Challenge to ABO blood type barrier in living donor liver transplantation
2020-09-21HirotoEgawa
Hiroto Egawa
Department of Gastroenterology, Tokyo Women's Medical University, Tokyo, Japan
Keywords:
ABSTRACT
Introduction
Professor Alexandre was a pioneer challenging the ABO blood type barrier in kidney transplantation [1].Afterwards some pioneers challenged this barrier in liver transplantation, due to miserable outcomes.They however stopped their effort s and gave up [2].In Japan where living donor liver transplantation (LDLT)was started in 1996, many technical innovations were accomplished.The Kyoto group challenged ABO blood type barrier in children presenting liver failure who could only be treated using blood type incompatible living donors.The initial experience in ABO incompatible (ABO-I) LDLT for younger children was progressively expanded to aged recipients.Initial strategies to overcome the ABO blood barrier were apheresis, splenectomy and overimmunosuppression (O-IS).These experiences revealed a profound impact of age on incidence and severity of antibody mediated rejection (AMR) [3].Even children aged 8 years or older developed,similarly to adult liver recipients, fatal AMR leading to massive hepatic necrosis or extensive intrahepatic biliary tract destruction and sclerosis (Fig.1) [3].Only two out of 10 patients who underwent ABO-I LDLT until 20 0 0 at Kyoto University survived.The other causes of death were AMR or severe infection secondary to O-IS.The Keio University group reported the beneficial effect of portal infusion treatment in ABO-I LDLT in their clinical works and the survival rate improved from 20% to 60% in Japan [4].Based on this favorable experience the Kyoto group next developed the arterial infusion therapy [5].The following step consisted of the Foundation Japanese Transplant Surgeons of the Society for ABOI kidney and liver transplantation which led to multicenter studies looking at the effect of rituximab in the context of living transplantations.The liver team learned a lot from their renal counterparts;all these experiences led to the implementation of a Japanese protocol for the use of rituximab for LDLT [6].Thereafter several minor upgrades were made, and based on the reported results and successes, this Japanese protocol rapidly found its way in all parts of the world.The obtained success rate after LDLT became equal to successes obtained after ABO compatible transplantation [7].The most important contribution of this Japanese effort was that it allowed to increase the chances for LDLT by 10% -30% in countries where postmortem donors are a scarce allograft source [ 8 , 9 ].Moreover, this protocol also has an economic impact (in Japan) as thecosts related to the use of rituximab is half of one session of plasm apheresis and one fifth of intravenous immune globulin treatment.

Fig.2.Location of blood type antigen.Blood type A antigen was expressed on the surface of red blood cell (RBC), the epithelium of the bile duct, and the endothelium of the portal vein.
In this review, we addressed the pathophysiologic and immunologic consequences of ABO-I related AMR, the optimal use of rituximab as well as the still existing limits of current protocol.Future challenges of ABO-I LT were also addressed briefly.
Mechanism of ABO-I related AMR
ABO blood type antigens are expressed on the surface of red blood cells, biliary, and vascular epithelia (Fig.2).Antibodies bind to antigens on the surface of the vascular epithelium and complement binds to Fc-r receptors leading to activation of complement cascade.The resulting inflammatory reaction destroys the capillary epithelium and the development of small thrombi will impair the blood circulation (Fig.3).When these circulatory disturbances are limited only to small arteries, intrahepatic biliary destruction and sclerosis will develop; when circulatory disturbances are massive,hepatic necrosis will develop.
Pathology of ABO-I related AMR
Haga et al.reported portal edema and necrosis (PEN) as a significant early finding of ABO-I related AMR [10]as well as the usefulness to look at C4d deposition in ABO-I related AMR in LDLT [11].Fig.4 A shows PEN as a significant finding of an early sign of ABO-I related AMR, and Fig.4 B shows C4d deposition on the endothelium of the portal vein and connective tissues in ABO-I related AMR in LDLT.
Inflammation of the small artery in a liver graft with intrahepatic biliary sclerosis is shown in Fig.5.
Role of B cell in ABO-I related AMR
In early experience of Kyoto, we noticed that peripheral B cell counts before transplant as well as hemagglutinin antibody titers before transplantation might be related to AMR.We performed ABO-I LDLT for a primary biliary cholangitis patient without rituximab desensitization because both B cell count and titer were very low.However, she developed fever and mental disorder on postoperative day (POD) 10 accompanied by increase of antibody titer and developed fatal AMR despite eventual use of rituximab and plasma pheresis (Fig.6) [12].This patient was the first case in whom B cell repertoire was examined.B cells began to increase 6 h after reperfusion and rituximab was administrated at POD 8 because the frequency of B cell increased to 50%.Interestingly her memory B cells were also increased.Although rituximab decreased dramatically both B and memory B cells, the antibody titer increased again thereafter.Once the memory B cells expanded, rituximab was unable to control the antibody production.Following this case rituximab was not skipped anymore even in case of low antibody titers before transplantation.This approach had the inherent disadvantage that changes in the B cell repertoire could not be monitored because B cells were already depleted in all cases due to the rituximab desensitization protocol.Rituximab would suppress not only pre-sensitized B cell clones lineage and plasma cells, but also the process of antigen recognition and the development ofdenovoantibody production by the host [13].
Best protocol
Our actually applied standard protocol is shown in Fig.7 [7].Rituximab with a dose of 375 mg/m2is administrated two weeks before transplantation and when B cell depletion is insufficient(more than 1% cells) additional administration is considered based on the patient's condition.The number of CD19 positive mononuclear cells is used to assess the B cell population instead of CD20.Tacrolimus (TAC) and mycophenolate mofetil (MMF) are started seven days before transplantation as TAC is known to deplete B1 cell proliferation in mice experiments [14].The trough TAC level is adjusted around 5 ng/mL.MMF is initiated from 500 mg/day.When tolerated, MMF will be increased to 10 0 0 mg/day.Two or three sessions of plasma pheresis are applied immediately beforetransplantation only for patients with antibody titer greater than 256 or 512.

Fig.3.Mechanisms of antibody mediated rejection.ABO blood type antigens are expressed on the surface of the red blood cells, the biliary epithelium, and vessel epithelium(Fig.2).Antibodies bind antigens on the surface of vessel epithelium and complement binds to Fc-r receptors leading to activation of complement cascade.Following inflammatory reaction destroys the capillary epithelium and small thrombus disturbs blood circulation.

Fig.4.Histological findings of AMR in ABO-I LDLT.A: portal edema and necrosis (PEN) as a significant finding of an early sign of ABO-I related AMR; B: C4d deposition on the endothelium of the portal vein and connective tissues in ABO-I related AMR in LDLT.AMR: antibody mediated rejection; ABO-I: ABO incompatible; LDLT: living donor liver transplantation.
Splenectomy and local infusion are avoided in the standard protocol.Induction with anti-T cell or IL-2 receptor antibodies are not standard in liver transplantation.The reason why a small dose of rituximab is administrated in ABO-I kidney transplantation is that such T cell targeted induction treatment is combined with rituximab in ABO-I kidney transplantation [15].In fact, it is not clear which is the minimal safe dose of rituximab to use in combination with T cell targeted induction in liver transplantation.
After transplantation, triple immunosuppression (IS) consisting of steroids, TAC and MMF is applied similarly to ABO-identical transplantation.To prevent infectious complication, it is utmost important to apply a prophylactic anti-fungal treatment using micafungin sodium at a dose of 50-100 mg for 14 days and cytomegalovirus antigenemia or genome monitoring instead of routine anti-cytomegalovirus (CMV) prophylactic treatment.Preemptive CMV treatment is indeed preferable to prophylactic treatment.When tolerated, ganciclovir can be prophylactically taken.One has to be aware of renal injury due to combination of overdose of TAC and prophylactic ganciclovir as well as of the bone marrow suppression due to the combination of MMF and ganciclovir.Dialysis requiring long placement of a central catheter and leukocyte depletion are high risks for infection.Strict management for intravenous lines is important to prevent blood stream infection which may turn into an immune cascade leading to evoking B cell immune reaction [13].Antibiotics are administrated as usual for 3 to 4 days.Further, prophylactic use of intravenous immunoglobulin (5 g) for 4-5 days after transplantation is also recommended.
Modification of timing and amount of rituximab
Although Egawa et al.showed that the timing of rituximab prophylaxis had a more significant impact on the B cell depletion when applied earlier than 7 days before transplantation, this obser-vation could not be confirmed in his following multicenter study neither on the incidence of AMR nor on patient survival [ 7 , 12 ].The main reason to recommend the administration two weeks before LT is that it becomes possible to administer a second dose in case the B cell depletion is unsatisfactory.

Fig.5.Inflammation of the small artery.Inflammation of the small artery in the liver graft with intrahepatic biliary sclerosis.
It has been reported that a small dose of rituximab combined with T cell induction is the preferred protocol in kidney transplantation [15].Generally speaking, liver recipients are more susceptible to infection than kidney recipients.Unfortunately, there has been no evidence about the safety of the combined use of T cell induction and rituximab in liver transplantation.In a small liver recipient cohort, Egawa et al.identified that the reduction of rituximab dose to 200 mg/m2still allows to prevent AMR [16].The discussion about the reduction of the rituximab dose based on the antibody titer needs to be further explored.As there are no solid arguments determining the minimal cutoffdose to use, 375 mg/m2remains the widely accepted dose.There are a 100 mg vial and a 500 mg vial of rituximab.It is felt that the dose could be determined based as follows: 1) calculate 375 mg/m2(for example,when body surface area is 1.1 m2, amount of rituximab is calculated as 1.1 m2×375 mg/m2= 412 mg.You can use 412 mg and discard 88 mg); 2) delete number of tens and ones (for example,412 mg -12 mg = 400 mg); 3) but assure that the dose remains greater than 200 mg/m2(for example, minimal safe amount is 1.1 m2×200 mg/m2= 220 mg.400 mg is greater than 220 mg and this dose is safe).We do not have to discard 88 mg of rituximab.
Necessity of local infusion and splenectomy in the rituximab era
Although local infusion opened the door for ABO-I LDLT [ 4 , 5 ], it is not as powerful as the well-timed rituximab prophylaxis [ 7 , 17 , 18 ].Clinical practice of local infusion is as follows: insert an intraportal catheter during transplantation, and perform continuous intraportal infusion of methylprednisolone(125 mg/day on day 0-7, 50 mg/day on day 8-14, then tapered and discontinued on day 21), prostaglandin E1 (0.01~0.02μg/kg/min on day 0-14), and gabexate mesilate (protease inhibitor; FOY,Ono Pharmaceutical Co., Ltd., Osaka, Japan, 10 0 0 mg/day on day 0-14).The mechanism of local infusion results from preventing microangiopathy through the depletion of inflammatory processes on the capillary endothelium.In the Japanese multicenter series,all 6 ABO-I LDLT for fulminant hepatic failure were treated with rituximab given immediately before transplantation followed by portal infusion and/or splenectomy.All patients survived without AMR, while 12 patients who got local infusion presented a 33%incidence of AMR and 58% of mortality; four patients who did not receive rituximab nor local infusion had a 75% of incidence of AMR and 75% of mortality [7].Local infusion and splenectomy might contribute to prevent AMR in case of an urgent transplantation.Based on the hypothesis that depletion of memory B cells by rituximab could prevent antibody production (as mentioned above), adding rituximab immediately after reperfusion might be helpful to palliate insufficient depletion of antibody production of rituximab administrated immediately before transplantation.In such a situation, splenectomy or local infusion might increase safety.Expanding this protocol in the field of LDLT might be transferable to ABO-I postmortem LT in case a blood type matching between donor and recipient is not available in urgent situations.
Rituximab does not increase hepatocellular carcinoma (HCC)recurrence
One could postulate that O-IS including rituximab could negatively impact on HCC.The Asan and Samsung Medical Centers in Seoul, Korea reported similar results in relation to HCC recurrence and patient survival in the ABO-I and ABO-compatible LDLT patient groups [ 19 , 20 ].In the Japanese series, overall and recurrence free survival rates were also similar between blood type compati-ble and ABO-I patients when using rituximab; the results remained the same when looking at HCC patients within and beyond Milan criteria [21].In conclusion, rituximab prophylaxis does not increase HCC recurrence after LT.
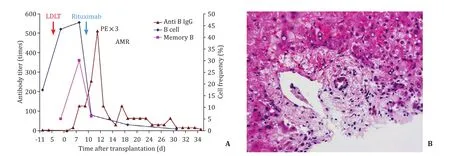
Fig.6.Change of B cell subset in AMR.A: Clinical course and changes of B cell and memory B cell after ABO incompatible LDLT; B: HE stain of liver specimen by needle biopsy showed periportal edema and necrosis accompanied by hepatic artery thrombus.LDLT: living donor liver transplantation; PE: plasma exchange; AMR: antibody mediated rejection; HE: hematoxylin-eosin.
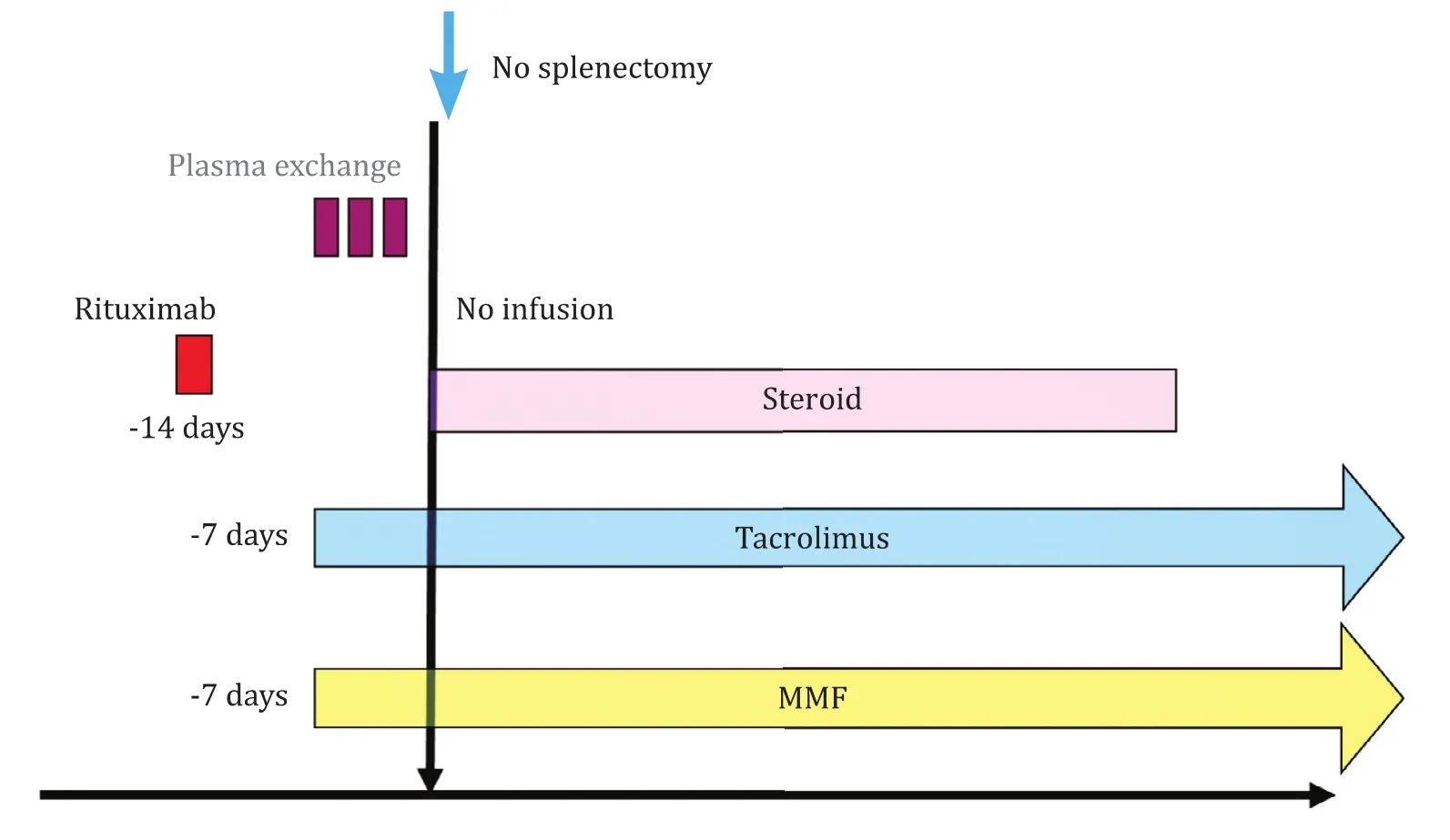
Fig.7.Standard regimen for ABO-I LDLT.The standard regimen consists of preoperative rituximab, tacrolimus, and mycophenolate mofetil (MMF) and triple immunosuppression after transplantation.Splenectomy or local infusion is not performed.Plasma exchange is considered only for cases with high hemagglutinin titers.ABO-I: ABO incompatible; LDLT: living donor liver transplantation.
Pediatric ABO-I LDLT
It was already reported in 1995 that ABO-I liver transplantation was feasible in pediatric cadaveric recipients [22].Egawa et al.showed a significant relationship between patient age and incidence and feature of AMR [3].The lowest age of a patient who developed intrahepatic biliary complications was 11 months and hepatic necrosis was 8 years.The age between 8 and 16 years was the most risky period regarding AMR.In order to avoid AMR, rituximab has to be administrated in patients older than 11 months.The Kumamoto group reported their experience in 11 pediatric patients older than two years who had rituximab prophylaxis [23].Although they did not reduce the dose, two patients developed fatal AMR.One of them, a 4-year-old boy, who had streptococcal peritonitis after rituximab infusion, was presented with very highly increased antibody titer after transplantation.Narumoto et al.reported a case of a child younger than two years who developed intrahepatic biliary complications [24].They speculated that streptococcal infection might have resulted in the reactivation of B cells,triggering the AMR.
There are no clear safety guarantees in the setting of absence of rituximab prophylaxis for patients aged between 11 and 24 months.
A discussion about the mechanisms of graft acceptance is ongoing.The Kyoto group suggested that a donor-specific hyporesponsiveness remains present after ABO-I liver transplantation, particularly in pediatric patients.Long-term persistence of blood antigens has indeed been shown to contribute to this donor-specific hypo-responsiveness [25].Ohdan et al.showed a lack of donor specific antibody production using a human PBMC -NOD/SCID chimera model [26].They identified a lack of antibody production against donor-A antigens of his father's allograft in the blood group-O recipients.
Impact of blood type on AMR
Impact of blood type on AMR is an interesting issue.It is well known in kidney transplantation that blood type A2 has less antigenicity.Nelson.et al.reported that transplantation of A2 or A2B kidneys into B and O patients is clinically equivalent to that of ABO-compatible transplantation when recipients are selected by low pretransplant anti-A titer histories [27].Some case reports were published in liver transplantation [28].There is a difference of frequency of blood type groups and that of A2 is 10% in White,8% in Black, and 0 in Asian people [29].In Japanese multicenter series, there is no statistically significant impact of blood type combination for incidence and clinical features of AMR [3], possibly due to absence of blood type A2.
Limitation and challenge
Even in the rituximab era, around 5% of recipients develop intrahepatic biliary complication, both in the Japanese and Korean experiences [7-9].These cohorts presented some differences in background.In the Japanese series, IS was heterogeneous as was the dose of rituximab, and recent Japanese series showed that an insufficient dose of rituximab contributes to the development of AMR [ 6 , 7 , 16 ].Conversely in Song et al.experience the dose of 300 mg/m2as well as other medications were standardized [9].A possible factor which might have contributed to their 5% incidence of AMR could be the non-use of preoperative administration of TAC and MMF.Indeed, preoperative administration of these drugs might have a potential to further reduce the incidence of AMR.
Although it has been shown numerous times by several teams that rituximab is useful for desensitization, it has no effects once the process of AMR is initiated.Plasma cell depleting agents, such as proteasome inhibitor, bortezomib (BTZ), are approved by the US Food and Drug Administration for the treatment of plasma cell dyscrasia, and may be used in ABO-I LT recipients with caution.BTZ selectively induces apoptosis among plasma cells, decreasing antibody production [30].Recently, again the Kyoto group reported a case who was rescued by two subcutaneous injection of BTZ [31].In their report, interestingly, the early plasma CD19 + /CD20-cells,remained increased after rituximab but were depleted after BTZ administration.Besides BTZ, complement inhibitors could have potential to attenuate AMR.In the field of HLA-related donor specific antibody (DSA), C1 and C5 inhibitors are useful in the prevention and treatment of AMR [ 32 , 33 ].
Challenges to reduce infectious complications
Even with cautious perioperative management, infection cannot be avoided.The Hiroshima group reported that IgG Fc gamma receptor (FcγR) single-nucleotide polymorphisms (SNPs) influence the effect of rituximab on B-cell depletion and are possibly predisposing factors for infectious complications after ABO-I LDLT [34].The affinity of FcγR for rituximab, an anti-CD20 IgG1, differs based on SNPs in FcγRs.They demonstrated that the effects of rituximab on B cells were more profound in FcγR2A [131H/H]than in FcγR 2A [131H/R or R/R]recipients of ABO-I LDLT.The high incidence of infectious complications in FcγR 2A [131H/H]recipients and their poor prognosis could potentially be attributed to this polymorphism.The combination of FcγR2A and FcγR3A SNPs further stratifies the incidence of infectious complications, e.g., FcγR2A[131H/H]and FcγR3A [158F/F or F/V]displayed the highest incidence of infectious complications after ABO-I LDLT.
Egawa et al.also reported that the CD8 repertoire prior to transplantation could predict a susceptibility for infection [35].Patients, in whom the peripheral effector cells are increased, display a high incidence of life-threatening complications.Especially those patients with effector memory dominance have a high incidence of infection.
Taken all knowledge together, it can be noticed that patients requiring such desensitization protocols need a strict peri-operative management to further improve outcomes in ABO-I (liver) transplantation.
Conclusions
Although ABO blood type barrier has been overcome in 95% of liver recipients by the application of effective desensitization using rituximab as the standard drug, issues such as identification of high risk patients for infection and AMR and also secure treatment strategies for evoked AMR need to be resolved.
Acknowledgments
None.
CRediT authorship contribution statement
Hiroto Egawa:Conceptualization, Funding acquisition, Writing -original draft, Writing - review & editing.
Funding
This study was supported by a grant from Japan Agency for Medical Research and Developement (20317617).
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Recent evolution of living donor liver transplantation at Kyoto University: How to achieve a one-year overall survival rate of 99%?
- Endoscopic papillary large balloon dilation with or without sphincterotomy for large bile duct stones removal: Short-term and long-tem outcomes
- Optimizing biliary outcomes in living donor liver transplantation:Evolution towards standardization in a high-volume center
- Hepatic vein in living donor liver transplantation
- Hepatobiliary&Pancreatic Diseases International
- Hepatic artery reconstruction in pediatric liver transplantation:Experience from a single group
