HA coating on Mg alloys for biomedical applications:A review
2020-12-18MostafizuRahmanYuncangLiCuieWen
Mostafizu Rahman,Yuncang Li,Cuie Wen
School of Engineering,RMIT University,Melbourne,Victoria 3001,Australia
Received 25 November 2019;received in revised form 9 April 2020;accepted 18 May 2020 Available online 23 June 2020
Abstract Magnesium(Mg)alloys are receiving increasing attention as biodegradable implant materials in recent years.However,their low corrosion resistance and fast degradation in the physiological environment remain challenges for a widespread application.Hydroxyapatite(HA)coating on Mg alloys can enhance their corrosion resistance,biocompatibility,and bioactivity of the Mg alloy substrates since the compositions of HA are similar to those of the hard tissue of natural bone.This review analyzes the challenges of Mg alloys for biomedical applications,the fundamental requirements for biodegradable metals,and the corrosion mechanisms of Mg alloys in the physiological environment.The benefit of HA coatings on Mg alloys,the most commonly used surface coating techniques and their advantages and limitations,and the in vitro and in vivo performance of Mg alloys with and without surface coatings are comprehensively elucidated.Multistep processes such as alkali treatment and then HA coating by electrochemical deposition on Mg alloys appear to be necessary to achieve a satisfactory surface coating on Mg alloys,which has been demonstrated to have the potential to improve the degrading behavior,bioactivity and biocompatibility.Multifunctional coatings are most effective in achieving safe and bioactive Mg alloy surfaces for promising biodegradable implant applications.© 2020 Published by Elsevier B.V.on behalf of Chongqing University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Magnesium alloys;Hydroxyapatite coating;Corrosion;Biodegradability;Biocompatibility.
1.Introduction
Magnesium(Mg)is a lightweight metal and naturally exists in bone tissue;it is important to the human metabolism.Mg is considered to be one of the most vital substances in the human body,and the toxicity level of Mg ions is within the tolerable limit[1,2].In addition,Mg,iron(Fe),and zinc(Zn)are needed as nutritional elements in the human body,with the recommended daily allowances for an adult in the ranges of 240-420mg,8-18mg,and 8-11mg,respectively[3].However,a higher intake of pure Zn of 100-300mg/day can cause health problems or even become harmful[4].The use of biodegradable Mg alloy implant materials is a groundbreaking approach in biomaterials science.Mg alloys were firstl proposed as implant materials for trauma and orthopedic surgery in the 1930s owing to their outstanding biocompatibility[5].A magnesium-cadmium(Mg-Cd)alloy was used for different fracture fixatio techniques by Tsitrin and Troitskii[6].
Mg and some of its alloys have attracted increasing attention in the recent years as biodegradable implant materials and are considered to an innovative research topic in the area of biomedical engineering[7-14].Conventional metallic implants,such as cobalt(Co)-based alloys,stainless steels(SS),and titanium(Ti)-based alloys,can be used as temporary implant materials for the reconstruction of bone tissue.They are not biodegradable,therefore necessitating a second surgery after a period of implantation.Hence,a biodegradable implant material that remains intact for a period of time to allow the diseased tissue in the human body to recover and is then progressively dissolved or absorbed,excreted or consumed,thus,avoiding a second surgery,is an attractive concept[15].Although,metallic implants are currently used for regeneration or replacement of damaged bone tissue for their acceptable biocompatibility and excellent mechanical toughness and strength[13,16,17],some problems have been mentioned[7,18,19].Table 1 lists the density and mechanical propertiesof natural bone and metallic implant materials.The limitations of conventional metallic materials are their higher Young's modulus of elasticity as compared to the hard tissue of natural bone and the possible release of toxic metallic ions through wear and corrosion phenomena.
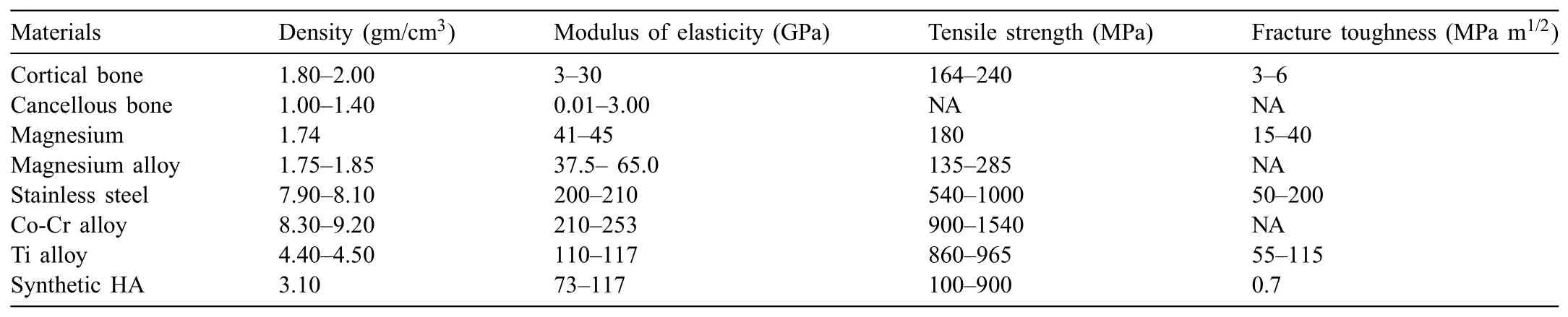
Table 1Density and mechanical properties of natural bone and metallic implant materials[17,20-24].
The lack of matching of the mechanical properties of the implant materials can cause adverse effects on the surrounding bone tissue.For instance,the Young's modulus mismatch between implant materials and the host bone tissue results in a stress shielding effect,i.e.the implants take more of the bulk load compared to the host bone tissue in the physical body,which leads to atrophy of the adjacent bone tissue[25,26].Researchers have tried to solve this problem by altering the surface structure of permanent metallic implant materials to reduce the elastic modulus.Permanent metallic implants,such as Co-based alloys and Ti-based alloys,have been fabricated with a porous structured surface using various coating methods to control the Young's modulus mismatch with natural bone tissue,but a considerable reduction in the fatigue strength has also been reported[27-30].Further,these porous metallic implant materials suffer from a number of other drawbacks,such as surface brittleness,inadequate control over the shape and size,phase impurity,and uneven porosity distribution,leading to limitations on their orthopedic applications[31].Another major drawback of metallic implant materials is that they can release toxic metallic ions into the physiological environment,leading to unexpected inflammator responses that reduce the biocompatibility and accelerate the tissue damage[32-35].As a result,researchers and clinicians have diverted their attention to biodegradable materials in order to overcome these limitations of conventional metallic implants.
In general,biodegradable materials should offer sufficien mechanical strength until the completion of the healing process.As can be seen in Table 1,the Young's modulus and density of Mg and its alloys are close to those of natural bone.However,Mg and its alloys rapidly corrode in the physiological environment,with a pH value in the range of 7.4-7.6,which results in a loss of mechanical strength,thereby causing premature failure of the implants and the generation of hydrogen(H2)gas around the implants through the corrosion process[6,36,37].In most cases whereas the temporary presence of a device or implant is required in the body,biodegradable materials could be a healthier approach than those that are inert or stable.Hence,the use of new biodegradable materials as innovative implants for orthopedic and cardiovascular applications could stimulate the healing process of damaged tissues[10,38-40].This article discusses the challenges to this approach,such as the rapid corrosion and the fast degradation rate of Mg and some of its alloys,and the HA coating,which can be used to slow down their degradation.The corrosion behavior,biodegradability,biocompatibility,bioactivity,and osseointegration of HA-coated Mg alloys are elucidated.
2.Hydroxyapatite(HA)coating on Mg alloys for biomedical applications
HA has a density of 3.16g/cm3and is the mineral compound of bone and teeth which is naturally occurring from calcium apatite and a crystal phase of calcium phosphates(CaP).CaP have attracted significan attention in the interdisciplinary area of sciences concerning chemistry,medicine,biology,and geology.In the mid of 18th century,Berzelius[41]firs attempted to identify the chemical composition of CaP compounds.A century later,Hausen[42]discovered the different phases of CaP crystal and the mixtures of CaP crystal phases named apatite.Table 2 lists the most common CaP salts found in different phases at ambient temperatures and in aqueous solutions.HA(Ca5OH(PO4)3/Ca10(PO4)6(OH)2)is the most variable CaP salt with a pH value between 4 and 12 at ambient temperature.Natural bone is a living and growing tissue which consists mostly of collagen and water,and CaP(mineral)[43].Similarly,Ca and P are the main components of HA which improve the osseointegration of host bone tissue.HA-coated metallic implants possess many advantages as biomaterials with outstanding osteoconductivity and bioactivity,and faster integration with host bone tissues[44].
Recently,HA compounds have attracted considerable attention in catalysis,pharmaceutical products,water treatment,applications of protein chromatography,and the development of biocompatible materials.In particular,HA crystals were found to easily calcify in hard tissues,such as the teeth and cranium bones due to being an inorganic compound with limited solubility in water[49-53].In addition,HA coating is an expedient processing technique with cost-effectiveness,and its chemical composition is closer to that of natural bone as compared to other CaP compounds[54,55].HA is a biodegradableand biocompatible inorganic compound of human bone with suitable oseointegration property for bone healing[56,57].Furthermore,HA has osteoinductive and osteoconductive capabilities,which are beneficia for orthopedic applications[58].The application of HA is limited to non-load bearing applications because of its intrinsic brittleness property[59].Therefore,it is challenging to fabricate crack free,uniform coating thickness,and conformal coatings of HA,nano-HA,or HA composite on Mg alloy substrates.
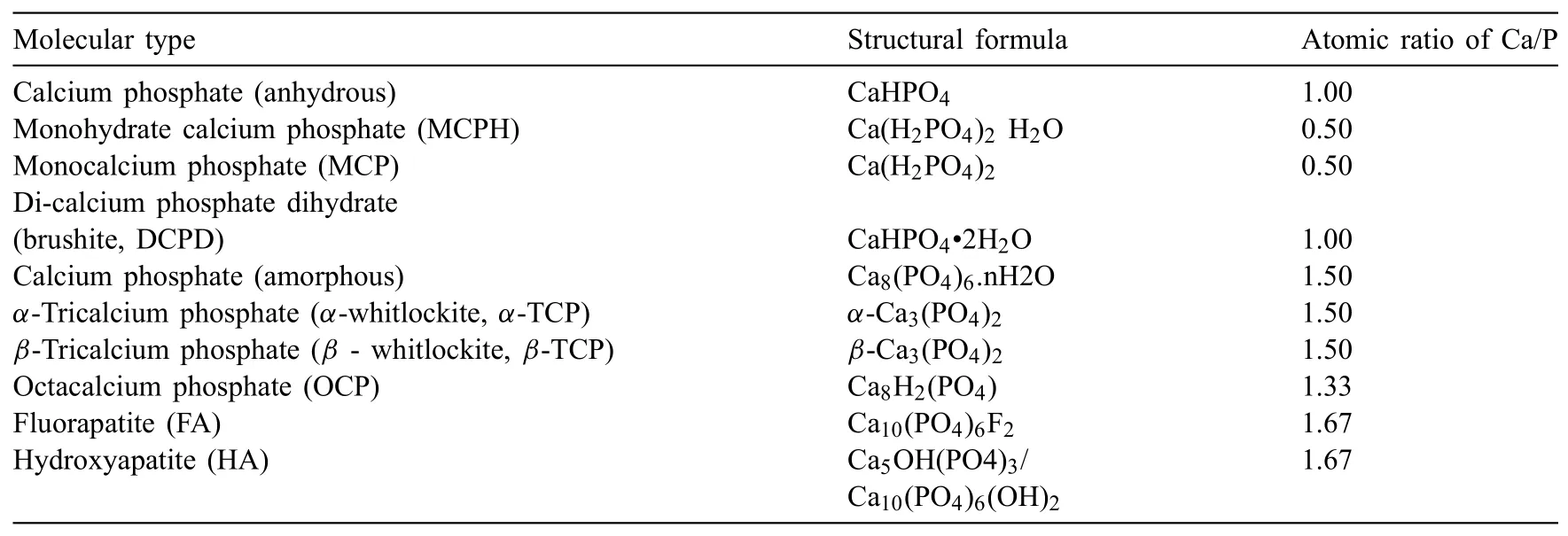
Table 2Different phases of CaP crystals[45-48].
2.1.Surface coating techniques for Mg alloys
So far many techniques,such as electrochemical deposition(ED),sol-gel processing,spray coatings,laser techniques,multistep coatings,have been studied for the preparation of surface coatings on metallic alloys for biomedical application[45,60-63].It is worth noting that the composition of electrochemical deposition and the chemical conversion coatings can effectively improve the corrosion resistance,bonding strength,bioactivity,and biocompatibility of the single layer coated biomaterials[64].Among the above-mentioned techniques,electrochemical deposition is the most studied method for the fabrication of an HA coating on Mg-based alloys due to its simplicity and cost-effectiveness.
2.1.1.Electrochemical methods
Jamesh et al.[65]synthesized an HA coating on pure Mg sample using an electro-deposition method in a mixture of electrolytes composed of 0.06M(NH4)3PO4,0.1M Ca(NO3)2,and 10mL/L of 30vol% H2O2at 27°C and a pH value of 4.Fig.1 illustrates the procedure of electrodeposition of HA coating on an Mg plate sample.After electro-deposition,the coating consisted of DCPD crystals.To obtain an HA coating,the as-deposited pure Mg samples were immersed in 1M NaOH solution for 120min at 80°C.A uniform fla e-like crystal structure was observed on the pure Mg surface which improved osseointegration.
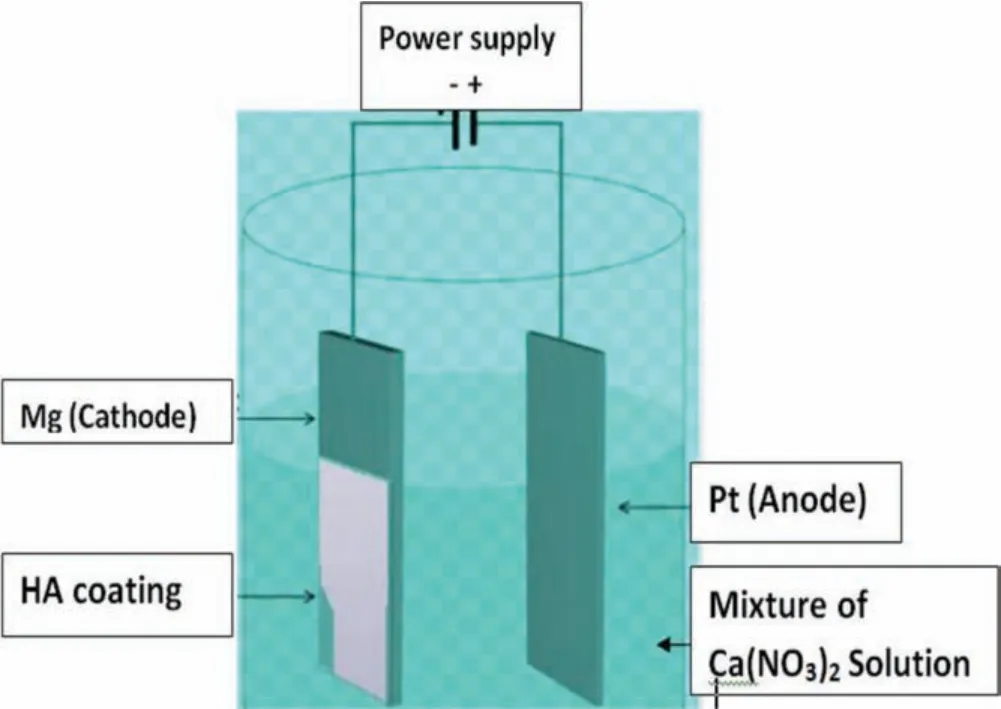
Fig.1.HA coating procedure through electro-deposition technique.
Tomozawa et al.[66]used a hydrothermal treatment process to synthesize an HA coating with different crystal structures on an Mg substrate.The HA coatings were produced by treating the Mg substrates in a mixture of Ca-EDTA and KH2PO4solution at 60°C;the pH of the solution was 8.9 and the processing time was 0.6-28.8 ks.Fig.2 illustrate the formation mechanism of HA crystal structures on Mg substrate.It can be seen that corrosion is initiated by certain chemical reactions in the HA-coated Mg sample after immersion in the treatment solution,and hence the pH value increases as shown in Fig.2(a).The fast nucleation of HA crystals with concurrent formation of an Mg(OH)2coating layer can be observed on the Mg sample surface due to the increase in the pH value.Ca2+ions are continuously supplied to promote the nucleation of HA crystal growth over the formation of an Mg(OH)2film As a result,a dome-shaped crystal structure can be observed due to the frequent nucleation of HA crystals,as shown in Fig.2(b).However,the corrosion rate is comparatively lower than the previous one due to the dense covering of the Mg surface with dome-shaped HA crystals and thus the discharge of Mg2+is suppressed,thereby increasing the local pH value.A rod-like HA crystal structure is fabricated on the surface of the Mg substrate,as shown in Fig.2(c)and(d),which is governed by the pH value,and rod-like HA crystals are synthesized during the hydrothermal process.Li et al.[67]fabricated glucose-induced Ca-P coatings including HA,Ca-deficien hydroxyapatite,and dicalcium phosphate anhydrous on pure Mg using alkaline solution and reported that the Ca-P coating significantl enhanced the corrosionresistance of pure Mg due to the addition of glucose in the alkaline solution.The electrodeposition process can be carried out at room temperature.There were some shortcomings with conventional electrodeposition process such as the gradual speed of ion transmission from the substrate to anode material which leads to the generation of H2in the cathode.This coating process has also been noticed to be more porous and less adherent.
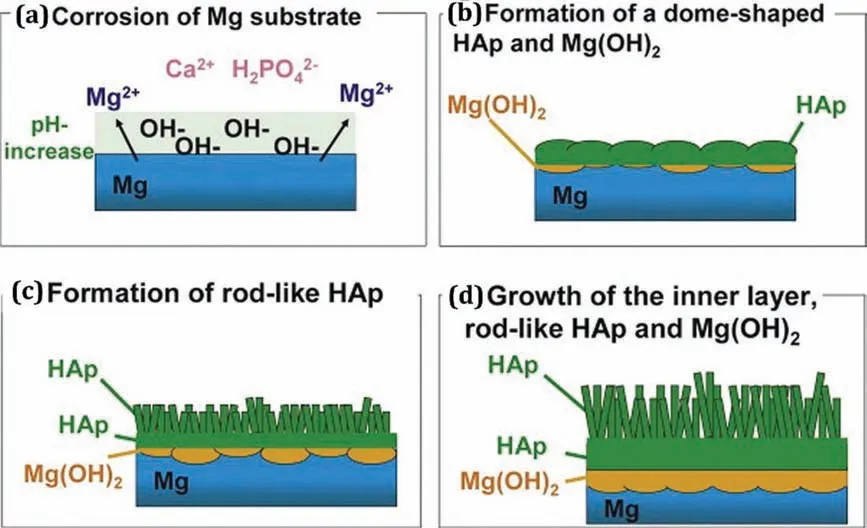
Fig.2.Schematic illustration of the formation mechanism of HA crystal structures on Mg substrate(adapted from[66]).
2.1.2.Sol-gel processing
Sol-gel processing has been mostly studied for surface modificatio of Mg alloys for increasing the corrosion resistance and adhesion strength between the surface layer and substrate materials.A sol-gel process is usually accomplished in four stages;(i)hydrolysis,(ii)condensation and polymerization of monomers to make a chain and constituent parts,(iii)particle enhancement,and(iv)heap of the structures of polymer pursued by the formation of a chain in the liquid media,which leads to producing a gel by increasing viscosity[68].There are two types of sol-gel process,such as inorganic and organic processes.An inorganic coating can be produced on Mg alloys by dipping the substrate in solution without forming chemical reactions.The organic process undergoes in a continuous liquid phase for making a network of suspended colloidal matters,the size of about 1-1000nm[69]and it also occurs in an alcohol or organic solvent where the dissolution of monomeric metal or precursor is metalloid alkaloid[70].
Lamaka et al.[71]fabricated an anticorrosion coating on AZ31B alloy using the sol-gel technique and reported that the nanostructured coating exhibited good corrosion resistance due to their stable chemical bonding with the Mg alloy.The electrochemical impedance spectroscopy confirme the anticorrosion properties of the coated AZ31B alloy.Similarly,another anticorrosion coating was produced on the pre-treated AZ31 Mg alloy using the sol-gel method,and reported that the corrosion resistance of Mg alloy was greatly improved due to the acid pickling,which was confirme by the gas volumetric measurement[72].
Rojaee et al.[73]produced a nanostructured HA coating using the sol-gel dip coating technique on AZ91 Mg alloy.The deposited substrate was dried at an ambient temperature for 24h and sintering at 400 °C for 6h.They reported that the desired coating thickness was maintained with the homogeneity and found a crack-free substrate surface.The nanostructured HA coating via sol-gel process increased the cell growth,promoted the bio-mineralization,and reduced the bio-corrosion but that the limited to bonding strength.
A CaP glass ceramic coating was prepared by Ren et al.[74]using the sol-gel dip coating method on the AZ31 Mg alloy.The deposited samples were dried at 60 °C for 2h and heat treatment was performed at different temperatures(400,450,and 500 °C)for 2h,and then it was cooled inside the furnace.The heat treatment temperatures dominated in the formation of surface structure and properties.It was found that the surface of the substrates was not compact at the heat treatment temperatures of 450 °C and 500 °C,but that the crystalline particles were distributed randomly,and the surface was compact at a heat treatment temperature of 400 °C.The crystalline particles homogeneously and smoothly distributed,which led to the formation of an entire glass phase.The CaP glass ceramic coating was produced using the heat treatment temperature of 400 °C;it exhibited good corrosion resistance in SBF solution compared to other heat treatment temperatures.In addition,a calcination temperature influ ences the crystallinity of the particles,and phase formation.A suitable calcination temperature is required to obtain the desired crystallinity and phases,for example,Piveteau et al.[75]reported that the crystalline particles were poorly distributed on the coating surface at a calcination temperature of 350 °C;however,Bogdanoviciene[76]reported that a pure HA coating can be obtained at a calcination temperature of 1000°C.The sol-gel coating technique mostly studied to be a suitable coating technique of Mg alloys for clinical purposes,and industrial applications.This coating is usually chosen for promoting the biocompatibility,and biomineralization,increasing the cell adhesion,and corrosion resistance.A heat treatment process is required to accomplish this technique,and the heat treatment temperatures are in the range of about 400 °C to 550°C,which is a great limitation for Mg-based alloys because it has a low melting point.The sol-gel process offers exclusive advantages including cost effectiveness,excellent adhesion strength between substrate and coating layer,and applicability to complex coating geometry.In addition,this process does not require special vacuum or heating equipment,nor a conductive substrate.The main disadvantage of this process is the difficult in maintaining a uniform coating thickness due to uneven dipping and withdrawing speed of the specimen holder in the sol-gel solution.Moreover,the sol-gel coatings are mostly porous with cracks due to quick evaporation of the solvent and residual water.
2.1.3.Spray techniques
The spray coating process consists of passing metal alloys,polymers,composites,and ceramics through a gun at a specifi pressure and temperature.Various types of spray coating include air spray coating,thermal spray coating,cold spray coating.Air spray coating is ideally done layer by layer while adjusting air fl w and temperature.Hahn et al.[77]produced an aerosol coating on the AZ31 alloy to increase the biocompatibility and corrosion resistance using the mixture of HA-chitosan powder at a room temperature.The mixture was sprayed on the surface Mg alloy through a vertical nozzle,which rotated along three directions.They disclosed that the deposited sample exhibited good corrosion resistance and well adhesion strength at a level of chitosan powder was applied,and,in contrast,showed a low corrosion resistance when a higher level of chitosan powder was applied.In the cold spray coating process,the ballistic impulsion of the particles results from the high velocity of gas f ow on the substrate surface.In the cold spray coating process,the suitable peening effect,particle size,and temperature are the important factors in obtaining the desired coating surface.Noorakma et al.[78]studied HA coating on the AZ51Mg alloy using the preheated HA powder,which was sprayed on the surface of the sample through a nozzle with a high pressure and at a room temperature.It was observed that the coating surface was uniform and the thickness of 25μm due to the high impact velocity of the nozzle and preheating of the HA powder,which exhibited good bioactivity after immersion in SBF solution but limited to the temperature.
Thermal spray is coating a process in which melted materials are sprayed onto the surface of the substrate using a gun.Unger et al.[79]fabricated a successful thermal spray coating and depositing a layer of Al on the AZ91D Mg alloy.They mentioned that this process significantl increased the corrosion resistance and anti-wear properties after post-heating at a temperature of 450°C.In addition,Zeng et al.[80]produced a cold spray coating in which TiO2was sprayed on the AM60 Mg alloy.They disclosed that the corrosion resistance was significantl improved in comparison to the bare Mg alloy after immersion in Hanks'solution.This experiment also indicated that the galvanic corrosion was observed between the substrate surface and the coating surface,which led to retarding the protective properties after coating.The spraying coating can significantl improve the corrosion resistance of the substrate and the process is excellent for its set-up simplicity,cost effectiveness,and nonprecious coating layer.
2.1.4.Laser techniques
The laser beam has high-density energy,which can be employed to melt the surface and create a metastable solid solution in order to improve the surface properties of the implant materials.The main objectives of laser surface modificatio are to refin the microstructure,dissolve precipitates,and create a homogeneous composition of the coating.The coating surface exhibits a uniform microstructure due to employing the rapid cooling.Abbas et al.[81]conducted a study on the WE43,AZ61,and AZ31 Mg alloys using the laser surface melting process(LSM)to improve the corrosion resistance.The corrosion tests were performed in 5% NaCl solution with a pH value of 10.5 at 20 °C and revealed that improved the corrosion resistance in terms of weight reduction at about 87,66,and 30% for the WE43,AZ61,and AZ31,respectively.The superior corrosion resistance was found in WE43 compared to other alloys because of containing the rare earth element as the alloying elements,and the better corrosion resistance found in AZ61 compare to AZ31 due to a higher amount of Al as an alloying element.The laser shock peening(LSP)is another technique for surface modificatio but it differs from the laser surface melting process in that it involves.The rapid irradiation at a power density of about 109-1012W/m2and the short pulse of about 1-50 is applied on the surface of the target material.Zhang et al.[82]performed an experiment on the AZ31 using the laser shock peening technique,and mentioned that the initiation of corrosion cracking of the substrate sample was retarded with the addition of 1wt% of NaOH solution at an ambient temperature for 500h.However,the laser-assisted mask-less micro-deposition is one of the surface modificatio methods in which micro-deposition occurs in the layer by layer and the atomized nanoparticles are injected into the moving substrate.Foroozmehr et al.[83]performed the same technique by incorporating of nanoparticles of Ag on the Mg.They revealed that a uniform coating thickness,and better biocompatibility was achieved due to the bioactive properties of the Ag.Unfortunately,a few cracks were found on the Ag fil that was the main limitation for biomedical applications.Banerjee et al.[84]studied the ZE41 Mg alloy and reported that the poor improvement in corrosion resistance was observed after immersion in 0.001M NaCl solution for an hr.They reported that few cracks were found on the surface of the Mg alloy which led to poor improvement in the corrosion resistance after the laser irradiation.Overall,the laser technique offers superior advantages for the fabrication of complex geometry materials,fast deposition time,maintenance of stoichiometry,higher fl xibility,and the ease of growing multilayered coatings.Some limitations were observed during this process including the stress concentration cracking around the coating,pinholes,and poor corrosion resistance.Therefore,researchers should focus on these aspects to minimize or to eliminate these constraints altogether.
2.1.5.Multistep coatings
Multistep coating(for example,chemical treatment followed by one or more coating layer)can be an effective way of improving the anticorrosion properties of Mg alloys.For instance,Tan et al.[85]produced multistep coating(firs anodization then sol-gel coating)on the AZ91D alloy to improve the corrosion resistance.The electrochemical impedance spectroscopy revealed that the corrosion resistance was greatly improved using the multistep coating and compromising the porosity level.
Tang et al.[86]fabricated an HA coating on AZ31 Mg alloy with a micro-fl wer crystal structure using a microarc oxidation process followed by a solution treatment to improve the degradation performance of AZ31 in the physiological environment.The solution treatment was produced with a mixture of potassium dihydrogen phosphate(KH2PO4)and ethylenediaminetetraacetic acid calcium disodium salt hydrate(EDTACa:C10H12N2O8Na2Ca).The solution concentrations of KH2PO4and EDTACa were 200 and 300mmol/L,respectively,and the pH value of the treatment solution was adjusted using NaOH solution.
Mousa et al.[87]fabricated an HA coating on an AZ31B Mg alloy sample using an anodization process followed by a hydrothermal treatment.The anodization process was performed in an SS bath in which the bath was acted as cathode and the Mg sample was acted as the anode.The whole process was completed in two steps:frst the AZ31B sample was immersed in 5M NaOH for 2h at 60°C and rinsed with distilled water;secondly the anodized samples were immersed in SBF solution made of commercial Hanks'solution with the addition of 0.350g of NaHCO3,0.097g of MgSO4,and 0.185g of CaCl2in one liter of distilled water to which 10% of HA nano-particles were added.After the hydrothermal treatment,the nucleation and growth of the HA deposited Mg sample were accelerated,and a plate-like structure was observed which exhibited excellent bioactivity;this was confirme by in vitro assessment using MC3T3-E1 cells as compared to uncoated AZ31B.
Gomes et al.[88]developed a multifunctional coating on an AZ31 alloy by conjoining an anodization process with the coating of a polymeric-based layer consisting of polyether imine reinforced with HA nanoparticles,aiming at improved control of the corrosion behavior and biological performance of the Mg alloy substrate.The AZ31 alloy after anodization plus HA-composite coating displayed excellent corrosion resistance and biological performance,including the formation of new bone tissue at the interface between implant material and host bone.They suggested that the multistep-coated Mg alloy is a more promising substitute compared to the uncoated or anodized alloy for clinical applications owing to their favorable degradation rate and the new bone formation.Layer by layer coating on Mg substrate is an effective approach to decrease the corrosion rate in the physiological environment.A triple-layered coating composed of Ca3(PO4)2,MgHPO4·3H2O,(Ca,Mg)3(PO4)2,and SnO2was synthesized by a multistep method on Mg-1Li-1Ca alloy which led to the formation of HA in Hanks'solution[89].The authors demonstrated that the HA-coated Mg alloy substrate showed antibacterial activity and improved corrosion resistance confirme by the plating counts and electrochemical test,respectively,due to the embedded SnO2.Multistep coating on Mg alloys is a very promising technique for the development of thick coatings in combination of organic and inorganic coating layers.Multistep coating possesses advantages such as bioactive and biocompatible top layers and highly corrosion-resistant bottom layers.The challenge of this process is to achieve optimum bonding strength between coating layers and substrate due to different coating composition for different coating layers.
2.2.Surface morphology of HA-coated Mg alloys
The surface morphology of biodegradable implant materials is a significan tissue in that the interaction of implant materials with adjacent tissues is essential for implant engagement and cell attachment[90].HA-coated Mg alloy implant materials exhibit outstanding biocompatibility and ion exchangeability,as well as a great affinit with biopolymer.HA is commonly used as an alternative to biomaterials for replacing human hard tissue[91].Ca and P are the main constituents of bone minerals.CaP coatings,particularly HA and TCP,can be regarded as osteoconductive minerals that faciliate the formation of new bone[92]and promotes osteointegration surrounding the Mg based implants.In the case of a bone implant,the nano-spheres and nano-plate structures of the HA coating act in the Mg based alloys interface as a biomimetic fil which mimics natural bone[93].Factors such as solution,pH value of the treatment solution,doping element like strontium(Sr),zirconium(Zr),argentum(Ag)into electrolyte and treatment time and temperature can influ ence the coating morphology and biomedical properties of the implant material.The Sr doped Zn-Ca-P coating on AZ31 Mg alloy provided significan control over the degradation and improved the biocompatibility of the Mg alloy[94].However,Song et al.[95]produced three types of coating on an Mg-Zn alloy and reported that a DCPD coating was produced at room temperature and pH 4.4,whereas an HA coating was produced at 80°C for 4h,with fast nucleation growth of HA crystals having a fla e-shape structure.Further,a fluoridate hydroxyapatite(FHA)coating was synthesized at about 60°C,and an apatite crystal structure on the surface of the Mg alloy was observed.The DCPD structure was dissolved in SBF solution and that some cracks and holes were found in the HAcoated Mg alloy sample;however,the FHA-coated sample was uniform and almost intact after two weeks of immersion.HA coating on fluoride-treate Mg-Zn-Ca substrate enhanced the corrosion resistance and improved the bioactivity of the alloy as confirme by immersion test and electrochemical test[96].Tomozawa et al.[97]synthesized an HA coating on pure Mg using a Ca-EDTA solution by means of hydrothermal processing.They reported that the crystallographic orientation and microstructure of the HA-coated Mg substrates varied according to the pH value of the treatment solution,as shown in Fig.3.The coating morphology was uniform and the fines structures in Figs.3(a)and(b)are for the pH values of 5.9 and 8.9,respectively.A chestnut-like structure due to the formation of the HA coating was observed at a pH value of 11.9,as shown in Fig.3(c).After ultrasonic cleaning,a smaller size(the finest crystal structure was found,shown in Fig.3(d),and greater nucleation at the pH value of 11.9 compared to the pH values of 8.9 and 5.9 can be observed in the figures Different structures,such as crystallographic orientation,crystal shape,and the size of the HA synthesized fla es and particles,have been extensively investigated[97-101].Wang et al.[102]prepared a Ca-deficien HA coating on Mg-Zn-Ca substrate with different surface morphologies depending on the treatment time using electrodeposition process and reported enhanced corrosion resistance due to the heterogeneous nucleation and growth of HA crystals.
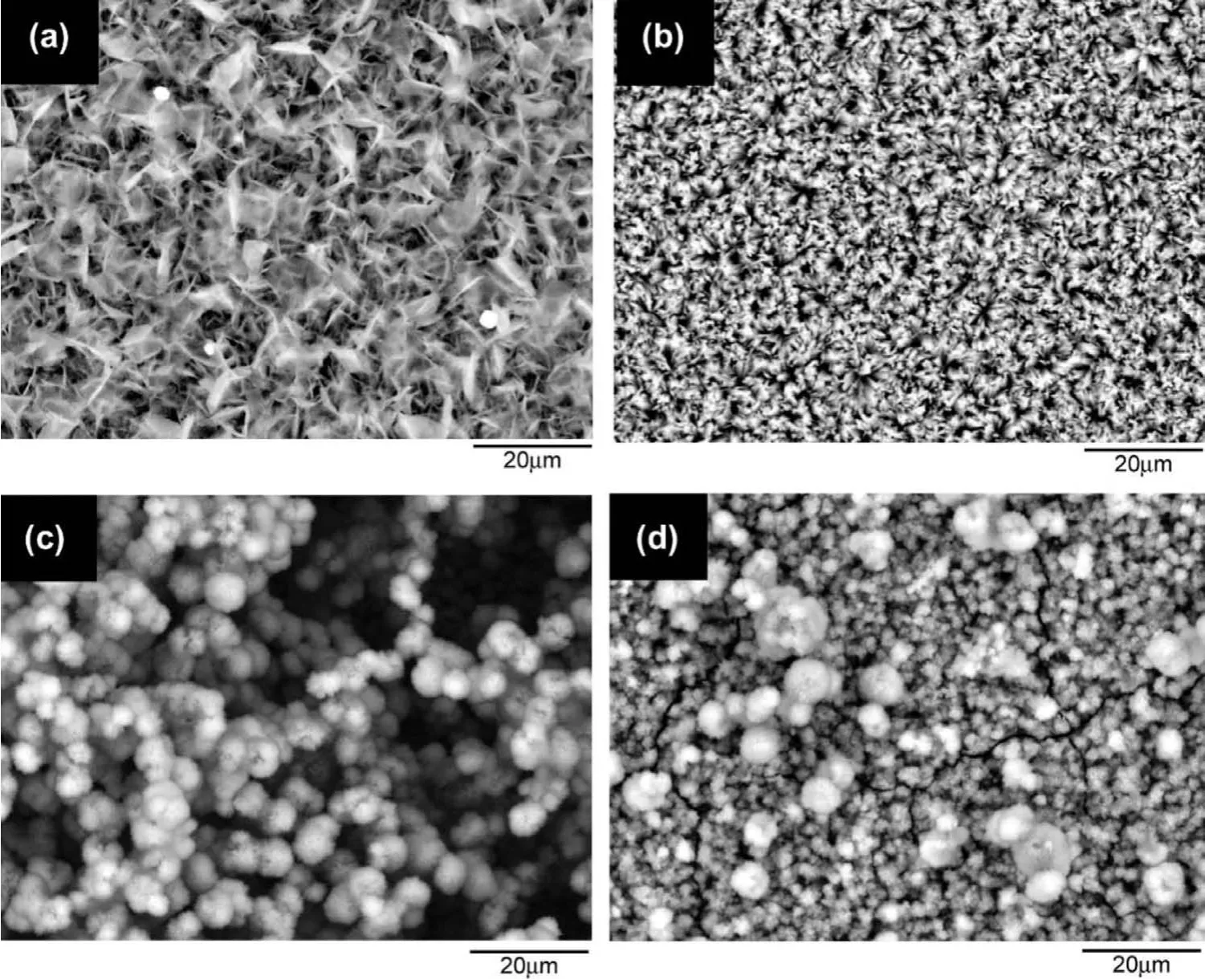
Fig.3.SEM images of HA coating on pure Mg at different pH values:(a)pH 5.9,(b)pH 8.9,(c)pH 11.9 and(d)pH 11.9 after ultrasonic cleaning(adapted from[97]).
2.3.Mechanical properties of HA coatings on Mg alloys
Metallic biomaterials are mostly employed as orthopedic implants because of their mechanical properties and loadbearing capability.Several test methods such as micro-,nanoindention testing can be used to determine elastic modulus,hardness,and fracture toughness of HA-coated or uncoated Mg alloys.Kumar et al.[103]synthesized different HA coatings on Mg-3Zn substrate using electrodeposition process for orthopedic applications and investigated the mechanical properties including elastic modulus,hardness,and fracture toughness of the different HA coatings using instrumented microindentation;in addition,a scratch technique was used to measure the adhesion strength of the HA coatings deposited under different conditions.
Li et al.[104]synthesized a HA-coating on a high-pressure torsion(HPT)-processed ZEK100 alloy using hydrothermal method and reported that the HPT processing refine the grain size and introduced a large number of twins,resulting in the enhancement of microhardness and Young's modulus of ZEK100;furthermore,the fin grain boundaries and twins in the HPT processed ZEK100 provided more nucleation sites for HA during in hydrothermal treatment,leading to a denser HA coating with higher thickness on the Mg alloy which showed significantl enhanced corrosion resistance during electrochemical impedance spectroscopy(EIS)measurements performed in phosphate buffered saline(PBS).Tian et al.[105]reported on HA coatings with nano-to-submicron structures(namely nHA and mHA)deposited on Mg plates and rods using a transonic particle acceleration(TPA)process under different conditions and investigated for their effects on Mg degradation in revised simulated body flui(rSBF).Their results demonstrated that nHA and mHA coatings enhanced corrosion resistance of Mg and retained 86-90% of ultimate compressive strength after in vitro immersion in rSBF for 6 weeks,much greater than non-coated Mg that only retained 66% of strength.Surmeneva et al.[106]prepared nanostructured HA coating on AZ31 alloy using a radio-frequency(RF)magnetron sputtering process and reported the nanoscale hardness(H)and Young's modulus(E)of the nanostructured HA coatings measured using nanoindentation technique;they also calculated the elastic strain to failure(H/E)(an indicator of a wear resistance)and the plastic resistance ratio H3/E2(an indicator of the resistance of the material to plastic deformation).

Fig.4.SEM images of AZ31 samples after immersion in SBF for 7 d:(a)uncoated;(b)as-deposited HA-coated;and(c)post-treated HA-coated(adapted from[110]).
Recently,HA has attracted much attention as a reinforcement material to improve the mechanical integrity of Mg alloys,for example,Dubey et al.[107]fabricated Mg-3Zn-5HA composite with 5wt% HA as reinforcement by powder metallurgy and reported that the ultimate compressive strength and yield strength of the Mg-based composite material were greatly enhanced after immersion in SBF for 3,7,and 14 d as compared to those of Mg-3Zn;it was also mentioned that Mg-3Zn and Mg-3Zn-5HA samples retained approximately 34% and 66% ultimate compressive strength after immersion for 3 d,and 6% and 8% of their ultimate compressive stress after immersion for 14 d in SBF.
2.4.Corrosion behavior of HA-coated Mg alloys
The surface morphology,microstructure,and composition are extremely significan in determining the bioactivity and corrosion performance of HA-coated Mg alloys.HA-coated Mg alloy substrates are expected to exhibit high corrosion resistance in the physiological environment due to their excellent thermodynamic stability[108].The poor corrosion resistance,and insufficien biocompatibility and bioactivity of conventional Mg alloys hinder their applications in orthopedics and cardiovascular devices.To enhance the corrosion resistance,biocompatibility,and bioactivity,an HA coating was synthesized on the surface of the AZ91D alloy using the electrochemical deposition process[109].It was reported that the deposited coating was composed of DCPD andβ-TCP,which was subsequently transformed into HA with improved corrosion resistance as well as a slower down degradation rate;this was confirme by the EIS analysis.Wen et al.[110]prepared an HA coating on AZ31 alloy using a mixture of electrolytes,0.1mol/L NaNO3,0.025mol/L NH4H2PO4,and 0.042mol/L Ca(NO3)2,with a pH value of 5,at 85°C,and an immersion time of 60min after which the coated Mg alloys were immersed in NaOH solution for 4h at 80°C and dried at 60°C for 4h.The HA coating on the Mg alloys exhibited the morphological architecture of a radiated plate-like structure in the as-deposited condition and a needle-like structure after posttreatment.Fig.4 shows the SEM images of the uncoated and HA-coated AZ31 after immersion in SBF solution for 7 d.Some pits and cracks can be observed in Fig.4(a)of the uncoated sample,whereas Fig.4(c),the post-treatment sample,gained more mass as compared to the as-deposited HA-coated sample in SBF(Fig.4(b)).The post-treated needle-like structure exhibited a greater contact surface for apatite formation and exhibited improved corrosion resistance and a reduced degradation rate,and showed better bioactivity compared to the other samples.
Similarly,Kang et al.[111]fabricated an HA coating on a biodegradable Mg with a needle-shaped crystal structure and it showed excellent corrosion resistance after immersion in SBF solution,indicating its potential for implant applications.
An alkali treatment can play a vital role in improving the corssion resisatnce of Mg alloys in the physiological environment.Rojaee et al.[112]synthesized an nanoHA(nHA)coating on an anodized AZ91 Mg alloy by means of an electrophoretic deposition process and reported that corrosion resistance significantl increased compared to uncoated Mg alloy,confirme by potentiodynamic polarization tests.The corrosion resistance and the biomedical properties of Mg alloys greatly increased due to a double layer coating[113].An HA coating was synthesized on a phosphate-treated Mg-Mn-Zn alloy to improve corrosion resistance and it was found that the coating thickness increased with the treatment time,resulting in a more corrosion resistant coating compared to that of the bare Mg-Mn-Zn alloy evaluated using the EIS test[114].
In another study,a nano fluorin doped hydroxyapatite(nFHA)coating was produced on the surface of an Mg-Zn-Ca alloy and the immersion tests and potentiodynamic polarization analysis confirme considerably greater corrosion resistance as compared to traditional cathodic deposition process and suggested that the nFHA coated Mg alloy is a promising biodegradable implant material for clinical applications[115].Due to poor corrosion resistance of Mg and its alloys hinder their application for clinical applications and hence,by inducing some protein,antibiotic and polymeric materials could enhance the corrosion resistance and antibacterial performance of Mg alloy.Liu et al.[116]fabricated DNA doped bioinspired Ca-P coatings on AZ31 Mg alloy to enhance bonding strength and corrosion resistance and demonstrated that the bonding strength and corrosion resistance were greatly improved due to DNA addition to the electrolyte.
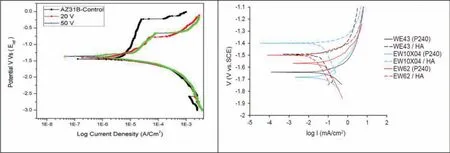
Fig.5.Potentiodynamic curves of HA-coated and uncoated Mg alloys:(a)AZ31B(adapted from[119]);and(b)WE43,EW10×40,and EW62(adapted from[120]).
Hiromoto et al.[117]compared the corrosion performance of HA coating and OCP coating on Mg alloys and found that the HA-coated AZ31 sample demonstrated greater resistance to corrosion in comparison to the OCP-coated AZ31 sample in NaCl solution due to the inner layer of the HAcoated AZ31 substrate was microscopically denser than the inner layer of the OCP coated sample.For biomedical applications,the long-term corrosion performance of Mg based alloys needs to be understood very clearly.HA-coated pure Mg with a highly crystalline structure exhibited significantl better corrosion resistance as compared to bare Mg,confirme by the cyclic wet and dry tests,as well as the polarization tests[118].Moreover,potentiodynamic curves revealed that an HA-coated AZ31B Mg alloy at 20V exhibited the best corrosion resistance as compared to uncoated AZ31B and HAcoated AZ31B at 20V,as shown in Fig.5(a)[119].Furthermore,Dunne et al.[120]fabricated HA coating on WE43,EW10×40,and EW62 Mg alloys using CoBlastTMprocess and reported that corrosion resistance was significantl increased with HA-coated samples as compared to bare sample of Mg alloys,confirme by the potentiodynamic polarization test(Fig.5(b)).
Double layer or multilayer coating either inorganic coating or organic coating on Mg alloys can improve corrosion resistance as compared to single layer coating.For instance,Ji et al.[121]fabricated a polyacrylic acid(PAA)/gentamicin sulfate(GS)-hydroxyapatite(HAp)coating on AZ31 substrate to enhance corrosion resistance and to generate antibacterial activity.Their results indicated that the multilayer coating not only greatly improved the corrosion resistance but also imparted the substrate with effective antibacterial ability due to the barrier type fil at the outer surface.A compact and dense coating of HA on Mg alloy substrate benefit the corrosion resistance and bonding strength between substrate and coating[122].Bakhsheshi-Rad et al.[123]synthesized a bi-layered nanostructured silica(SiO2)/silver-doped fluoro hydroxyapatite(AgFHAp)on Mg-1.2Ca-4.5Zn substrate using electrodeposition(ED)process followed by physical vapor deposition(PVD)and reported that the SiO2/Ag-FHAp coated substrate exhibited superior corrosion resistance due to the formation of compact coating structure with adequate thickness as compared to single layer coating and uncoated substrate.
During degradation,the increase in alkalinity and the generation of H2gas are both degradation consequences of bioabsorbable Mg alloys.This necessitates the development of Mg alloys with well-controlled degradation rates.Moreover,biocompatible composite coatings or another layer of HA coating along with alkaline treatment can slow the commencement of corrosion,which would protect the mechanical integrity of the Mg based implant materials,thereby enabling them to remain intact until the advanced stages of healing.Chen et al.[124]synthesized a composite coating of HA,OCP,and oxide on an Mg-Zn-Ca alloy to promote the bone response and corrosion resistance,and demonstrated that fast release of Mg ions from the substrate was prevented at the interface,and degradation of the implant occurred gradually by virtue of the composite coating on the surface of the Mg alloy sample.Further,composite coatings on an Mg alloys were demonstrated to significantl enhance the corrosion resistance,promote fast bone response,and induce formation of new bone tissue.In another study,a composite coating of HA and satiric acid was produced on AZ91D Mg alloy using the electro-deposition method and the composite coating was found to be porous,exhibiting good osseointegration properties[125].The composite coating also provided excellent protection for AZ91D Mg alloy in SBF solution with increased corrosion resistance.
2.5.In vitro and in vivo assessment of HA-coated Mg alloys
The in vitro and in vivo degradation behavior and related mechanisms of biodegradable Mg based implant materials have been assessed in recent years.In vitro and in vivo assessments are important in accumulating the data of Mg alloys to be used in clinical applications,although a greater focus needs to be placed on better understanding of the corrosion and degradation mechanisms of Mg based alloys in the biological environment in the future.In vitro assessment studies of Mg based alloys in the physiological environment primarily focus on the degradation rates,corrosion products,corrosion types,and the effects of compositions of body fluid on the degradation of the materials.In vivo studies mainly analyze the biological response of the host environment,such as bone tissue engagement,cell attachment,new bone growth,and the degradation performance of the implant in terms of service lifetime.Guan et al.[126]produced an HA coating on the surface of an Mg-Zn-Ca-Zr alloy to evaluate corrosion performance,degradation rate,cytocompatibility,and hemolysis compared to uncoated Mg alloys.In vitro and in vivo tests revealed that corrosion resistance was greatly increased,and the degradation rates were slowed down after immersion in SBF solution compared to those of the untreated Mg alloy.The corrosion potentials of HA-coated Mg-Zn-Ca-Zr,uncoated Mg-Zn-Ca-Zr,pure Mg,and Mg-Ca were−1.51,−1.72,−1.95,and−1.97V,respectively,which indicates that the HA-coated Mg-Zn-Ca-Zr alloy possessed greater resistance to corrosion.The hemolysis rates of the uncoated and HA-coated Mg alloy samples were 4.12,and 4.35 indicating that there was no significan hemolysis as it was less than 5%.The hemolysis rates of Mg alloy met the requirements for biomedical applications as implant materials.Moreover,Ca-P coating on Mg alloys can improve corrosion resistance and biocompatibility with addition of Sr or Ag into the electrolyte.Makkar et al.[127]successfully fabricated Sr-doped Ca-P coating on Mg-Zn-Ca alloy in order to improve corrosion resistance,bioactivity,and biocompatibility and reported that the Sr-doped Ca-P coating significantl enhanced the corrosion resistance,in vivo cytocompatibility,and bone regeneration of the Mg alloy.
Furthermore,a plate-like-shaped,rod-shaped,sphereshaped,needle-shaped of crystal structure of HA coatings could be fabricated on the Mg based alloys,which could improve corrosion performance as well as biological properties due to increased surface contact area in a physiological environment.Both in vitro and in vivo analysis were performed on HA-coated pure Mg to improve its bio-corrosion,and promote bone response,and biocompatibility[128].A needle-shaped crystal structure of HA coating was formed on the surface of Mg which improved its bio-corrosion resistance in SBF because of the greater contact surface area.In vivo assessment revealed that the biological response,together with cell engagement,differentiation and proliferation on the HA-coated Mg sample were significantl improved in comparison to the uncoated Mg sample,demonstrating that the corrosion rate and degradation were reasonably reduced,and that HA-coated Mg has excellent mechanical stability as an implant material.A comparative study was performed on AZ31 alloy to investigate the performance of OCP and HA coating both in vitro and in vivo in order to evaluate the corrosion behavior,degradation performance,and bone tissue response of the coated alloy[129].Corrosion product covered the uncoated AZ31 implant with some cracks after immersion in the medium and implantation in a mouse,as shown in Fig.6(a)and(b),respectively.The in vitro experiment revealed that the growth of plate-like crystals of the OCP-coated on the alloy promoted bioactivity,as shown in Fig.6(c)and(d),and that the residues accumulated adjacent to the rod-like crystals of HA after immersion in the artificia biological environment for duration of 14 weeks and 52 weeks,respectively,as shown in Fig.6(f)and(g).In vivo assessment demonstrated that plate-like crystals of the OCP-coated implant were becoming smaller and thinner as shown in Fig.6(e),whereas the rod-like crystals of the HAcoated implant did not change significantl after implantation in a mouse as compared to the other implants,as shown in Fig.6(h).
Most of the studies of HA-coated Mg based alloys have shown retarded corrosion rates,and degradation rates but researchers have given little attention to the improvement of mechanical integrity.For example,Wang et al.[130]fabricated a Ca-deficien HA coating on an Mg-Zn-Ca alloy using the pulse electro-deposition technique to reduce the degradation rate and adjust the mechanical integrity during the healing process.A considerably retarded degradation rate and improved mechanical integrity were observed and that was revealed by both in vitro assessment and the slow strain rate tensile testing method.They also mentioned that the Cadeficien HA coating exhibited excellent adhesion to the Mg-Zn-Ca implant material.
Biocompatible coating such as HA coating is beneficia for promoting cellular response in the physiological environment.Zhou et al.[131]fabricated a biofunctional composite coating based on polycaprolactone and nanohydroxyapatite for controlled corrosion behavior and enhanced biocompatibility of AZ31 alloy and reported that the nano-HA-contained coating not only enhanced corrosion resistance measured by immersion and potentiodynamic tests,but also significantl improved cellular response in terms of alkaline phosphatase activity,cell adhesion,and proliferation of osteoblastic cells as compared to uncoated and single polydopamine layer coated substrates.Du et al.[132]synthesized a calcium silicate and calcium phosphate(CaSiO3/CaHPO4·2H2O)composite coating on Mg-Zn-Mn-Ca alloy to improve its biocompatibility and reported good cell adhesion,high growth rate and proliferation characteristics of osteoblast cells on the coated-Mg alloy.Peng et al.[133]fabricated an HA/graphene oxide(GO)composite coating on AZ31 alloy where HA coating at the inner and GO at the outer layer to improve biocompatibility and demonstrated excellent cell adhesion and proliferation of MC3T3-E1 cells due to the bi-layer coating,and recommended that HA/GO-coated AZ31 substrate is promising for biodegradable implant applications.

Fig.6.SEM images of AZ31 sample surfaces:(a)and(b)without coating;(c)-(e)with OCP coating;and(f)-(h)with HA coating;(c)and(f)as-prepared;(a),(d),(g)immersed in the artificia biological medium;(b),(e),(h)after implantation in the mouse(adapted from[129]).
3.Multifunctional coatings
Multifunctional coatings can offer more benefit than the single-functional coatings of metallic alloys.For instance,an FHA coating was produced on a biodegradable Mg-Zn alloy by an electrochemical deposition process to improve both the degradation behavior and the nucleation of osteoconductive minerals[134].In vitro assessment confirme the higher cell viability and no toxicity was shown;in vivo assessment confirme significantl enhanced bioactivity and indicated its promise as an implant material for orthopedic applications.Similarly,Bakhsheshi-Rad et al.[135]produced an FHA coating on Mg-Ca alloy using the electrochemical deposition technique to improve degradation performance.A uniform and dense f lm of an FHA coating f lm was fabricated on the surface of the Mg alloys.In vitro assessments including immersion and electrochemical measurement tests revealed improved corrosion resistance and slowing of the initial degradation rate of the Mg-Ca alloy.In vitro degradation assessment revealed that the corrosion resistance improved due to the development of a barrier-type film which resulted in the retarded degradation rates of the Mg alloy.The degradation rate was significantl retarded due to the combination of the HA coating and barrier-type layer compared to the HA coating alone.
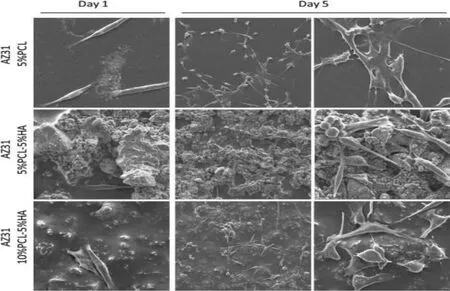
Fig.7.Surface morphology of the attachment of MG63 osteoblastic like cells on AZ31 Mg alloy after incubation for 1 and 5 d(adapted from[138]).
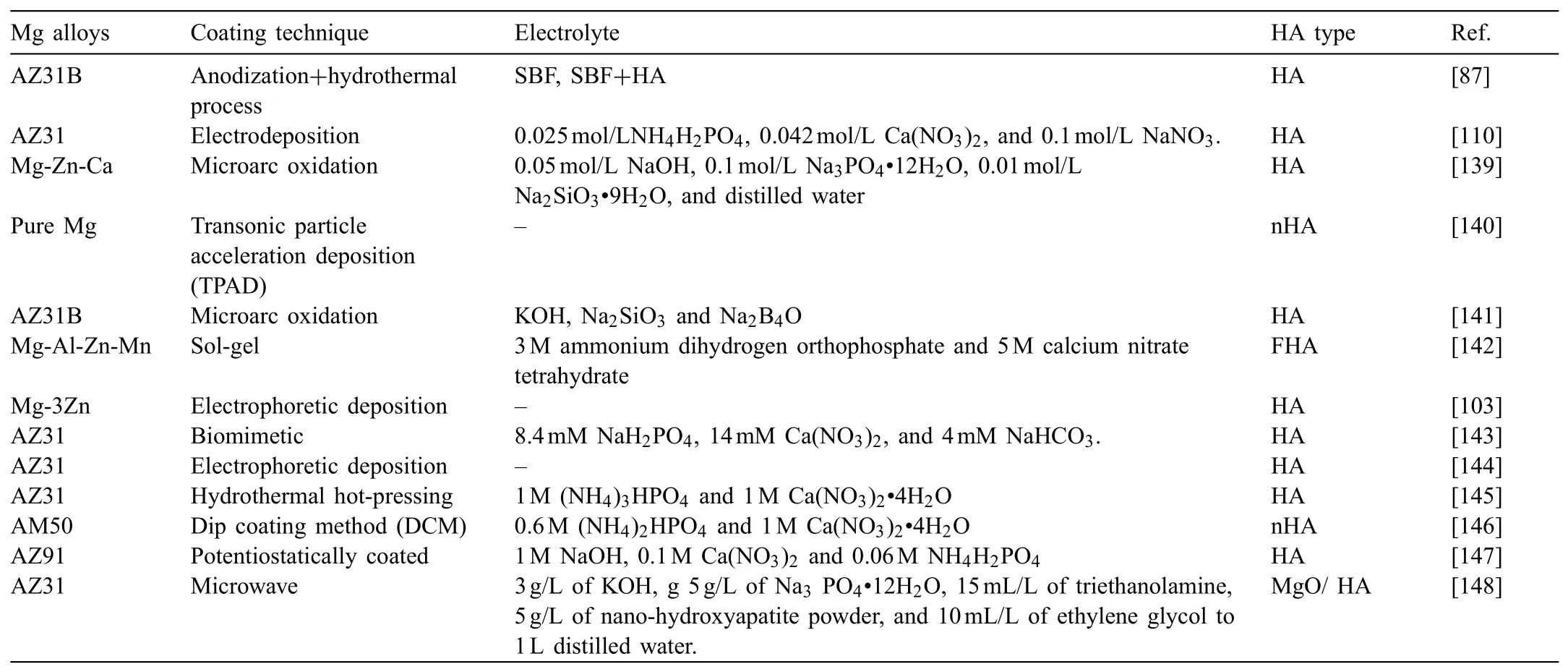
Table 3Overview of different HA coatings on various Mg based alloys,the coating techniques and the electrolytes used for the coatings.
HA-composite coating is a combination of HA and one or more constituents,and it is becoming most popular to be a promising coating since it provides multifunctional effects to the base substrate.The combination of the coatings can be a ceramic/metal,ceramic/polymer,or polymer/ceramic/metal.In the literature,many HA-coated hybrid coatings were prepared on the Mg alloys and Ti alloys owing to the outstanding bioactivity,biocompatibility and excellent corrosion resistance.Tian et al.[136]fabricated nanocomposite coating with HA/graphene oxide(GO)on pure Mg and reported that the nanocomposite coating significantl reduced degradation rate,enhanced mechanical properties,and improved bone healing ability due to their osseointegration property.However,Redepenning et al.[137]produced a composite coating using the electrochemical deposition,a combination of the HA and chitosan on Ti.They observed that pure HA coating was less biocompatible compared to the composite coating.Zomorodian et al.[138]fabricated a bifunctional composite coating combining nanoHA and polycaprolactone(PCL)on AZ31 Mg alloy in order to enhance the corrosion resistance and cell adhesion.They reported that the composite coating greatly improved the corrosion resistance and promoted the biocompatibility and cell adhesion compared to uncoated AZ31 substrate.Fig.7 shows the cellular morphology on the composite-coated AZ31 sample after culture for 1 d and 5 d.It was observed that the AZ31 substrate with the composite coating showed significantl increased cell proliferation as compared to the un-coated substrate.The ceramic coating exhibits good biocompatibility,and high corrosion resistance but poor biodegradability.A ceramic composite coating was fabricated by Du et al.[132]with the combination of calcium phosphate and microporous calcium silicate(CaSiO3-CaHPO4·2H2O)on the Mg-Zn-Mn alloy by undergoing a chemical reaction.The in vitro assessment confirme that this composite coating showed a significan increase in the cell adhesion and improved the cytocompatibility therein.Multifunctional coating has more benefit including enhanced corrosion resistance,mechanical integrity and biocompatibility due to the combination of different coating layers.The limitation of this coating is the possibility of poor bonding strength because of organic and inorganic coating compositions thereby causing removal of the coating layer and quick corrosion damage on the surface of the substrate.
Overall,in the future,further in vitro and in vivo studies are required for a better understanding of the corrosion rate,degradation mechanism,biocompatibility,bioactivity,and osseointegration of Mg based alloys with and without surface coatings(in particular,HA coatings)for biomedical applications.Table 3 lists an overview of the different HA coatings on various Mg based alloys,the coating techniques and the electrolytes used for the coatings.
4.Summary
HA coating on Mg alloys is a very promising approach for improving their corrosion resistance,slowing down their degradation rate,and enhancing their bioactivity,biocompatibility,and osseointegration.A few techniques including electrochemical deposition method,sol-gel processing,spray techniques,laser techniques,and multistep coatings on Mg alloys have been analyzed in this review.Although the mechanism of HA formation on Mg alloys has currently not been fully explored in terms of the coating processes and processing parameters,which influenc the coating thickness,coating uniformity,crystal structure,and morphology.Electrochemical deposition is one of the most effective methods to improve the corrosion resistance of Mg alloys.The composition and concentration of the electrolytes during electrochemical deposition have a great influenc on the thickness,porosity,morphological structures,mechanical properties,and corrosion resistance of the HA coating.Therefore,a combined approach such as a chemical conversion coating followed by multilayer/multifunctional coatings on Mg alloys seems to be a promising strategy to fabricate uniform and barrier-type coatings which can provide improved corrosion resistance,reduced degradation rate,and enhanced biocompatibility of Mg alloys.The development of congenial techniques for biocompatible coatings on Mg based alloys deems great potential for the development of biodegradable implant materials for clinical applications.
Acknowledgments
The authors would like to acknowledge the financia support to this research by the Australian Research Council(ARC)through the Discovery Project DP170102557 and Future Fellowship Project FT160100252.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Microstructure and tensile properties of magnesium nanocomposites fabricated using magnesium chips and carbon black
- The effect of K2SiF6 on the MgH2 hydrogen storage properties
- Influenc of graphene oxide(GO)on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting
- The creep behavior of Mg-9Al-1Si-1SiC composite at elevated temperature
- Constitutive modeling of f ow behavior and processing maps of Mg-8.1 Gd-4.5Y-0.3Zr alloy
- Fabrication of high-strength duplex nanoporous Cu by dealloying a dual-phase Mg-Cu precursor alloy
