The effect of K2SiF6 on the MgH2 hydrogen storage properties
2020-12-21IsmilYhySzeleeAliHlimYpMustf
M.Ismil,M.S.Yhy,N.A.Szelee,N.A.Ali,F.A.Hlim Yp,N.S.Mustf
a Energy Storage Research Group,Faculty of Ocean Engineering Technology and Informatics,University Malaysia Terengganu,21030 Kuala Terengganu,Malaysia
b Faculty of Innovative Design and Technology,Universiti Sultan Zainal Abidin,Gong Badak Campus,21300 Kuala Nerus,Terengganu,Malaysia
Received 28 October 2019;received in revised form 9 March 2020;accepted 17 April 2020 Available online 1 June 2020
Abstract The catalytic effect of K2SiF6 on MgH2 was frst timely studied.The MgH2+5 wt.% K2SiF6 was prepared via the ball milling technique.The catalyst had lessened the initial decomposition temperature by 134 °C and 48 °C as compared to both pristine and milled MgH2 samples,respectively.In 2 minutes,4.5 wt.% of hydrogen was absorbed(250 °C)by the doped composite,which was 0.8 wt.% higher than the milled MgH2.Meanwhile,for the desorption kinetics(320 °C,1 atm),the amount of desorbed hydrogen was increased by 2.4 wt.% and 2.3 wt.%for the firs 10 and 20 minutes.Besides,contracting volume and Johnson-Mehl-Avrami models were used to analyse the kinetics sorptions.The decomposition activation energy calculated based on Kissinger equation was 114 kJ/mol.As for the active species,Mg2Si,MgF2 and KH were formed during the heating process.These active species are speculated to be responsible for the improvement of the hydrogenation properties of the composite.© 2020 Published by Elsevier B.V.on behalf of Chongqing University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Catalytic effect;Magnesium hydride;Hydrogen storage;Sorption properties;Contracting volume model.
1.Introduction
Global energy demands have risen terrifyingly.Quite recently,in 2018,the U.S Energy Information Administration(EIA)projected that global energy consumption will exceed 700 quadrillions of British Thermal Unit(BTU)by 2040[1].In this context,experts are looking for solutions not only on the limited fossil fuel resources but also how to reduce the CO2emissions.Thus,the exploitation of sources that can be renewed like sun and wind is seen as one of the recognizable methods for this matter.Therefore,a significan amount of efforts have been dedicated globally on the development of reliable power systems based on renewable energy sources like solar PV systems[2],wind power systems[3]and hydropower system[4].As a result,about 18.1% of global energy consumption in 2017 was accounted for these kinds of energy sources[5].In addition,as for the global electricity production,26.2% came from renewable sources by the end of 2018,which was 1.7% higher as compared to the global electricity production in 2016.
Renewable energy sources like solar and wind are intermittent and fluctuate In this context,energy storage is critically needed in order to improve the performance of these power systems in meeting the load demand.Energy storage helps to reduce the‘supply-demand'mismatch as well as improving the efficien y and reliability of the systems that lead to more cost-effective power systems[6].Furthermore,energy storage is one of the critical integrants in energy preservation for multi-sector applications.In this particular issue,hydrogen has come to light as auspicious energy storage because of its high energy capacity;three times higher than the petroleum with a value of 120 to 142 MJ/kg[7].Hydrogen,which is considered as an energy carrier rather than primary renewable energy sources is inexhaustible and can be produced from water as well as any primary energy sources[8].It can be exploited to produce power mechanically like by the combustion engines or electrochemically like using the fuel cells[9].Furthermore,the waste product from these power generation methods is water vapour,which is environmentally friendly.
Generally,hydrogen can be stored in gas,liquid or solid forms.Among these,the solid-state form is the least mature as compared to the gas or liquid hydrogen storage.Unlike the gas and liquid storage,the solid-state hydrogen storage does not require high compression pressure as well as low storage temperature(-253 °C)[10]like the liquid storage which limits its utilization.Thus the solid-state storage is seen as an auspicious alternative for the hydrogen storage which will widen its application in the future[11,12].
Magnesium hydride(MgH2)is a well-known candidate for the solid-state hydrogen storage.Favourable properties like lightweight,high hydrogen capacity(7.6 wt.%)through the chemisorption process,reversible,abundant(2.3% in earth crust)and low raw material cost(USD 2-3/kg)[13,14].Unfortunately,these favourable characteristics are counterbalanced by drawbacks like unfavourable hydrogen sorption properties and decompose at high-temperature that impedes the utilization[15].In order to tackle these drawbacks,improvement approaches like modifying the physical structure of the particles using the ball milling technique[16]and catalyst(s)introduction[17-27]have been explored by others.
One of the potential catalysts that are well known for metal hydrides is potassium(K).Extensive studies have been carried out to investigate the catalytic effect of the potassium on metal hydrides.As an example,Wang et al.found the favourable effect of KH on the decomposition of NaAlH4-Ti composite[28].The dehydrogenation rate of the composite was stated to improve by 5 times faster due to the addition of KH.In addition,Wang et al.[29]claimed the Mg(NH2)2/2.0LiH composite decomposition peak temperature was reduced by 50°C due to the introduction of KH(3 mol%).Meanwhile,several potassium composites(KBr,KCl,KF and KOH)were investigated by Dong et al.[30]on the effect of kinetic desorption enhancement for the LiH-NH3composite.Potassium composites that were studied were able to boost the desorption characteristics,particularly by the KBr.
Silicon has shown effective catalytic effects on metal hydrides hydrogen sorption properties.To start with,Jalil et al.[22]reported a study on nano-SiO2as a catalyst for the MgH2system.They found that 5 wt.%of nano-SiO2had lowered the MgH2decomposition temperature to 338 °C within 14.8 min.Meanwhile,in their other work,Jalil et al.[31]mentioned that 5 wt.% of SiO2that was obtained from beach sand had reduced the MgH2decomposition temperature from 409 °C to 307.1 °C.
In addition to the SiO2catalyst,fluoride-base catalysts have shown promising catalytic effects on MgH2and have been extensively explored over the years.For instance,the favourable effect of NbF5had been studied by Pighin et al.[32].The MgH2+NbF5mixture was milled for 40 hours and 2 wt.% of hydrogen was absorbed in just 200 s at 250 °C while for the uncatalyzed sample,only 1 wt.% of hydrogen was absorbed.Then,Jain et al.[33]claimed the effect of 5 mol% of MgF2on the hydrogen storage properties of MgH2.The mixture had absorbed hydrogen for about 0.7 wt.%at 145°C in 30 minutes and under the hydrogen pressure of 10 atm.At 285 °C,under the similar hydrogen pressure,the mixture had absorbed hydrogen for about 5.5 wt.% in 11 minutes.Meanwhile,Malka et al.[34]investigated the ZrF4,NbF5and TaF5catalysts.For the MgH2+ZrF4and MgH2+NbF5mixtures,they were able to absorb about 6 wt.% of hydrogen in less than 2 minutes at 325 °C while under the similar operating temperature,the MgH2+TaF5mixture took about 3 minutes to absorb 6 wt.% of hydrogen.In addition,the isothermal desorption at 325 °C shown that the MgH2+ZrF4,MgH2+NbF5and MgH2+TaF5mixtures took about 2,5 and 7 minutes to release 6 wt.% of hydrogen,respectively.In addition,Jin et al.[35]reported a study on a series of 1 to 10 mol% of NiF2,TiF3,VF4,NbF5,ZrF4,CrF2,FeF2,CuF2,CeF3and YF3catalysts on the MgH2system.They concluded that the hydride phases that were formed because of the reaction of MgH2and the catalysts after the ball milling process were the key factor on the hydrogenation kinetics improvement rather than the fluoride itself.
It is entrancing to have potassium,Si and metal fluorid as a single catalyst compound based on the literature reviews.To the best of our knowledge,investigation on the improvement done by K2SiF6on the hydrogen storage properties of MgH2has not been explored.Therefore,in this work,improvements on the decomposition temperature,sorption properties,changes of the microstructure and the possible chemical reaction have been carried out.
2.Methodology
MgH2for hydrogen storage grade(98% purity)as well as potassium hexafluorosilicat powders were provided by Sigma-Aldrich.No purificatio or modificatio was done on the starting materials.Other than the ball milling process,samples'preparations were carried out in the MBraun Unilab glove box with argon gas to minimize the oxidation and moisture.
The MgH2+K2SiF6mixtures were prepared via the mechanical ball milling method(NQM-0.4 planetary ball mill).Each mixture(200 mg in total)was ball milled for 1 hour at a speed of 400 rpm and the minimum weight ratio between the steel balls and the mixture was 80 to 1.Similar milling method was carried out for the milled MgH2sample as a reference.
Characterizations were carried out on the milled MgH2and its mixture to explore the improvements done by K2SiF6on the MgH2system.Decomposition temperature and hydrogen sorption characterisations of the samples were achieved using the pressure-composition-temperature(PCT)equipment(Sievert type,Advanced Materials Corporation).About 70 mg of each sample was used for the PCT characterizations and the sample was heated at 5 °C/min from room temperature to 450 °C for the temperature decomposition.Meanwhile,for the sorption kinetics,hydrogen pressure was limited to 30atm and 1 atm for the absorption and desorption kinetics at a specifi temperature,respectively.
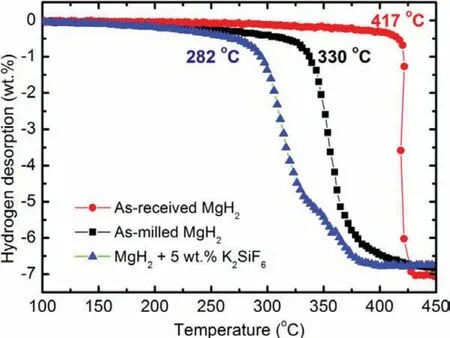
Figure 1.Desorption curves(hydrogen desorption vs temperature)of the pure,milled and MgH2+5 wt.% of K2SiF6.
The decomposition activation energy was calculated based on a set of data obtained from the‘Mettler Toledo TGA/DSC 1'differential scanning calorimetry(DSC)equipment.DSC measurements were done at heating rates of 15 °C/min,20°C/min,25 °C/min and 30 °C/min.Temperature range of this DSC measurement was set from room temperature up to 500°C under the influenc of 50 ml/min of argon gas f ow.
X-ray diffraction using Rigaku MiniFlex II(Cu Kαradiation)had been utilized to understand the phase structure of the samples.Each sample was covered with a layer of tape on a glass plate to protect it from moisture and oxidation.They were scanned from 20oto 80oand at 2o/min of the scan speed.Furthermore,scanning electron microscope(SEM)from JEOL JSM-6360LA was used to understand the physical microstructure of the samples.Samples were coated with a thin layer of gold prior to the SEM characterization.
3.Results and discussion
3.1.Temperature of desorption
Three desorption curves of the hydrogen weight loss versus temperature based on the PCT characterization for the pure MgH2,milled MgH2and MgH2+5 wt.% K2SiF6are presented as in Figure 1.The onset desorption temperatures of the pure and the milled MgH2are about 417 °C and 330 °C.The onset temperature reduction(87°C)on the milled sample signifie the consequence of ball milling on the MgH2system.This mechanical procedure had altered physical structure on the MgH2sample like the surface areas increment due to smaller particles size and surface defect formations as well as formations of fresh micro/nanostructure on the interior or exterior(surface)of the samples.Similar observations on these physical alterations were reported by others to successfully aid the hydrogen diffusion from the material[16,36].Worth to note,the hydrogen capacity was 0.2 wt.% lower than the pure MgH2sample.Referring to previous researchers[37,38],the milling process had similarly caused some of the hydrogen to be released from the compound.
Further improvement on the desorption temperature owing to the introduction of 5 wt.% K2SiF6can be discerned as in Figure 1.The doped sample began to desorb hydrogen at a temperature of 282 °C,48 °C lower than the milled sample.This outcome exhibits that K2SiF6is a promising catalyst that able to reduce the initial desorption temperature of the MgH2system.
3.2.Sorption kinetics
Investigations on the hydrogen sorption kinetics were carried out to understand the effect of K2SiF6on the hydrogen sorptions property.The firs investigation was carried out for the hydrogen sorption capabilities at 320°C and 30 atm of hydrogen pressure.Figure 2(a)collates the hydrogen absorption curves vs time(60 min)for the milled MgH2and the doped sample at 320 °C and 250 °C.In general,the milled MgH2sample has higher hydrogen absorption capacity as compared to the doped sample at both operating temperatures.This is expected since MgH2is known to have high absorption capacity at high operating temperature[16,39].
However,at 250°C,the doped sample shows faster absorption kinetics as compared to the milled sample for the frst 5.5 min(Figure 2(b)).For the firs 0.5 min,the doped sample is able to absorb 1.4 wt.% more hydrogen as compared to the undoped sample.Meanwhile,at 2 min,the absorption of the doped sample is 4.5 wt.%,which is 0.7 wt.% higher than the undoped sample.Then,after 5.5 min,despite having a similar amount of hydrogen for both samples,the milled MgH2starts to show higher hydrogen absorption kinetics compared to the doped sample as the MgH2+K2SiF6sample is reaching its saturation point.Thus,for the absorption property,although the amount of hydrogen capacity of the doped sample is lower than the milled MgH2due to the dead weight of the catalyst which does not contain hydrogen[40,41],the introduction of K2SiF6into the system has significantl increased the absorption kinetics at 250 °C.
The second investigation on the sorption kinetics was carried out for the hydrogen desorption performance at 320°C.Comparison of the hydrogen desorption performance is mapped as in Figure 3.It is worth to note that the pure MgH2has sluggish hydrogen desorption kinetics[42,43].In this context,the catalyst addition is found to be able to boost the hydrogen desorption capacity as well as the desorption kinetics.Referring to Figure 3,the doped sample is able to release 1.7 to 2.4 wt.%more hydrogen than the undoped sample for the frst 30 minutes.In addition,after 60 minutes,the MgH2+K2SiF6sample is able to release 0.8 wt.% more hydrogen than the milled MgH2sample.This investigation shows that the used catalyst is beneficia for the enhancement of the desorption property of the MgH2system.
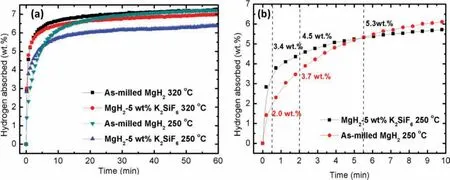
Figure 2.(a)Absorption kinetics of the milled MgH2 and MgH2 doped with 5 wt.% of K2SiF6 at 320 °C and 250 °C(30 atm).(b)Absorption kinetics of the milled MgH2 and doped MgH2 at 250 °C(30 atm)for the frst 10 minutes.

Figure 3.Desorption kinetics of the milled MgH2 and MgH2 doped with 5 wt.% of K2SiF6 at 320 °C.
The behaviour of the sorption kinetics can be further analysed using kinetic models in order to understand the kinetic mechanism of the studied material.For instance,in 2005,Chou et al.[44]had proposed a kinetic model based on characteristic absorption/desorption time for Mg1.95Ag0.05Ni and LaNiMg17alloys.Meanwhile,Luo et al.[45]had carried out a comparative study between Chou model and Jander model for the Mg-Ni based alloys.As for Pan et al.[46],they had analysed the kinetic mechanism of Mg-LaNi5composites using Chou model.Furthermore,a number of kinetic models have been extensively reviewed and summarized by Pang and Li[47]in their work.
For the current work,the kinetic mechanisms were analysed using Contracting Volume(CV)model and Johnson-Mehl-Avrami(JMA)model as summarized in Table 1.These models were selected as they can be fitte with the experimental data and accurate since no additional assumption or approximation is needed as claimed by Pang and Li[47].Furthermore,these models were previously used by other researchers[48-53]in understanding the rate-limiting steps of the studied materials.The rate-limiting step of the kinetics can be deduced from the experimental data with the kineticequations like CV and JMA.In this context,the best linear plot of the experimental data with the kinetic equations determines the rate-limiting step.
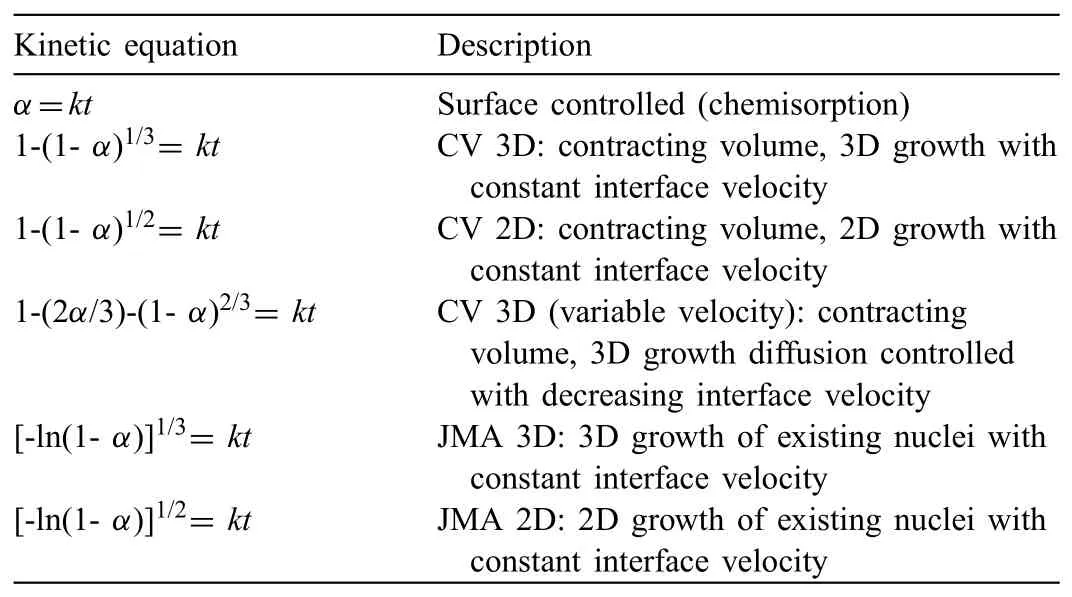
Table 1Kinetic models used for sorptions kinetic of current study.α=reacted fraction,t=time,k=reaction rate constant[48,49]
Figure 4(a)to(c)depict the kinetic curves that were calculated from equations as in Table 1.The kinetic curves for the absorption and desorption of the doped composite were calculated for the reacted fraction in the range of 0 to 80%[52].From these figures it can be deduced that the rate-limiting step for both the absorption processes(320 °C and 250 °C)can be best described by the 3D growth diffusion controlled with decreasing interface velocity.Meanwhile,for the desorption process of the doped sample at 320 °C,the rate-limiting step is given by the contracting volume 3D growth with constant interface velocity.
3.3.Decomposition activation energy
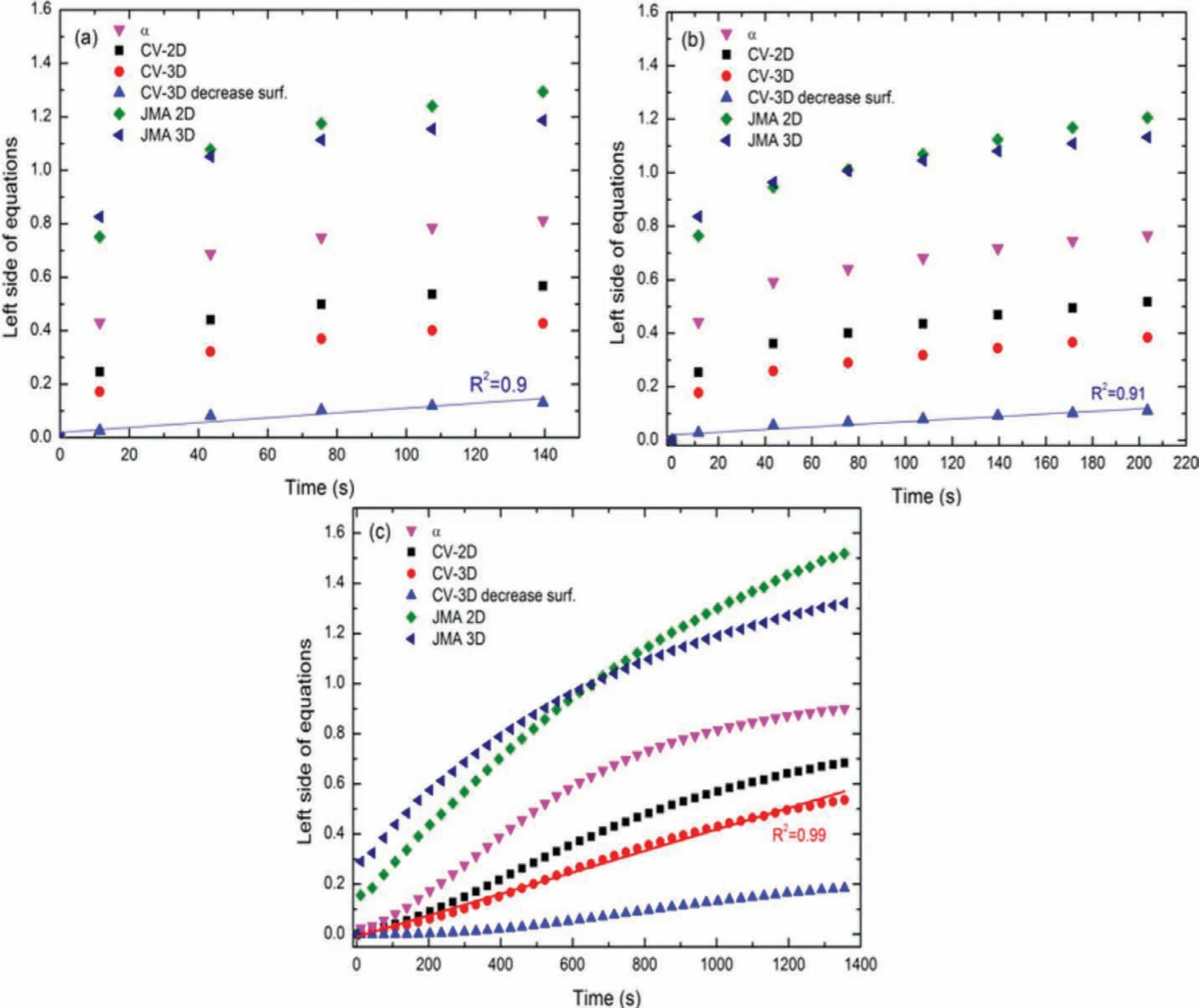
Figure 4.The resulting calculation of different kinetic equation described in Table 1 for(a)absorption kinetics of doped sample at 320 °C,(b)absorption kinetics of doped sample at 250 °C and(c)desorption kinetics of doped sample at 320 °C.
The decomposition activation energy(Ea)of the doped system is investigated using the differential scanning calorimetry(DSC)method.In order to instigate any chemical reaction in a system,critical minimum energy is needed which can be define by theEa[54].In this context,the decompositionEais the least energy that is required to instigate the decomposition process of the studied MgH2and its doped system.Furthermore,it is directly related to the onset decomposition temperature of the system.However,this energy cannot be directly measured but possible to be quantifie with the aid of a method initiated by Homer E.Kissinger in 1957.For this energy quantificatio method,a set of decomposition temperature data of the studied samples from the DSC measurements are needed and are shown as in Figure 5(a)and(b).The Kissinger equation can be written as[55]

According to this Kissinger equation,theβvalue is the heating rates used in the DSC measurement.In addition,the value of peak temperature from the DSC curves is denoted byTp.ThenRis the value of gas constant whileAis a linear constant.Finally,the decomposition activation energy,Eais determined according to the declination slope of the Kissinger's graph which is plotted by ln[β/T2p]vs 1000/Tpand as shown as in Figure 5(c).
The calculated decompositionEafor the milled MgH2is 135.3 kJ/mol.Meanwhile,the doping process has reduced theEavalue of the doped composite to 114 kJ/mol.The lower value ofEaexplains why the MgH2+K2SiF6composite had decomposed at a lower temperature than the milled MgH2.Table 2 tabulatesEafor several doped MgH2systems that can be found in the literature.The currently studied composite showed in this table is not the lowestEavalue concerning previous studied MgH2systems.However,by taking into account several other factors like the energy and milling time taken in preparing the composites via the ball milling,K2SiF6is an enticing and promising catalyst which has the possibility to be further improved via other methods like co-doping.
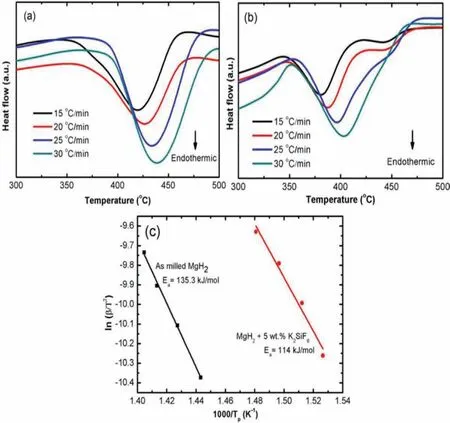
Figure 5.DSC decomposition temperature profile of the(a)milled MgH2 and(b)MgH2+5 wt.% K2SiF6.(c)is the Kissinger plots for the milled and doped MgH2.
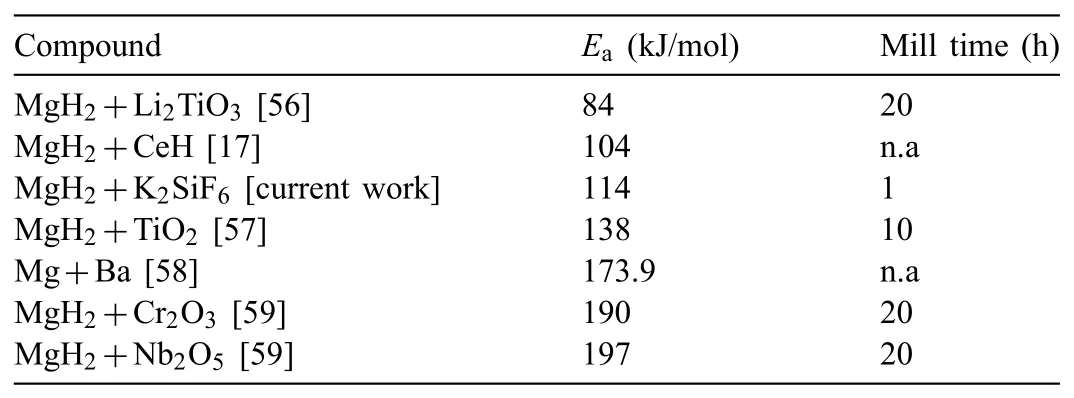
Table 2Ea of decomposition of doped MgH2 systems(n.a-no milling time reported).
3.4.Scanning electron microscopy
Scanning electron microscopy characterization was carried out to perceive the ball milling effect on the MgH2system.Furthermore,this characterization was carried out to observe the effect of the catalyst on the microstructure of the milled composite.The pure MgH2and K2SiF6,as well as the milled and doped MgH2composite of the SEM images,are given as in Figure 6(a)to(d).
Referring to Figure 6(a),the shape of the pure MgH2particles are solid with fla e-like particles.The particles are irregular in sizes which are more than 50μm.Meanwhile,the pure K2SiF6as in Figure 6(b)are made of rectangular/cubicle like particles with the size about 100μm or less.On the other hand,for the milled MgH2(Figure 6(c)),the solid and fla e-like particles are started to clump and agglomerated after the milling process because of the cold welding[60].In addition,the relatively smooth particles'surfaces(prior the milling process)are superseded with surface defects and asperities.These modifie surface structures are the factor that caused the milled MgH2to decompose at a lower temperature with respect to the pure MgH2[16].Meanwhile,for the MgH2-K2SiF6composite(Figure 6(d)),the agglomeration of the particles are significantl reduced with the size of about 1μm.The reduction of particles'size is very important since it will reduce the length of hydrogen diffusion path and will significantl increase the total surface area for a higher density of hydrogenation process[61,62].Furthermore,it is worth to note that the hardness of K2SiF6is 2.5 Mohs[63],which is lower than the hardness of MgH2(4.0 Mohs[64]).Therefore,it is speculated that instead of introducing the pulverization effect,this catalyst has acted as a lubricant during the milling process which has helped to reduce the agglomeration of the particles.

Figure 6.SEM images of the(a)pure MgH2,(b)pure K2SiF6,(c)milled MgH2 and(d)MgH2+5 wt.% of K2SiF6.
3.5.X-ray diffraction
The phase composition of the doped composite was identifie with the aid of the XRD method.Characterizations were carried out at different phases;(a)after milling process,(b)after dehydrogenation process at 450 °C and(c)after the rehydrogenation process at 320 °C(Figure 7).Referring to the post milling process(Figure 7(a)),the only peaks that available are the MgH2peaks.Meanwhile,no K2SiF6peak is detected and is speculated to happen due to the small amount of catalyst.Similarly,for the dehydrogenated and rehydrogenated samples(Figure 7(a and b)),only Mg and MgH2peaks can be observed.These results suggested that the catalyst amount is too low to be detected by the XRD.
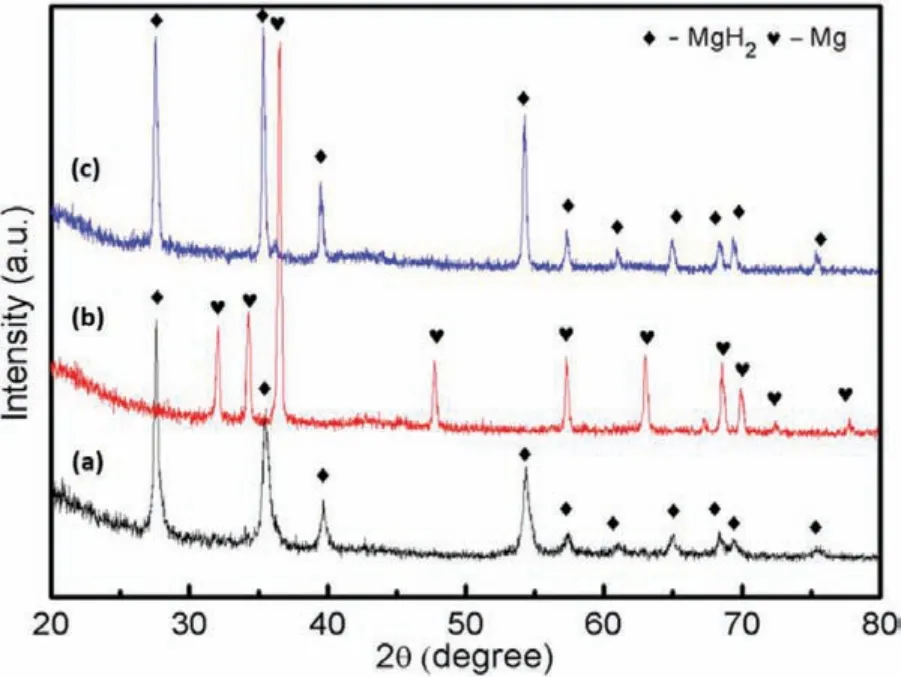
Figure 7.XRD curves of the 5 wt.% doped composite(a)after milling process,(b)after dehydrogenation process at 450 °C and(c)after the rehydrogenation process at 320 °C.
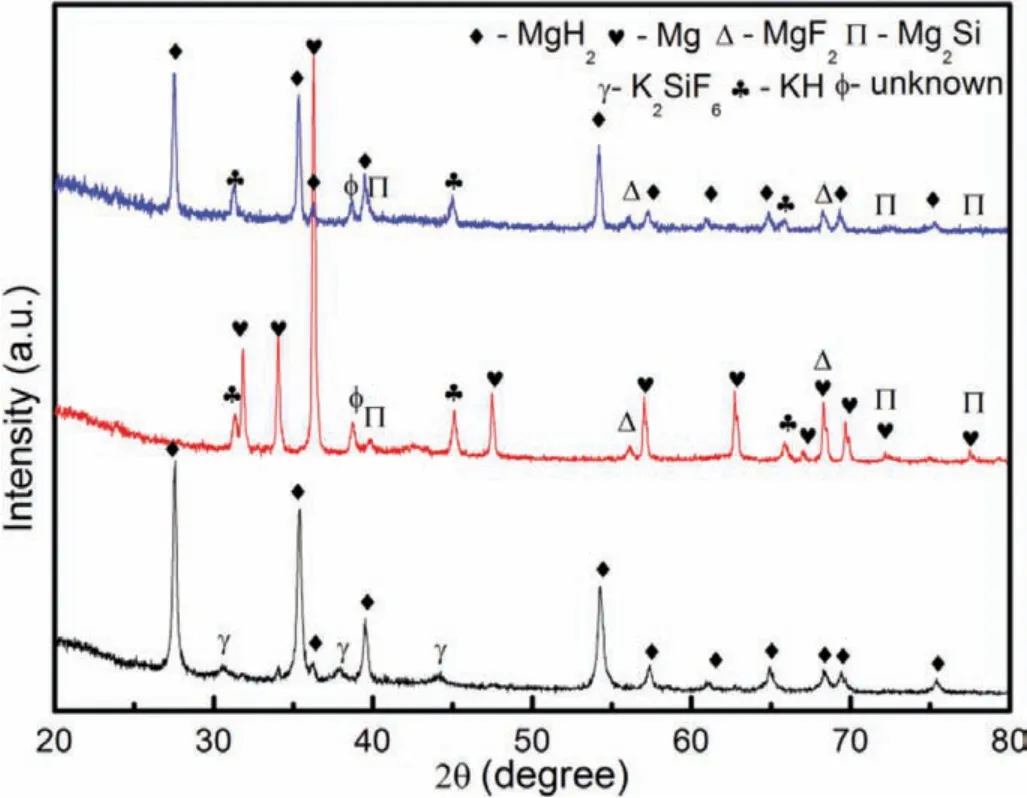
Figure 8.XRD curves of the 20 wt.% doped composite(a)after milling process,(b)after dehydrogenation process at 450 °C and(c)after the rehydrogenation process at 320 °C.
Further XRD characterization is carried out on the MgH2+20 wt.% of K2SiF6in order to have a better understanding of the chemical reaction of the doped system.Figure 8(a)to(c)present the XRD peaks of the MgH2+20 wt.%K2SiF6composite at different phases;(a)after the milling process,(b)after the dehydrogenation process at 450 °C and(c)after the rehydrogenation process at 320 °C.Unlike the doped sample,peaks of K2SiF6,as well as the MgH2peaks,are discernable in the ball-milled sample.The existence of the K2SiF6peaks manifests the non-reaction condition between MgH2and K2SiF6during the ball milling process.Meanwhile,for the dehydrogenated sample,a chemical reaction has happened and it is deduced on the manifestation of new XRD peaks that represent KH,MgF2and Mg2Si.Meanwhile,for the rehydrogenated sample,similar peaks are presented except Mg peaks are superseded by the MgH2peaks.The occurrence of the MgH2peaks connotes that the Mg is reversible,which is one of the key criteria that is necessary for hydrogen storage.A possible reaction that has happened in the heating process based on this XRD characterization can be best described as follows:
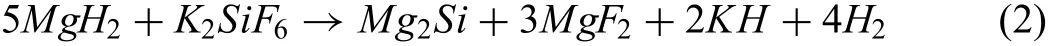
Referring to these outcomes,the MgH2+5 wt.% K2SiF6composite storage properties are improved due to several factors.First,the modificatio of the physical characteristic of the sample.The reduction of particles'sizes and less agglomeration has increased the total surface areas for faster H re/decomposition process[65].In addition,it will provide shorter hydrogen path for faster hydrogenation process[66].Secondly,the effect of active species that were formed during the heating process.For instance,the Si component in K2SiF6is able to destabilize strong bound hydrides like MgH2as reported by Vajo et al.[67].Meanwhile,for the MgF2compound,it helps to improve the hydrogen sorption properties of the MgH2based system as claimed in Ref[68,69].The F-anion will aid by weakening the Mg-H bonding,thus improving the hydrogen sorption of the doped composite[34].In addition,the formed KH active species is speculated to be one of the key factors on the improvement of the MgH2+5 wt.% K2SiF6composite storage properties.This is deduced according to the study by Wang et al.[29]where they claimed the decomposition temperature of the Mg(NH2)2/2.0LiH system can be further lowered by a mean of 50 °C due to the addition of KH.Meanwhile,according to the report by Luo et al.[70],they stated that the Li2Mg(NH)2system that was catalysed with<4 mol% of KH was able to be fully rehydrogenated in 0.1 hours under the operating temperature of 200 °C while the non-catalyzed system was only able to be rehydrogenated up to 70% only in 0.5 hours under the same operating condition.
4.Conclusion
Investigation on the improvement done by the 5 wt.%K2SiF6compound on the hydrogen storage performance of the MgH2system is presented.The doped MgH2composite was found to has a lower onset dehydrogenation temperature as compared to the milled MgH2by 48 °C.In addition,the MgH2+5 wt.% K2SiF6composite had absorbed up to 3.4 and 4.5 wt.% of hydrogen in the absorption kinetics for the firs 0.5 and 2 minutes at 250 °C,respectively.In comparison,the milled MgH2had absorbed 2.0 and 3.7 wt.% under the same operating condition.On the other hand,for the desorption kinetics,the doped composite had released 3.2,4.7 and 5.1 wt.% of hydrogen for the firs 10,20 and 30 minutes at 320 °C.While the undoped system was only able to release 0.8,2.3 and 3.4 wt.% of hydrogen under the same time and operating condition.For the decompositionEa,the calculated value of the doped composite was 114 kJ/mol,which was 21.3 kJ/mol lower than the milled MgH2.In terms of phase composition,the addition of K2SiF6had developed KH,MgF2and Mg2Si as the active species.Based on this study,the K2SiF6compound is believed to be a potential catalyst for the improvement of the hydrogen storage properties of the MgH2system.
Acknowledgements
This research was supported by the Universiti Malaysia Terengganu(UMT)through the Golden Goose Research Grant(GGRG)(VOT 55190).The authors also like to acknowledge UMT for the facilities to perform this research.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Microstructure and tensile properties of magnesium nanocomposites fabricated using magnesium chips and carbon black
- Microstructural evolution of Mg-Al-Re alloy reinforced with alumina fiber
- Predicting and controlling interfacial microstructure of magnesium/aluminum bimetallic structures for improved interfacial bonding
- Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys:Comparison of coatings formation mechanism
- Effects of annealing treatment on microstructure and tensile behavior of the Mg-Zn-Y-Nd alloy
- Microstructure and performance of biodegradable magnesium alloy tubes fabricated by local-heating-assisted dieless drawing
