Hepatic vein in living donor liver transplantation
2020-09-21DenizBalciElvanOnurKirimker
Deniz Balci , Elvan Onur Kirimker
Ankara University School of Medicine, Department of Surgery, Ankara Universitesi Tip Fakultesi Ibni Sina Hastanesi Akademik Yerleske K-4 Sıhhiye, Ankara,Turkey
Keywords:
ABSTRACT
Introduction
Living donor liver transplantation (LDLT) is the treatment of choice to overcome graft shortage in patients awaiting for liver transplantation (LT).While LDLT is a viable alternative, it is technically more demanding and requires an in-depth understanding of donor liver anatomy, careful preoperative preparation, as well as meticulous surgery.
Donor liver parenchymal transection must be performed in such way that it results not only in an adequate liver graft and future liver remnant (FLR) volume but also in well preserved graft with healthy vascular and biliary structures.The vasculo-biliary sheaths which were “…fromthesurgicalpointofview,themostimportant structuresofliveranatomy”according to Couinaud [1]were first described by Johannis Walaeus.Francis Glisson published his “Anatomia hepatis”and called these structures as Walaean Sheath [2].During donor surgery, these vascular and biliary structures are to be shared with precision and balance between blood supply (inflow) and hepatic venous (HV) drainage (outflow).Eventually the graft harvest often results in short stumps and multiple hepatic and portal veins, as well as hepatic arteries and bile duct(s).Graft selection in LDLT is a complex decision-making process, taking into account not only recipient metabolic demands and well-preserved inflow and outflow anatomy but also the FLR volume and hence donor safety which clearly has the utmost importance (Fig.1).The latter requires accurate volumetric calculation using a high-quality dedicated software which is a key component of comprehensive surgical planning and assessment in LDLT.
In adult patients, graft size is a significant limitation, and a left lobe graft (LLG), representing usually less than 40% of the total liver volume (TLV), may be insufficient for an adequate donorrecipient size-match.Therefore, right lobe LDLT is a major development in adult LDLT allowing a significant increase of the donor pool by providing larger graft size and decreasing risk of smallfor-size graft syndrome.However, right lobe anatomy is very complex from both inflow and outflow perspective.The right hepatic vein (RHV) enters the inferior vena cava (IVC) separately, while the middle hepatic vein (MHV) and the left hepatic vein (LHV) usually (65%-85%) share a common trunk [3].Therefore, the right lobe graft (RLG)-LDLT is more complex than a whole liver transplant or LLG-LDLT from a venous outflow reconstruction standpoint (Fig.2).
The first two decades of LDLT have been characterized by an overwhelming concern about anatomical variations, technical reconstruction modalities and adequacy of the inflow [4].The relatively high incidence of hepatic artery thrombosis and the fatal course of this complication resulted in a dominating infloworiented approach [5].Although venous complications occur, they tend to be more subtle ultimately leading to acute graft failure which is not always easy to diagnose.LLG that includes MHV usually has a sufficient outflow when a technically sound reconstruction paying attention to the orientation of the anastomosis isperformed.However, the outflow dynamics of RLG, compared to the full-size liver transplant and LLG, can be quite different and complicated due to its complex functional anatomic characteristics harbouring multiple RHVs that need to be taken into account.For the integrity of graft function, venous drainage of the graft is as important as hepatic inflow.From an anatomical point of view, there are numerous anastomoses between RHV, inferior RHV(IRHV) and MHV.They may play an important role after liver resection by taking over the territorial drainage once these veins are ligated [6].Hribernik et al.showed in corrosion cast studies the presence, mainly in segment 8, of small diameter (i.e.1-3 mm) intrahepatic venous collaterals between MHV and RHV.It has been shown that these intrahepatic venous connections can restore venous collateral flow in about one-fourth of cases.These multiple collaterals having a diameter of more than 2 mm in 30% of cases can potentially enlarge in case an outflow block is present [7].Kawaguchi et al.showed that the incidence of liver hypertrophy in left lobe including the MHV was significantly higher in remnant livers with small outflow obstructed regions than those with large outflow obstructed regions.In this study, intersegmental venous communications developed postoperatively in 52 (66.7%) of 78 donors having a positive effect on the remnant liver hypertrophy [8].However, in the LDLT setting, these small collaterals may not develop within a week despite a major venous blockage.Therefore, for optimal graft and recipient outcomes, it is required that all drainage routes connected with significant territories of the RLG should be reconstructed.If not, venous drainage patterns may change dramatically, causing significant congestion with reversed arterial flow in the congested area and hyperdynamic circulation in the posterior section leading to graft dysfunction and liver failure [9].Complex outflow reconstruction thus requires an optimal venous anatomical mapping with 3D reconstruction using a specialized software which enables a better interpretation of the anatomy.Furthermore, preoperative calculation of venous drainage territories with 3D reconstructions is helpful in planning the outflow reconstruction.Therefore outflow reconstruction may even require a more careful preoperative evaluation and planning than selection and reconstruction of the inflow where meticulous technique surpasses creativity in most occasions.
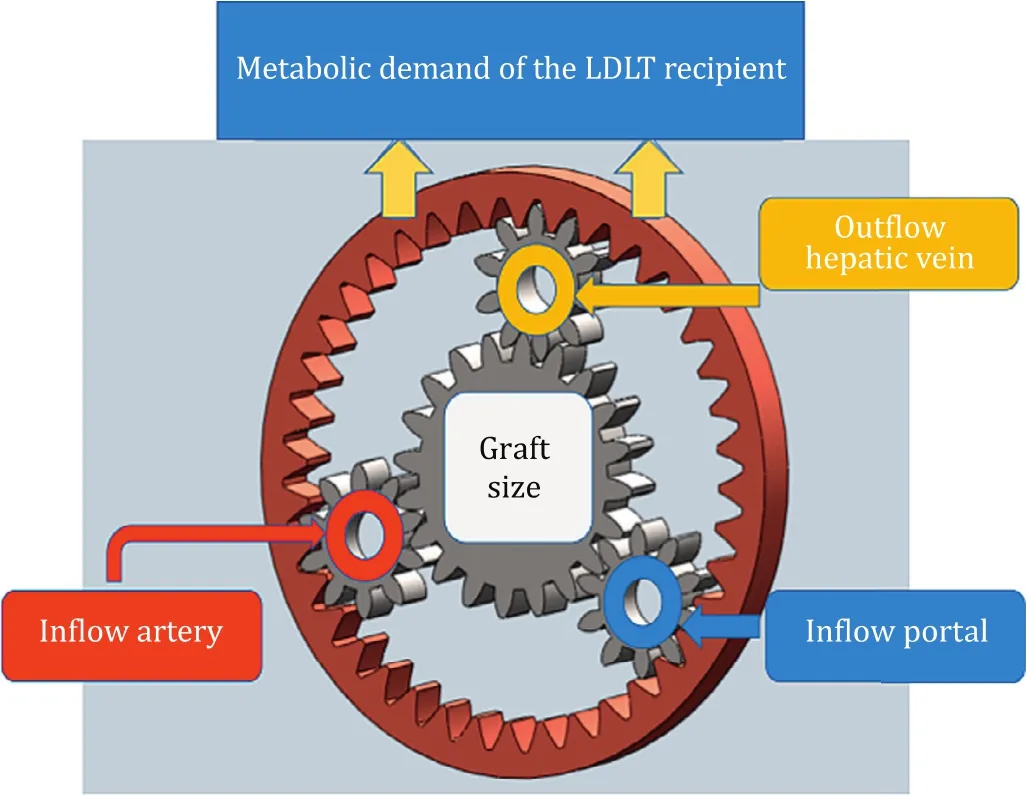
Fig.1.Compound epicyclic gear model to understand complex interactions of graft size, inflow and outflow to meet the metabolic demand of the recipient in LDLT.This graph reflects a complex high efficiency model that explains multiple kinematic combinations of relevant parameters showing interdependency of graft size with inflow and outflow.LDLT: living donor liver transplantation.
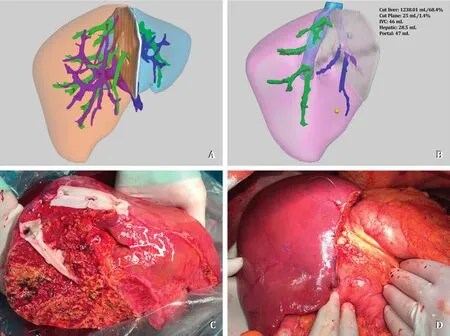
Fig.2.A: 3D software *reconstruction of donor liver anatomy; B: 3D software *reconstruction of the graft demonstrating complex venous outflow with three IRHVs; C :Back-table reconstruction: Segment 5 and 8 veins and RHV were unified with a cadaveric iliac vein graft and three IRHVs were unified with a cadaveric vein graft; D :Congestion-free modified right lobe graft after implantation.RHV: right hepatic vein; IRHV: inferior right hepatic vein; V8: segment 8 vein; V5: segment 5 vein; MHV:middle hepatic vein; LHV: left hepatic vein.IVC: inferior vena cava.*Livervision Ltd., London, UK.
Evolution of outflow reconstruction in LDLT
A historical debate in RLG-LDLT has been the potential anterior section (AS) congestion due to deprivation of segment 5-8 HV (V5-V8) tributaries.Numerous techniques were developed since the early LDLT era when outflow reconstruction of the RLG consisted of an RHV-IVC anastomosis only.It was believed that graft AS congestion could be tolerated with the development of intrahepatic collateral anastomosis.Consecutively, it was documented that when the MHV was not reconstructed, flow signals in the V5-V8 were present only after postoperative day 6 and a viable reverse flow from AS to RHV after 9 days; however, these intrahepatic anastomoses did not develop in every RLG [10].This phenomenon was recognized immediately after arterial reperfusion with a dusky appearance of the anterior section as a consequence of outflow blockage in the graft.This situation would translate into early allograft dysfunction with impaired hemodynamics, elevated liver enzymes and insufficient liver regeneration.
To prevent congestion in the AS, several technical modifications have been developed.The Hong Kong group adopted an extended RLG including the MHV [11].The Toronto group described a case showing reconstruction of the partial MHV using recipient renal vein as a jump graft to the IVC [12].The most significant contribution that expanded the use of RLG with simultaneous increase of the donor safety came from Asan Medical Center group in Korea.The historical paper by Lee et al.[13]first defined the AS congestion problem and also its impact on the graft function.Furthermore, they described the clinical consequences of this phenomenon on the recipient outcomes with the potential solution of using interposition vascular grafts for the AS, namely V5-V8 HV reconstruction termed “modified RLG”[14].
Technical considerations for outflow reconstruction in LDLT
The RLG consists of 2 sections and 3 HV routes for drainage that requires reconstruction in a standardized manner in order to achieve a congestion-free graft [15].The long-term patency of the RHV and IRHV drainage is as important as MHV reconstruction for the integrity of RLG function.Therefore, the standardization of RLG outflow reconstruction has potential advantages of shortening back-table time, reproducibility and teaching and most importantly, reducing technical complications with insufficient outflow in the recipient.For this purpose, depending on the availability, various types of autologous and homologous venous grafts have been used and recommended for this type of venous reconstruction (i.e.internal iliac vein, umbilical vein, internal jugular vein, renal vein, portal vein, aorta and its branches and IVC,etc.) [ 16 , 17 ].Also, the usage of prosthetic grafts [dacron, expandedpolytetrafluoroethylene (e-PTFE), hemashield etc.]has been well described [ 18 , 19 ].Both native and prosthetic grafts have their own advantages, disadvantages and patency rates (Table 1) [18-28].
RLG outflow reconstruction standardization consists of a combination of four scenarios depending on the venous anatomy of the donor liver.Preoperative planning is utmost important in order to have native or prosthetic grafts readily available during surgery:
1.RHV reconstruction: A combination of caudal-side deep incision on the IVC to enlarge the outflow opening and patch plasty of the graft RHV to remove the acute angle between the RHV and IVC is required in most cases.In case of multiple superior RHVs, these can be well unified as well to create a single large opening and anastomosis.An important consideration is to match the relative location of the RHV orifice of the graft and the recipient RHV orifice in the IVC which is determined by the relationship between the depth of the right subphrenic fossa and the size of the graft.Many RHV stenoses were associated with anastomosis caused by inappropriate matching of the sites of insertion of the RHV of the graft and the IVC.Consequently, rapid regeneration of the graft causes ventromedial shifting with potential distortion of the anastomosis [29].
2.MHV reconstruction: Extended RLG grafts with MHV whether partial or complete can be joined to RHV with a native vessel graft for all-in-one anastomosis or can be separately anastomosed to a unified LHV-MHV orifice using a native or prosthetic interposition graft.Although a well-constructed, triangle anastomosis (RHV + MHV-IVC) under total IVC clamping provides excellent outflow in a single anastomosis, separate anastomosis of RHV-IVC and MHV-LHV + MHV orifice have the theoretical advantage of preventing the anastomosis site distortion when graft regeneration occurs.This will ultimately reduce the chance of jeopardizing the outflow of the graft (Fig.3).
3.V5 and V8 reconstruction: Anterior section HV reconstructions,whether single or multiple orifices represent the ultimate outflow design work required at the back-table.It is strongly suggested and now commonly agreed that all drainage veins of segments 5 and 8, larger than 5 mm in diameter should be reconstructed [30].When there is multiple V5 or V8, adjacent tributaries could be united into a single orifice.
Although, widely accepted by transplant centers, this arbitrary measure following Poiseuille's Law tries to predict the flow that is proportional to the diameter of the vein and hence with the corresponding liver parenchymal drainage territory volumes.This could also be tested during donor surgery after artery and the relevant hepatic vein(s) are clamped, resulting with a dusky congestion area in the drainage territory.However, one probably would accept that it would be much more useful to have this information preoperatively, using a dedicated 3D-reconstruction software that could show the congestion area or drainage territorial volumes of each drainage hepatic veins when not reconstructed (Fig.4).This technology gives the edge to the transplant surgeon to design the graft outflow in the preoperative period, not only by taking into account the morphologic characteristics such as the diameter of the veins of the graft, but also having the 3D image of the final reconstruction in mind when scrubbing for the operation and preparing the material required.AS reconstructions, using either native or prosthetic grafts, with a meticulous surgical technique show 80%-100%early (1 month) patency rates which is the critical period for the graft regeneration (Table 1).Long-term patency of the interposition grafts of V5 and V8 drainage is not a major concern given that late graft obstruction usually has limited clinical implications [27].
4.IRHV reconstruction: Posterior section HV drainage requires unification venoplasty for multiple short hepatic veins for a single wide orifice anastomosis to a corresponding orifice on IVC instead of individual anastomosis of these veins.It should be kept in mind, that the orientation of this anastomosis is crucial not only from the outflow blockage perspective but also because the graft when congested could compress on the IVC as well.

Table 1Patency rates for various interposition grafts utilized in right lobe outflow reconstruction.

Fig.3.A: Intraoperative picture showing anterior section congestion of the modified right lobe graft harvested after V5 and V8 division; B: Preoperative 3D software *reconstruction of the graft showing estimated congestion area (dark red); C: Back-table reconstruction with dacron interposition graft for anterior section and unification venoplasty for two IRHVs; D: Implanted modified RLG with dacron interposition graft for anterior section outflow reconstruction.IRHV: inferior right hepatic vein; RLG:right lobe graft; V5: segment 5 vein; V8: segment 8 vein.*Livervision Ltd., London, UK.
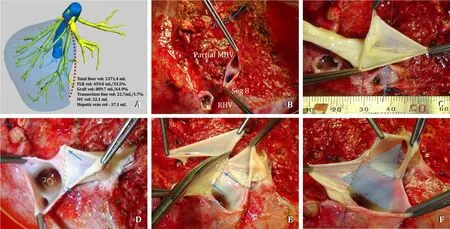
Fig.4.Extended RLG with partial MHV.A: 3D software reconstruction *showing parenchymal transection line (red dotted line) for extended right lobe graft with partial MHV preserving segment 4b vein; B: Unification venoplasty for partial MHV and segment 8 vein orifices; C: Cadaveric iliac vein used as an interposition graft with RHV; D:Caudal incision for blunting RHV-IVC angle; E: RHV patch plasty for enlarged anastomosis (blue arrows); F: Triangle orifice for all-in-one anastomosis between RHV-MHV and recipient IVC.RLG: right lobe graft; MHV: middle hepatic vein; LHV: left hepatic vein; RHV: right hepatic vein; IVC: inferior vena cava.*Livervision Ltd., London, UK.
MHV
Including MHV with the graft (extended RLG, ERLG) has numerous advantages.The MHV may receive more than 15 tributaries from segment 5 and segment 8 of which, most would likely to be severed during parenchymal transection along the course [30].Therefore, an ERLG provides better outflow drainage than a modified RLG.The recipient with severe portal hypertension, high model for end-stage liver disease (MELD) score and with significant metabolic demand i.e.acute-on-chronic liver failure recipient, will greatly benefit from an ERLG with perfect outflow.However, the same graft without appropriate AS drainage will only be sufficient to sustain a lower MELD score patient without significant portal hypertension, a graft-to-recipient weight ratio>1 and, hopefully, receiving a liver from a younger donor.Donor age is a critical surrogate marker both for graft function and donor safety.Donor safety must be the primary drive of donor and graft selection.Potential donors for right-lobe hepatectomy are generally healthy volunteers under the age of 60 years (ideally younger than 50 years).The 60 years of age threshold is critical because patients older than 60 years were shown to harbour occult medical conditions, present less regenerative liver capacity that may lead to more significant postoperative complications [31].The Florence Nightingale group algorithm for including RLG with MHV includes donor age<35 years, steatosis<15%, FLR>30% of TLV, the presence of segment 4b vein and right AS veins<5 mm [32].However,in these series, including the MHV in the RLG with FLR<30% led to an increased morbidity (57%) and a prolonged hospital stay.The recent Korean Transplant Registry analysis of 773 RLG showed that only 5% of grafts were ERLG with MHV [33].It remains a complex decision-making process to harvest an ERLG, despite providing better outflow and immediate graft function.It should be noted that as LDLT experience increases, the percentage of ERLG tends to decrease [ 23 , 27 , 30 ].
Conclusion
Outflow reconstruction is one of the key requirements for a successful LDLT.Over three decades, numerous technical advances have been made to increase donor safety and decrease complications for RLG-LDLT.Finally the inclusion of the MHV with the RLG,procuring an ERLG, produces an optimal outflow drainage however at the expense of an increased donor morbidity.
Acknowledgments
The authors would like to thank Mr.Cumhur Ceken for the 3D reconstructions using LiverVision software and Nicolas Goldaracena for proofreading.
CRediT authorship contribution statement
Deniz Balci :Conceptualization, Writing - original draft,Writing - review & editing.Elvan Onur Kirimker :Writing -original draft, Writing - review & editing.
Funding
None.
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Small-for-size syndrome in liver transplantation: Definition,pathophysiology and management
- Endoscopic papillary large balloon dilation with or without sphincterotomy for large bile duct stones removal: Short-term and long-tem outcomes
- Recent evolution of living donor liver transplantation at Kyoto University: How to achieve a one-year overall survival rate of 99%?
- Optimizing biliary outcomes in living donor liver transplantation:Evolution towards standardization in a high-volume center
- Hepatobiliary&Pancreatic Diseases International
- Hepatic artery reconstruction in pediatric liver transplantation:Experience from a single group
