Predicting and controlling interfacial microstructure of magnesium/aluminum bimetallic structures for improved interfacial bonding
2020-12-18ZingZhuRenhiShiAndrewKlrnerAlnLuoYiqingChen
Zing Zhu,Renhi Shi,Andrew D.Klrner,d,Aln A.Luo,c,∗,Yiqing Chen
a Materials Science and Engineering,The Ohio State University,Columbus,OH,USA bMaterials Science and Engineering,Hefei University of Technology,Hefei,Anhui,China
c Integrated Systems Engineering,The Ohio State University,Columbus,OH,USA
d now at M Cubed Technologies,Newark,DE,USA
Received 12 January 2020;received in revised form 29 February 2020;accepted 9 March 2020 Available online 27 May 2020
Abstract In this study,an overcasting process followed by a low-temperature(200°C)annealing schedule has been developed to bond magnesium to aluminum alloys.ProCAST software was used to optimize the process parameters during the overcasting process which lead to Mg/Al bimetallic structures to be successfully produced without formation of Mg-Al intermetallic phases.Detailed microstructure evolution during annealing,including the formation and growth of Al-Mg interdiffusion layer and intermetallic phases(Al12Mg17 and Al3Mg2),was experimentally observed for the frst time with direct evidence,and predicted using Calculation of Phase Diagrams(CALPHAD)modeling.Maximum interfacial strength was achieved when the interdiffusion layer formed at the Mg/Al interface reached a maximum thickness the without formation of brittle intermetallic compounds.The precise diffusion modeling of the Mg/Al interface provides an efficien way to optimize and control the interfacial microstructure of Mg/Al bimetallic structures for improved interfacial bonding.© 2020 Published by Elsevier B.V.on behalf of Chongqing University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Bimetallic structure;Interfacial bonding;CALPHAD and diffusion modeling;Aluminum alloys;Magnesium alloys.
1.Introduction
Lightweight aluminum(Al)and magnesium(Mg)alloys are increasingly used in transportation,electronics and energy industries due to their excellent specifi properties,which offer significan mass savings compared with iron and steel components[1,2].However,each metal has its own unique properties;Mg has a high damping capacity and good castability,while Al has high stiffness and good creep resistance.Typically,a single metal cannot simultaneously meet the complex performance and low cost/mass requirements in modern industry.Bimetallic structures combining Al and Mg alloys often provide better solutions than a monolithic alloy[3].
There are two main types of methods presently used to join Al and Mg alloys,but with limited success.One is fusion welding(such as arc welding and laser welding),which is highly efficien and can be applied to complex shaped parts[4,5].However,the formation of a thick layer of brittle Al-Mg intermetallic compounds(IMCs)such as Al3Mg2and Al12Mg17at the interface is a challenge for Mg/Al welding systems due to its detrimental effect on the interfacial properties.The other process for Mg/Al joining is solid-state welding(such as friction stir welding,diffusion bonding,accumulative roll bonding(ARB),and explosion cladding),which has a relatively thin IMC layer but low efficien y and limited applications[6-8].
Overcasting is a key enabling technology that joins dissimilar metals by casting molten Al and Mg alloys onto dissimilar metal substrates(steel,Al or Mg).This can be achieved by a conventional casting process[9,10],such as gravity casting or high pressure die casting(HPDC)[11,12]which is a highefficien y manufacturing process capable of producing complex and thin-walled Al and Mg components.The“shrink-fit between the cast layer and the substrate,due to the large solidificatio shrinkage during overcasting,results in a good mechanical bond,but lack a metallurgical bond[10].For the Mg/Al overcasting system,the aluminum substrates can be treated by“electropolishing+anodizing”process[13,14]to remove the natural oxide fil[15]on Al surfaces and promote wetting between molten Mg and Al subtracts,promising strong metallurgical bond in the interface.
In the last two decades,the design of new materials and development of manufacturing processes have gradually transitioned from the traditional“trial-and-error”methodology to computation-based approaches[16-19].CALPHAD(Calculation of Phase Diagrams)modeling[18-20],as part of Integrated Computational Materials Engineering(ICME)framework[21],has been used to understand the thermodynamic phase equilibria and kinetic atomic diffusion in materials design and process development for a wide range of structural and functional systems.
In this study,a novel“overcasting+annealing”process has been developed to bond magnesium AM50(Mg-5wt.%Al-0.4wt.%Mn)and aluminum 6061(Al-1wt.%Mg-0.6wt.%Si-0.3wt.%Cu)alloys.Using the optimal process conditions obtained from the HPDC overcasting process simulation,molten AM50 Mg alloy was firs cast around 6061 Al alloy rings.In an annealing process following overcasting,the AM50/6061 bimetallic structures were heated to a low temperature(200°C in this study)to control the diffusion layer while avoiding the formation of intermetallic phases.This process combines the advantages of HPDC process(low cost,high efficien y and the ability of producing complex shapes)and diffusion bonding(controllable IMC layer thickness)to improve the mechanical properties of Mg/Al bimetallic structures.Diffusion modeling using CALPHAD(Calculation of Phase Diagrams)software and an open-source Python library code,named“pydiffusion”[22]were used to predict the formation and growth of Mg-Al diffusion layer and intermetallic phases.Lastly,the simulations results were validated by experiments.
2.Experimental procedures and simulation methods
2.1.Die design and HPDC process parameters optimization
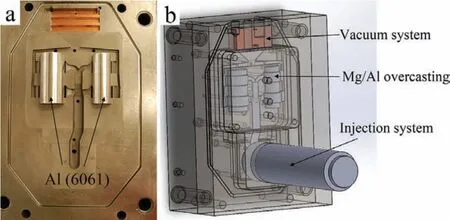
Fig.1.Photographs of(a)HPDC die;and(b)Schematic of a vacuum-assisted HPDC system for Mg/Al overcasting experiments.
Commercial magnesium AM50 alloy(high-energy absorption,high elongation,and good castability)and aluminum 6061 alloy(good weldability,corrosion resistance,and low cost)were used in this study.Fig.1(a)shows a HPDC die designed to produce overcasting samples.Prior to casting,two 3.3 mm thick and 99 mm long 6061 Al tube inserts with diameters of 38.1 mm and 50.8 mm were put into the die and preheated.Fig.1(b)shows a schematic of a vacuumassisted HPDC system for producing Mg/Al overcasting samples.First,AM50 alloy was ladled into the injection chamber.After the plunger moved past the pouring hole and sealed off the die cavity,the vacuum valve was activated and a lower than atmospheric pressure was created in the die cavity.The cavity was evacuated continuously from the beginning of die fillin to the end.ProCAST is a finit element method(FEM)-based casting simulation package that can be used to predict the f ow field temperature fiel and various defects during casting,which can heighten productivity and make more economical benefit[23].In this study,ProCAST was used to optimize the process parameters(such as gate velocity,die and insert preheating temperatures and metal temperature)before conducting HPDC experiments.Based on these simulation results,Mg/Al overcasting samples were produced using a vacuum-assisted 250-ton Buhler HPDC machine at The Ohio State University.A Mg/Al overcasting sample was then annealed at 430°C for 4 hours for diffusion bonding,based on diffusion simulation results.Samples in as-cast and annealed were sectioned and polished to examine the cross-sectional interface microstructure using optical microscopy(OM).
2.2.Diffusion behavior in low temperature annealing
Too thick intermetallic layers usually have a detrimental effect on the mechanical properties of bimetallic joints[9],thus most research has focused on reducing IMC formation during processing at a temperature range of 350-475°C[24-26],so the reaction rate between Al and Mg is generally high and thick IMC layers can form even with very short time.Since diffusion coefficient are exponentially increased with temperature,high-temperature exposure of Al-Mg bimetallic samples would cause fast formation and high growth rate of IMC at the Al/Mg interface[27].Therefore,a significantl lower temperature of 200°C was selected in this study to provide better-controlled diffusion experiments to suppress/reduce the formation of IMCs during annealing.
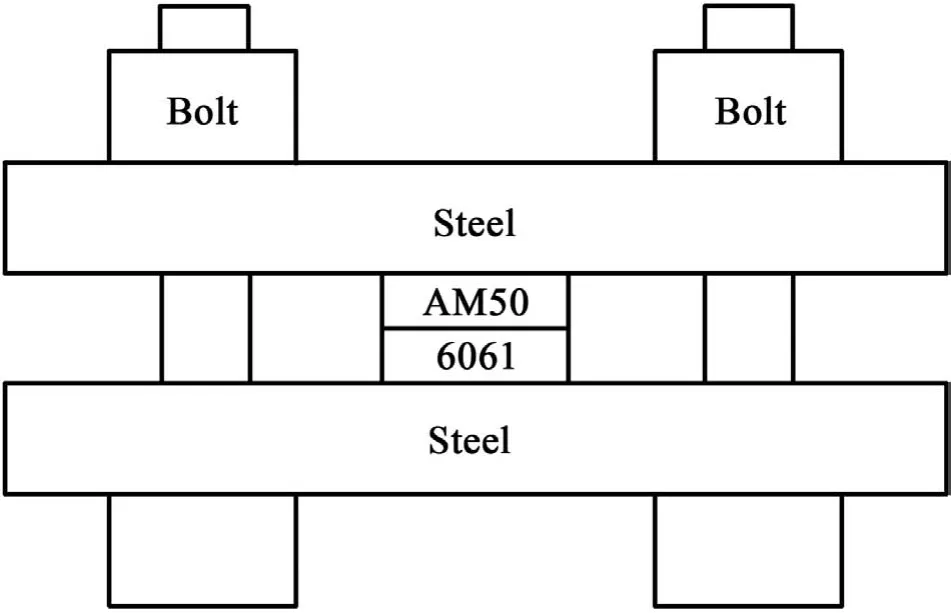
Fig.2.A schematic assembly of the AM50/6061 diffusion couple.
In order to explore the interdiffusion and the growth of Mg-Al IMC in the Al and Mg interface at 200°C,a Mg/Al diffusion experiment was performed.Both 6061 and AM50 alloy sample blocks were prepared to the dimensions of 10×10×3 mm using electrical discharge machining.The sample surfaces were ground and polished starting with 180-grit SiC paper and finishin with 0.25-μm colloidal silica.Diffusion couples of 6061(Al)vs.AM50(Mg)were assembled in a steel jig consisting of two steel plates and four steel bolts,as shown in Fig.2.During the diffusion experiments,clamping was maintained to ensure good contact between the Al and Mg alloy blocks.Each diffusion couple assembly was placed in a furnace and annealed at 200°C for different durations.After annealing,the diffusion couples were quenched in water to retain their microstructure and compositional profiles The annealed diffusion couples were sectioned parallel to the direction of diffusion and metallographically prepared for scanning electron microscopy(SEM)observations.The interfacial microstructure and compositions of the diffusion couples were characterized using a FEI Apreo LoVac SEM equipped with an energy dispersive spectroscopy(EDS)system at the accelerating voltages of 5-10 kV and the beam currents 13-26 nA.
2.3.Prediction of diffusion layer and intermetallic phases
It is important to predict and control the formation of the Mg-Al diffusion layer and intermetallic phases because they are generally the“weakest link”and determine the strength of Al-Mg bimetallic structures.However,currently there is no precise and effective method to estimate the growth of intermetallic phases.In this study,CALPHAD(Calculation of Phase Diagrams)and diffusion modeling was used to simulate the diffusion process of Al and Mg atoms during annealing,which can help to clarify the formation mechanisms of intermetallic phases and provide critical guidance in determining the anneal temperature and time for optimal interfacial strength.
2.4.Shear testing
Fig.3 shows a schematic of the shear strength test setup for bimetallic samples,which uses a MTS EM test system.The samples were put on a fla supporting surface and the magnesium ring was pushed on by a steel cylindrical ring punch,concentric with the sample,at a cross-head displacement rate of 0.2 mm min−1.Then the shear strength of the interface with different thickness of diffusion layer or intermetallic phases layer were compared.

Fig.3.Test setup of Mg/Al bimetallic samples.
3.Results and discussion
3.1.Overcasting process simulation
Various combinations of HPDC process parameters were tested in overcasting simulation using ProCAST software.It was determined that an optimal set of parameters included a gate velocity of 40 m/s,die preheating temperature of 100°C,Al tube insert preheating temperature of 250°C,and molten Mg temperature of 690°C.In this process condition,Mg melt temperature was kept at the low end of desirable casting temperatures to reduce the possibility of reactions in Mg-Al interface while ensuring a good quality of Mg/Al overcasting samples.Fig.4(a)shows the liquid metal flui velocity during the die filling Under the high speed and high pressure,the time of f lling process is only 0.185 s.In this condition,the casting can be fille very well.Fig.4(b)shows the distribution of solid fraction during solidificatio process.The solid fraction is consistent across different Mg rings.The solidifi cation sequence indicates that there is sufficien melt in the gate to compensate when the rings are solidified thus helping form a qualifie bimetallic product.
3.2.Interfacial microstructure of Mg/Al samples

Fig.4.Overcasting process simulation using ProCAST:(a)metal flui velocity during die filling and(b)percent fraction of solid during solidifica ion.

Fig.5.Photographs of(a)Mg/Al overcasting produced via vacuum-assisted HPDC;(b)cross-sectional interface of Mg/Al overcasting ring;(c)and(d)optical micrographs of as-cast interface and the interface after annealed at 430°C for 4 hours.
The Mg/Al overcast samples were produced using a vacuum-assisted HPDC system using the optimal process conditions described in the last section,shown in Fig.5(a).The AM50 casting was fille completely,which is consistent with the simulation results.Fig.5(b)shows a cross-sectional interface photograph of two Mg/Al overcast rings,with Mg casting thicknesses of 1.5 mm and 3 mm formed on 6061 tubes.The AM50 alloy was evenly cast on the 6061 tubes surfaces without any detectable macro defects.Fig.5(c)shows that the Mg and Al alloys created a clean interface,but no IMC layer was found at the interface in as-cast conditions due to the fast solidificatio of molten Mg during the HPDC process.The nearly perfect fi found between Mg and Al is also called“shrink-fit which is attributed to the larger solidifica tion shrinkage of Mg compared to the solid-state shrinkage of Al substrate[9].Fig.5(d)shows that a continuous IMC layer of about 105μm thick formed at the interface of an overcast sample after being annealed at 430°C for 4 h.These results indicate that using this novel“overcasting+annealing”process can avoid the formation of excessively thick intermetallic layers in traditional bimetallic compound process while offering the possibility of precise control of the formation and growth of interdiffusion layer or intermetallic layer in the annealing process.
3.3.Process of intermetallic growth
The backscatter electron(BSE)SEM images in Fig.6(a)-(e)show the interfacial microstructure evolution during annealing of the diffusion couples at 200°C for 3,4,12,61 and 235 hours,respectively.Fig.6(f)shows the EDS results of element line scanning at the cross-sectional interface of each Al-Mg diffusion couple,which indicate the change of Al concentrations with diffusion time.For the diffusion couple annealed at 200°C for 3 hours,there is essentially no intermetallic phase formation observed at the interface,shown in Fig.6(a).However,the Al solute concentrations at 1-3 hours in Fig.6(f)has an obvious transition,suggesting that diffusion has occurred at the interface,as Al solute atoms gradually diffused into the Mg side while Mg solute atoms diffused into the Al side.Thus,an interdiffusion layer has grown to about 1.5μm into each side,forming a 3μm region of Al-Mg solute enrichment.
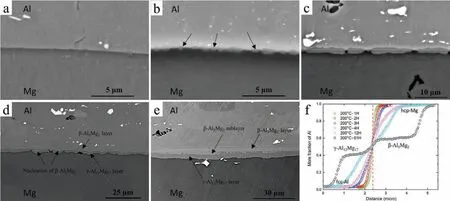
Fig.6.Backscatter electron(BSE)SEM images of 6061/AM50 diffusion couple interface annealed at 200°C for(a)3 hours,(b)4 hours,(c)12 hours,(d)61 hours and(e)235 hours,respectively;and(f)mole fraction of Al at the diffusion couple interface annealed at 200°C for 1-61 hours.
After annealing at 200°C for 4 hours,many small ovalshaped particles appeared at the interface,as shown by the black arrows in Fig.6(b).The thickness of particles measured perpendicular to the interface were 0.22μm to 0.74μm.Apparently,these particles were intermetallic phases formed from the Al and Mg block substrates from the enrichment of Al and Mg atoms due to diffusion.Then these particles gradually grew along the interface and eventually formed two continuous layers,as shown in Fig.6(c)for 12 hours and(d)for 61 hours.The thickness of these layers was 1.9μm at 12 hours and 4.2μm at 61 hours.Based on the EDX results in Fig.6(f)and Al-Mg equilibrium phase diagram analysis,these two layers were determined as the intermetallic phasesγ-Al12Mg17(near the Mg)andβ-Al3Mg2(near the Al).Therefore,the particles observed in Fig.6(b)were actually an initial stage ofγ-Al12Mg17andβ-Al3Mg2phase nucleation and growth.With the increase of the annealing time from 12 to 61 hours,it can be observed from Fig.6(d)that someβ-Al3Mg2particles formed at the interface and appeared in theγ-Al12Mg17side,suggesting thatβ-Al3Mg2phase might have nucleated in theγ-Al12Mg17layer.After a long annealing time of 235 hours,a sublayer ofβ-Al3Mg2phase eventually formed in theγ-Al12Mg17layer between the initialβ-andγ-phases,as shown in Fig.6(e).The thicknesses ofγ-Al12Mg17(3.11μm)andβ-Al3Mg2(2.87μm)layers as well as theβ-Al3Mg2sublayer(1.83μm)are greater than the original ones(1.65μm,1.78μm and 0.76μm,respectively),which means the growth mechanism ofβ-Al3Mg2is simultaneously transformedγ-Al12Mg17and fcc-Al intoβ-Al3Mg2.This is consistent with a previous report[28]that theβ-Al3Mg2phase has a higher growth rate.
3.4.Diffusion simulation results
In this study,the diffusional behavior of Al and Mg atoms was simulated via atomic diffusion mobility(Mi)[29]in DICTRA software[30].The atomic diffusion mobility database in Mg-Al binary system used in this work was taken from our recent work[31].Assuming the mono-vacancy mechanism for diffusion and neglecting the correlation factor,the tracer diffusion coefficienD∗iwas related to the diffusion mobility by the Einstein relation:D∗i=RT Mi.For a diffusion couple,the partial molar volume of each component was assumed to be a constant.The interdiffusion coefficien ˜Dnpqwas related to the flu of elementpto the concentration gradient of elementq,while elementnis the dependent element.The interdiffusion coefficien also can be derived by:where Kronecker deltaδip=1 wheni=pandδip=0 otherwise.In the present low-temperature annealing simulation,an Al/Mg diffusion couple similar to the experimental diffusion couple was constructed in DICTRA software and shown in Fig.7(a).The simulated concentration profile at 200°C for 1-3 hours are shown in Fig.7(b)and agree well with experimental results,indicating no formation of IMC layer at 200°C for 1-3 hours and only the interdiffusion layer measuring about 1.5μm exists at each side of interface.
After the formation of intermetallic phases at the Al/Mg interface,pydiffusion[22]was used to predict diffusion length and growth of Al-Mg intermetallic phases.pydiffusion is a powerful method for analyzing various types of diffusion,such as solid-solid and solid-liquid diffusion couples[32].The key features of pydiffusion include extraction of diffusion coefficient from diffusion couple experiments using forward simulation analysis(FSA)[22]and then fast simulation of multiphase diffusion.In order to determine the effectiveness of this method at different temperatures,the simulations and experiments of Mg/Al diffusion were performed at three temperatures,i.e.200°C,300°C and 400°C.First,three AM50/6061 diffusion couples were assembled and annealed at 200°C for 235 hours,300°C for 159 hours and 400°C for 100 hours,respectively.Fig.6(e),Fig.8(a)and(b)show the backscatter electron(BSE)SEM images for them,respectively.Based on the EDX results in Figs.8(e)and(f)and Al-Mg equilibrium phase diagram analysis,intermetallic phasesγ-Al12Mg17(near the Mg)andβ-Al3Mg2(near the Al)were determined in Fig.8(a)and(b).In addition,a thinε-Al30Mg23layer betweenγ-Al12Mg17andβ-Al3Mg2layers is also determined in Fig.8(a).It was observed that theε-Al30Mg23phase was present in the 300°C sample which was consistent with a prior report[33].Then the profile of Al concentrations were measured by EDS element line scanning and imported into the pydiffusion.The interdiffusion coefficient of all phases extracted by performing FSA in pydiffusion are shown in Fig.8(c)and compared with available literature data[27,34].The results for hcp-Mg,γ-Al12Mg17,β-Al3Mg2and fcc-Al calculated in this study are in general agreement with literature.The differences in hcp-Mg and fcc-Al may be due to the diffusion couples used in this study being AM60(95%Mg)/6061(99%Al)instead of pure Mg and Al.The extracted interdiffusion coefficient were then used to simulate the diffusion process for all experiments.The simulation results in Fig.8(d)-(f)show an excellent agreement with the experimental data on both diffusion profile and IMC thicknesses.These results validated that the simulation method used in this study can quickly predict the diffusion length and IMC phase growth of intermetallics for Al/Mg bimetallic structures at typical annealing temperature and times.
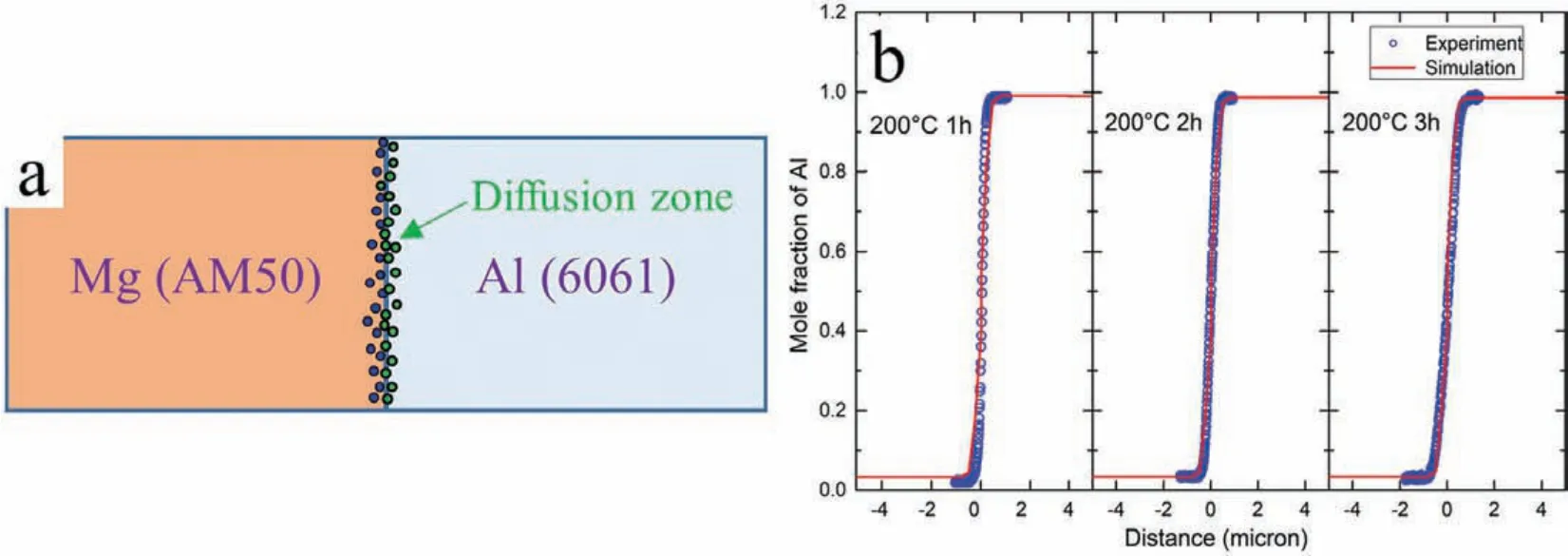
Fig.7.(a)model of diffusion couple in DICTRA;and(b)concentration profile at 200°C for 1-3 hours.
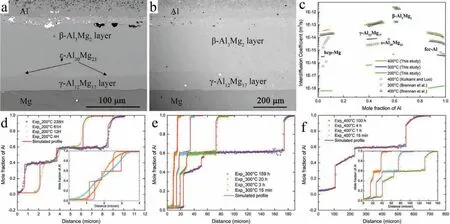
Fig.8.Backscatter electron(BSE)SEM images of 6061/AM50 diffusion couple interface annealed at(a)300°C for 159 hours and(b)400°C for 100 hours,respectively;(c)interdiffusion coeff cients as a function of the Al concentration for hcp-Mg,γ-Al12Mg17,ε-Al30Mg23,β-Al3Mg2 and fcc-Al phases obtained from this study in comparison with the results of Kulkarni and Luo[34]and Brennan et al.[27].Experimental and stimulated concentration profile at(d)200°C;(e)300°C;and(f)400°C for longer annealing time.
Fig.9 shows the measured layer thickness is plotted against the square root of time(hours1/2)for bothγ-Al12Mg17andβ-Al3Mg2phases for the three temperatures.The parabolic increasing layer thickness for both intermetallic phases suggests diffusion-controlled growth mechanisms.Both the calculated and measured thicknesses of the IMC layer were∼23μm after 15 mins at 400°C and∼6μm after 15 mins at 300°C,which are thicker than the 0.7μm IMC layer after 4hrs at 200°C.We can conclude that the solute enrichment period is very short at 300°C and 400°C,suggesting the IMC layer is unavoidable in these high temperature conditions.Thus,a low annealing temperature of 200°C is a better choice for diffusion bonding in Mg/Al dissimilar joining to avoid the formation of IMC layers.
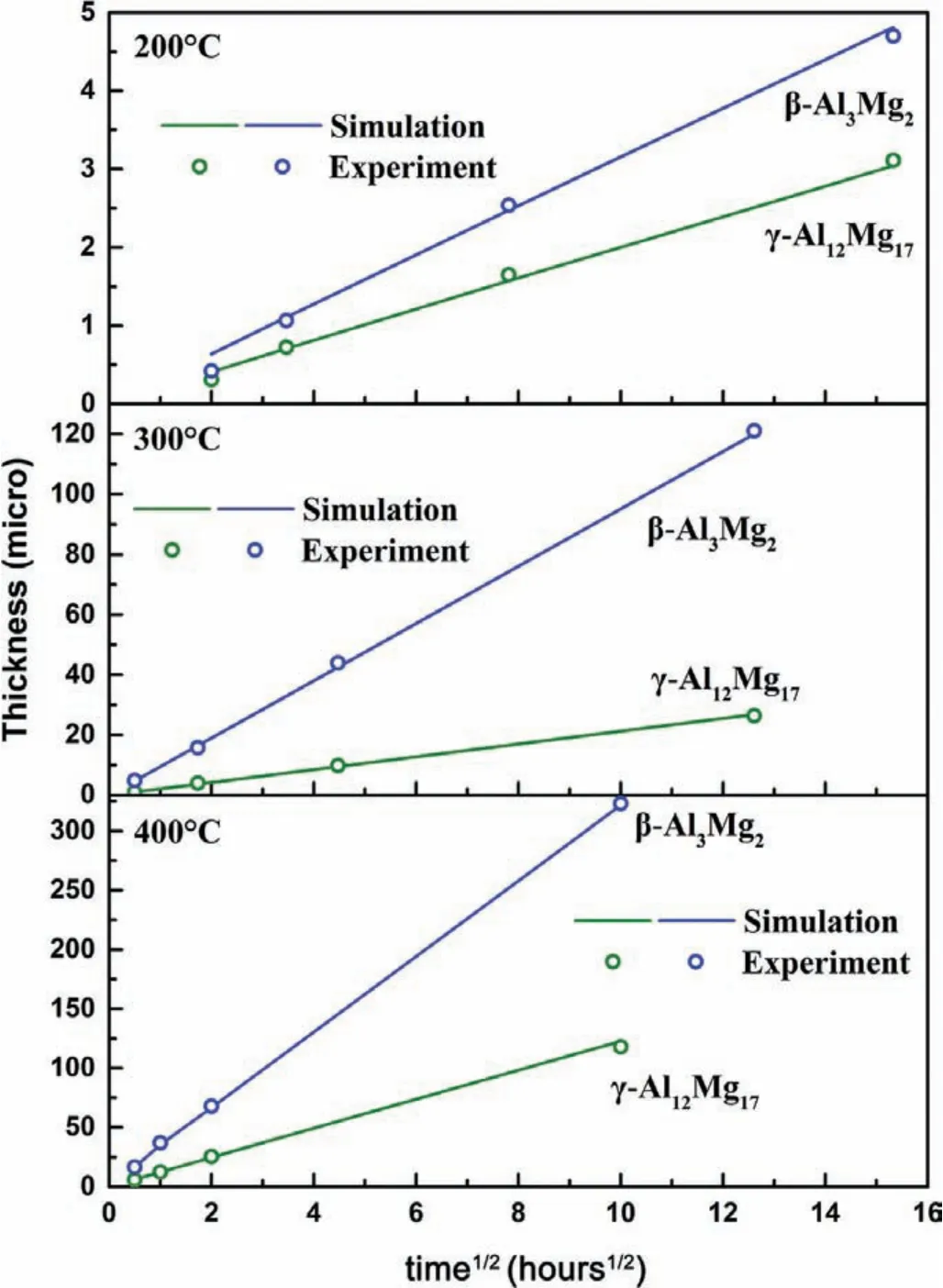
Fig.9.Simulated layer thickness of IMC vs.diffusion time compared with experimental data.
3.5.Interfacial microstructure and strength
Fig.10 illustrates the overall interfacial microstructure evolution process of Al-Mg interdiffusion layer and intermetallic phase(γ-Al12Mg17andβ-Al3Mg2)formation during lowtemperature annealing and the corresponding shear strengths of the bimetallic samples.Fig.10(a)shows graduate enrichments of Mg atoms in Al and Al atoms in Mg at the interface,also called the“interdiffusion layer”,as a result of Mg-Al interdiffusion.When such enrichments reach critical concentrations,nucleation ofβ-Al3Mg2phase in the Al side andγ-Al12Mg17phase in Mg side will start at the interface simultaneously,and they fi closely with each other(Fig.10(b)).With continuing time,γ-Al12Mg17andβ-Al3Mg2particles grow along the interface and gradually thicken into the diffusion layer(Fig.10(c))and eventually they form a continuous IMC layer at the Mg/Al interface.Further continuing,β-Al3Mg2particles nucleate in theγ-Al12Mg17side and grow along the interface(Fig.10(d)).Theseβ-Al3Mg2particles will also thicken toward the Mg side,which is driven by the concentration gradients of Al and Mg and will eventually form aβ-Al3Mg2sublayer(Fig.10(e)).This indicates that the growth ofβ-Al3Mg2is toward both the Mg and Al sides simultaneously achieved by consumingγ-Al12Mg17and fcc-Al,while theγ-Al12Mg17phase grows only toward the Mg side by consuming hcp-Mg.
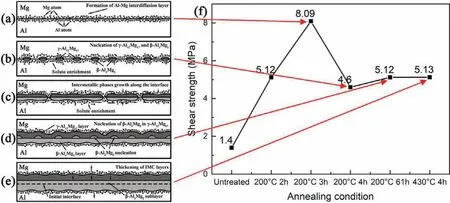
Fig.10.Schematic illustration showing:(a)formation of Al-Mg interdiffusion layer;(b)nucleation ofγ-Al12Mg17 andβ-Al3Mg2 in hcp-Mg and fcc-Al,respectively;(c)growth of intermetallic phases along the interface;(d)nucleation ofβ-Al3Mg2 inγ-Al12Mg17;and(e)formation ofβ-Al3Mg2 sublayer and the thickening of IMC layer;and(f)corresponding interfacial shear strength.
Fig.10(f)shows the shear strength measurements of bimetallic samples for various annealing conditions,corresponding to different stages of Al-Mg interdiffusion layer and intermetallic phase formation.The shear test setup is shown in Fig.3.The as-cast(untreated)sample only had a low strength of 1.4 MPa,while increasing annealing time resulted in significan increases in shear strength due to the interdiffusion of Al and Mg at the Mg/Al interface.The shear strength reached a peak value of 8.09 MPa when the interdiffusion layer is at its maximum at 200°C for 3 hours,after which it started to decrease due to the formation of brittle intermetallic phases.This result confirm that the formation of the interdiffusion layer instead of the intermetallic layer provides a higher joint strength in Al-Mg bimetallic structures.
4.Concluding Remarks
Mg/Al overcast samples without intermetallic phase formation were successfully produced by a vacuum-assisted HPDC system.Process simulation is an effective tool to optimize the process parameters in HPDC process.The low-temperature annealing(200°C in this study)following Mg/Al overcasting process can promote metallurgical bonding by forming a Mg-Al interdiffusion layer at the interface.Further annealing at this temperature will lead to the formation and growth of Al-Mg intermetallic phases(γ-Al12Mg17andβ-Al3Mg2),which should be avoided due to their brittleness and detrimental effect on strength.
The low-temperature annealing also fully revealed detailed microstructure evolution for the frst time with direct evidence and presented the formation and growth of Mg-Al interdiffusion layer and intermetallic phases.This process involves:(1)formation of Al-Mg interdiffusion layer;(2)nucleation ofγ-Al12Mg17in hcp-Mg andβ-Al3Mg2in fcc-Al;(3)growth of intermetallic phases along the interface;(4)nucleation ofβ-Al3Mg2inγ-Al12Mg17;and(5)formation ofβ-Al3Mg2sublayer and the thickening of intermetallic phases layer.
This research suggests that precise control of the annealing temperature/time after overcasting can provide optimum interfacial strength by maximizing the interdiffusion layer while minimizing or avoiding the intermetallic phase formation.Such a precise control requires accurate and reliable prediction of Mg-Al diffusion and IMC formation during annealing.CALPHAD modeling using DICTRA and pydiffusion codes proved efficien in predicting the diffusion profile and lengths in Mg/Al diffusion couple and bimetallic samples during annealing.Compared with the growth of Al-Mg intermetallic phases in different annealing temperatures(200°C,300°C and 400°C),the intermetallic phases are almost inevitable at 300°C and 400°C due to their high growth rates.The“overcasting+low temperature annealing”process as well as the diffusion simulation method provides an important technology for optimizing and controlling the interfacial microstructure of Mg/Al bimetallic castings for improved interfacial bonding.
Declaration of Competing Interest
None.
Acknowledgements
The authors gratefully acknowledge The Ohio State University(OSU)for supporting this research.Professor J-C Zhao and Dr.Zhangqi Chen of University of Maryland provided the support in using the pydiffusion code.Z.Zhu also expresses his gratitude to the China Scholarship Council for supporting his stay at OSU as a visiting scholar.Y.Chen and Z.Zhu are grateful to the support from the National Natural Science Foundation of China[grant number 51571080].
杂志排行
Journal of Magnesium and Alloys的其它文章
- Microstructure and tensile properties of magnesium nanocomposites fabricated using magnesium chips and carbon black
- The effect of K2SiF6 on the MgH2 hydrogen storage properties
- Influenc of graphene oxide(GO)on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting
- The creep behavior of Mg-9Al-1Si-1SiC composite at elevated temperature
- HA coating on Mg alloys for biomedical applications:A review
- Constitutive modeling of f ow behavior and processing maps of Mg-8.1 Gd-4.5Y-0.3Zr alloy
