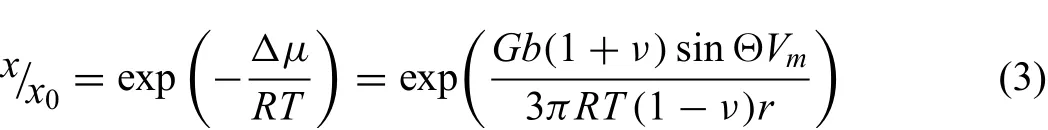
wherex0is the equilibrium solubility,Ris the gas constant,Tis the temperature,Vmis the molar volume of the solute atom,Gis the shear modulus,bthe Burgers vector,νthe Poisson ratio of the host metal,andΘthe angle about the glide plane.
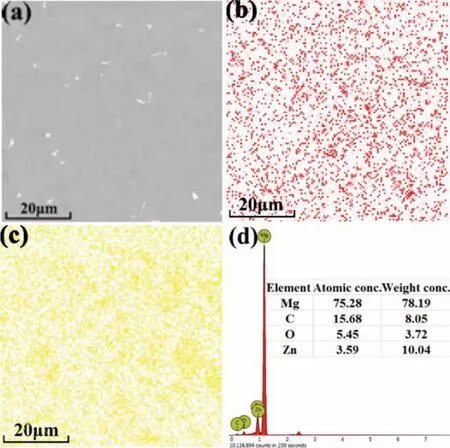
Fig.13.(a)SEM micrograph of ZK30-0.9GO,(b)the corresponding X-Ray map of carbon,(c)oxygen,and(d)EDS results.
The effect of stress upon the chemical potentialμof a solute atom is given by Eq.(4),and the stress acting on a solute atom located at a distance r from a dislocation core is given by Eq.5[39]:

As a consequence there resulted fewer and more discrete precipitates in the microstructure with increasing the content of GO as shown in Fig.4.
4.2.Corrosion

Fig.14.(a)Illustration of corrosion mechanism,(b)corrosion product and its EDS spectra of SLMed ZK30-xGO.
Each of the ZK30-xGO was a multiphase composite,in which the different micro-constituents such as the Mg matrix and the second phase particles(including the MgZn2and GO particles)may form micro-galvanic cells[33-35,40-42].Thus,the corrosion behavior can be understood with the corresponding schematic illustration shown in Fig.14a.After the ZK30-xGO samples were immersed in the SBF solution,micro-galvanic corrosion occurred due to the presence of the MgZn2and GO particles,with Mg matrix as the anode and the MgZn2and GO particles as the cathode,respectively.The Mg matrix was corroded to Mg ions by the anode reaction given by Eq.(6).The electrons generated from the dissolution of matrix moved from grains to second phases,where cathodic reaction occurred by Eq.(7).The formation of the hydroxide layer occurred by precipitation according to Eq.8[43,44].

The red circle shows that the second phase particles receiving electrons.Mg2+can pass through the loosened Mg(OH)2layer and form a new MgO/Mg(OH)2layer on the exterior surface[43].SBF solution components can also penetrate the loosened surface and react with the interior Mg substrate,leading to further degradation.
As the corrosion proceeded, hydroxyapatite Ca10(PO4)6(OH)2formed due to the reaction between phosphate ions(HPO42−or PO43−)and Ca2+in the solution,which caused Ca/P compounds to precipitate on the surface of hydroxide layer according to Eq.9[44].This is substantiated by the EDS results of the corrosion products as demonstrated in Fig.14b,in which the corrosion products of the corroded surface were composed of O,Mg,P and Ca.
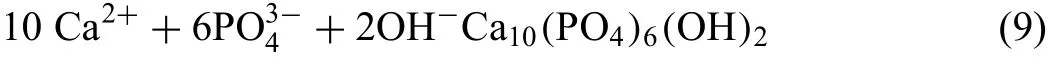
As stated above,the MgZn2distribution and grain size underwent major changes in microstructure as GO was added into ZK30 by SLM.These factors,i.e.,grain size,MgZn2particles and GO particles,were considered to affect the corrosion resistance of SLMed ZK30-xGO.The grain size was obviously refine due to the rapid solidificatio and the rapid cooling of SLM,and grain boundary pinning by GO.The fine-graine microstructure contained more grain boundaries and acted as a physical corrosion barrier to prevent corrosion[45,46].In addition,the grain refinemen could reduce the mismatch stress between the surface layer and magnesium substrate to inhibit pitting initiation[47,48].The second phase is also a key factor in determining the corrosion behavior for Mg alloys.The second phases typically accelerate the corrosion rate of Mg alloys,because they usually act as a cathode to accelerate the corrosion[33].The MgZn2became fewer and more discrete in the microstructure with increasing the GO content as shown in Fig.4,which was beneficia for a lower corrosion rate.On the other hand,the good dispersion of GO in the matrix could be obtained by SLM as stated above,and accordingly micro-galvanic corrosion occurred everywhere between the GO and the matrix.More GO addition resulted in more galvanic corrosion.For SLMed ZK30-xGO,the influenc of the grain refinemen and the fewer and more discrete MgZn2particles with increasing the GO content decreased the biodegradation rate and counteracted the influenc of the increased GO content on the increase of the biodegradation rate.Hence the SLMed ZK30-0.6GO had the lowest corrosion rate.
5.Conclusions
1.ZK30-xGO composites with GO as the reinforcement phase were successfully fabricated by selective laser melting,in which GO was well dispersed in the matrix.
2.The addition of GO significantl influence the microstructure(i.e.grain size and the amount and distribution of MgZn2 particles)and the hardness of SLMed ZK30-xGO composites.
3.The content of the GO was recommended to be 0.6wt%.The SLMed ZK30-0.6GO presented the lowest corrosion rate,because the influenc of the grain refinemen and the lower amount of the MgZn2 particles with the increase of the GO content on the decrease of the corrosion rate counteracted the influenc of the increased GO content on the increase of the corrosion rate.
4.The SLMed ZK30-xGO composites had good cytocompatibility.
Declaration of Competing Interest
None.
Data for reference
All data included in this study are available upon request by contact with the corresponding author.
Acknowledgments
Financially supported by Natural Science Foundation of China(No.51874368).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Microstructure and tensile properties of magnesium nanocomposites fabricated using magnesium chips and carbon black
- The effect of K2SiF6 on the MgH2 hydrogen storage properties
- The creep behavior of Mg-9Al-1Si-1SiC composite at elevated temperature
- HA coating on Mg alloys for biomedical applications:A review
- Constitutive modeling of f ow behavior and processing maps of Mg-8.1 Gd-4.5Y-0.3Zr alloy
- Fabrication of high-strength duplex nanoporous Cu by dealloying a dual-phase Mg-Cu precursor alloy
