Synthesis and thermal stability of latent fragrant compounds phenolic acids menthyl esters
2020-07-28LIUTaoHUANGYuanFUChenghuaZHOUFengDURongbin
LIU Tao, HUANG Yuan, FU Chenghua, ZHOU Feng, DU Rongbin
(College of Chemistry and Chemical Engineering, Anqing Normal University, Anqing 246133, Anhui, China)
Abstract: The synthesis and application of menthyl ester have become one of the research hotspots in the field of fine chemicals. Menthyl esters with a light fruit aroma were designed and prepared by the acetylation, esterification, and reduction of menthol with syringic acid and vanillic acid. The total yields of the menthyl esters were 74.8% and 70.2%. The structures were characterized by 1H NMR,13C NMR, MS, and IR techniques. The thermal stability of each of these menthyl esters under an air atmosphere was investigated by thermogravimetric-derivative thermogravimetric-differential scanning calorimetry (TG-DTG-DSC) analysis. The results show that these three menthyl esters have high thermal stability and have potential as new latent fragrant compounds.
Keywords: menthyl esters; phenolic acids; acetylation; esterification; reduction; thermal stability
L-menthol is a cyclic monoterpene with a cool and refreshing taste, analgesic effects, the ability to calm-itching, and the ability to promote infiltration[1,2]. Menthol is commonly used in cigarettes, cosmetics, toothpaste, and medicine. Due to its disadvantages of volatility and poor water solubility, its application is limited, and thus various derivatives[3], such as menthyl acetate, menthyl lactate, monomenthyl succinate, menthyl ferulate[4]and valine menthyl ester[5], have been prepared. The development of these derivatives[6]for use in daily life such as food, tobacco and medical fields are main research targets in this field.
Most of the phenolic acids[7]in plant food have pharmacological activities[8,9]. Vanillic acid and its derivatives[10-12]are known to have antibacterial, anti-inflammatory, and antioxidative effects as well as the ability to inhibit tyrosinase activity, allelopathy, and nerve action in addition to other biological activities. Syringic acid is a naturally occurring phenolic acid that is widely used in the perfume, medicine, and pesticide industries and in organic synthesis[13]. It is used as an antibacterial agent, a sedative and a local anesthetic, and the synthesis and application of its functional derivatives have also attracted substantial attention[14-16].
Combinations of the physical and pharmacological characteristics of syringic acid, vanillic acid, veratric acid and L-menthol, by chemical means, namely, esterification, afforded menthyl esters that were used to evaluate these combinations. They were assessed in terms of their applications in the spice, cosmetics, medical, food and other industries in order to obtain basic data.
1 Experiments
1.1 Materials and instruments
All the starting materials, reagents, and solvents were obtained commercially and were used without further purification. Melting points were determined with an X-4 digital display microscopic melting point apparatus and are uncorrected. Specific optical rotations were determined with an SGW-3 automatic spectropolarimeter. The NMR spectra were recorded on a Bruker AVANCE 500 spectrometer using tetramethylsilane (TMS) as an internal standard. The GC-MS spectra were recorded on an Agilent 7890A/5975C gas chromatograph-mass spectrometer. The IR spectra were recorded on a Nicolet IS50 ATR spectrometer. The thermal stabilities of the synthesized compounds were recorded on a Netzsch STA409PC thermal analyzer.
1.2 Synthesis and characterization
We tried to synthesize vanillic acidmenthyl ester and syringic acid menthyl ester via acetylation, esterification and deacetylation (Scheme 1).
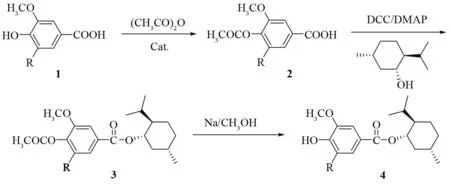
Scheme 1 Synthesis of menthyl esters. Raw materials: vanillic acid (1a): R=H; syringic acid (1b): R=OCH3
1.2.1 Synthesis of acetyl vanillic acid (2a)
Compound1a(8.00g, 47.58 mmol) and acetic anhydride (50.0 mL) were added to a 100 mL three-necked flask equipped with a mechanical stirrer. A solution of triethylamine (TEA) (18.0 mL, 129.50 mmol) was added slowly dropwise, and the temperature was maintained below 50 ℃. The mixture was stirred for 7 h at 50 ℃, and then the solvent was evaporated. Water (20 mL) was added to the residue. The mixture was filtered, washed with water, and dried to give white solid2a(yield 95.9%);m.p. 213.4-213.5 ℃.
1.2.2 Synthesis of acetyl vanillic acid menthyl ester (3a)
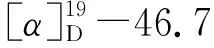
1.2.3 Synthesis of vanillic acid menthyl ester (4a)
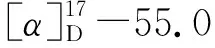
1.2.4 Synthesis of acetyl syringic acid (2b)
Compound1b(6.72 g, 33.91 mmol), acetic anhydride (15 mL), and TEA were added to a 100 mL three-necked flask and mechanically stirred. The mixture was stirred for 7 h at 50 ℃, and then the solvent was evaporated. Water (20 mL) was added to the residue. The mixture was filtered and washed with water to give white solid2b(yield 96.2%);m.p.194.1-194.3 ℃.
1.2.5 Synthesis of syringic acid menthyl ester (4b)
According to the literature[17,18], acetyl syringic acid menthyl ester3band compound4bwere synthesized from compound2b.
2 Results and discussion
2.1 Acetylation
Because the esterification reaction was conducted using DCC/DMAP, syringic acid and the phenol hydroxyl moiety of vanillic acid can undergo competitive esterification, the phenolic hydroxyl moiety must be protected. Acetylation with acetic anhydride is commonly used to protect phenol hydroxyl groups, and the traditional conditions use methylene chloride as the solvent. In this study, acetic anhydride was used as the solvent and reactant. The reaction was conducted with the appropriate catalyst, under mild reaction conditions, and using simple postprocessing steps, and the obtained yield was high.
2.1.1 Acetylation of vanillic acid
The acetylation of vanillic acid was optimized based on a four-factor and three-level orthogonal experimental design, and the catalyst type, reaction temperature, reaction time, catalyst loading were selected as the four factors (Table 1). The effects of each of the four factors were evaluated based on the yields calculated from the orthogonal experiments (Table 2). The order of the magnitudes of the effects of the four factors on the reaction is catalyst loading, catalyst type, reaction temperature, and reaction time. The optimized conditions (A3B3C2D3)are as follows: molar ratio of N(C2H5)3to syringic acid of 2.9 in acetic anhydride as the solvent and a reaction temperature of 60 ℃ for 5 h with triethylamine as the catalyst. The yield of acetyl syringic acid under these conditions was 95.9%, 1.4% higher compared with the No.4 experimental condition with the highest yield in four factors and three levels.
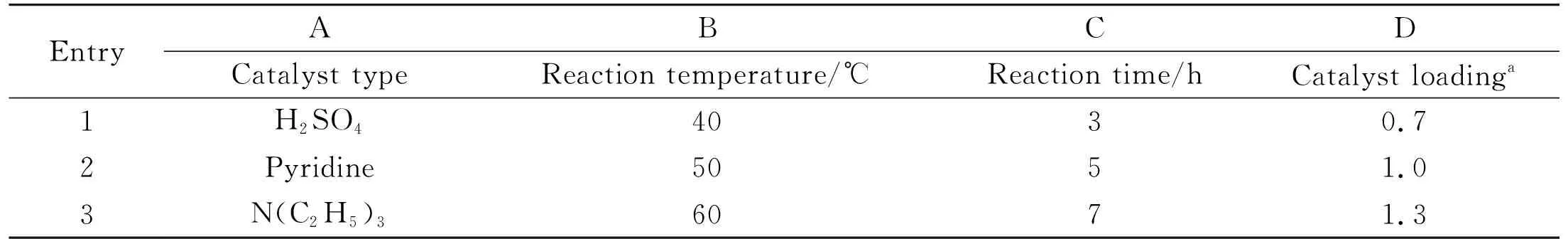
Table 1 Factor and level of orthogonal experiments for the acetylation of vanillic acid

Table 2 Results of orthogonal experiments for vanillic acid acetylation
2.1.2 Acetylation of syringic acid
The acetylation of syringic acid was optimized based on a four-factor and three-level orthogonal experimental design, and the catalyst type, reaction temperature, reaction time, and catalyst loading were selected as the four factors (Table 3). The effects of the four factors were evaluated based on the obtained yields calculated from the results of the orthogonal experiments (Table 4). The order of magnitudes of the effects of these four factors on the reaction is catalyst type, reaction temperature, catalyst loading and reaction time. The optimized conditions (A2B2C3D2) are as follows: molar ratio of N(C2H5)3to syringic acid is 2.7 in acetic anhydride as the solvent, a reaction temperature of 50 ℃ and times of 7 h with triethylamine as the catalyst. The yield of acetyl syringic acid under these conditions was increased from 82.0% to 96.2%. Compared with the No.5 experimental condition with the highest yield in four factors and three levels, yield is 1.2% more.

Table 3 Factor and level of orthogonal experimental design for the acetylation of syringic acid
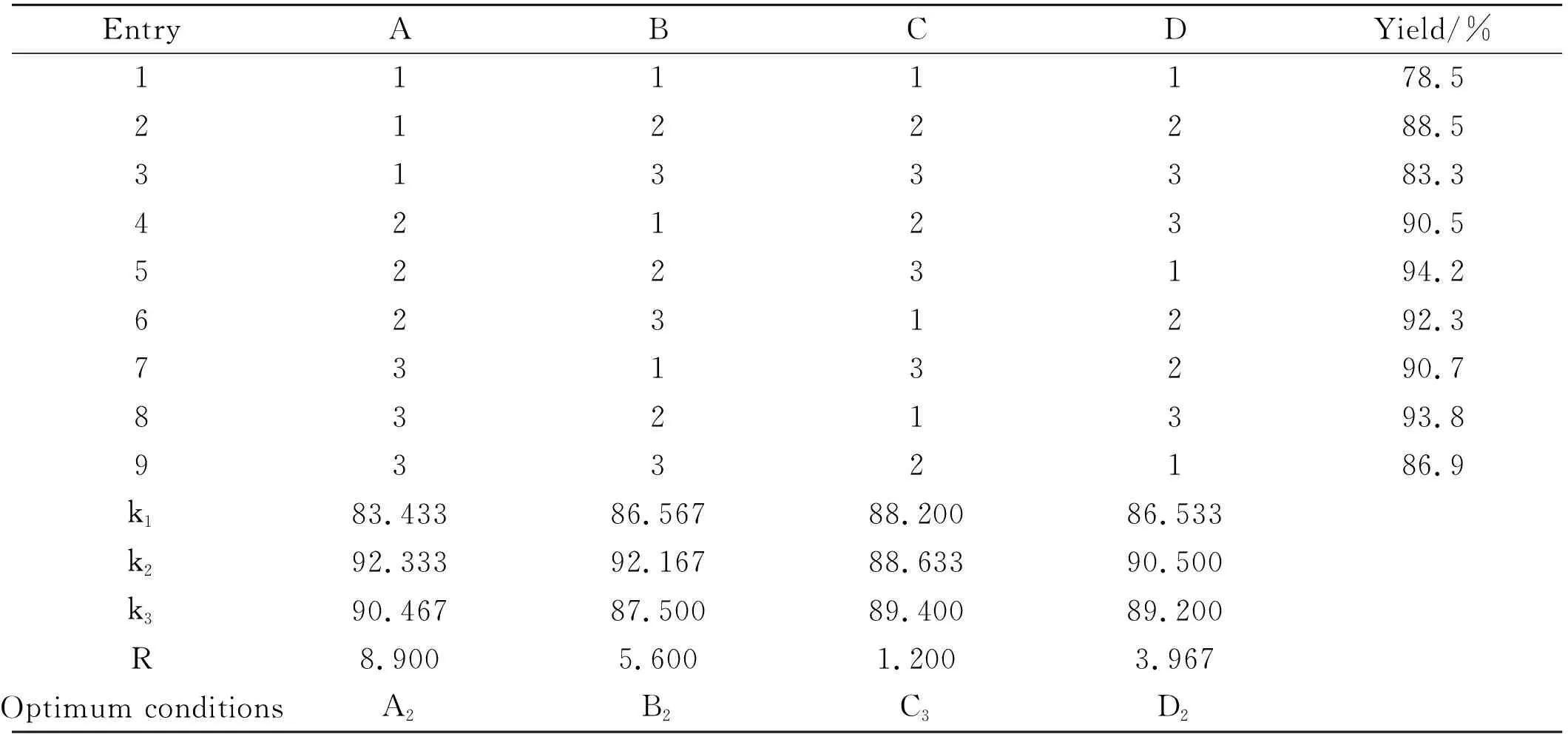
Table 4 Results of orthogonal experiments for syringic acid acetylation
2.2 Esterification
The menthyl esters prepared herein are mint aromatic acid esters. The carboxyl group on the aromatic ring and the hydroxyl group on the cyclohexyl of menthol result in substantial steric hindrance, and thus, the esterification reaction requires the highly active DCC/DMAP system.
2.3 Reduction
The Bouveault-Blanc reaction[18-20]is the direct reduction of an aliphatic carboxylic acid ester with metal and methanol to generate the corresponding primary alcohol. It is mainly used for the reduction of saturated fatty acid esters. Vanillic acid menthyl ester and syringic acid menthyl ester in the protic solvent methanol were synthesized via deacetylation under mild conditions, and the synthesis was fast and efficient.
2.4 Thermal stabilities
The thermal stabilities of the menthyl esters were investigated by thermogravimetric-derivative thermogravi-metric-differential scanning calorimetry (TG-DTG-DSC) analysis within the range of 20-600 ℃ at a heating rate of 10 ℃/min under an air atmosphere.
The TG-DTG-DSC curve of4ais shown in Fig.1. The TG curve shows that at 20-215 ℃, a small amount of weight is lost (3.8%). At 215-357 ℃, the main weight loss process occurrs, and the weight loss ratio is 91.0%. The DTG curve shows an obvious peak at 326 ℃, and the maximum weight loss rate reachs 19.3%/min. The area of the DSC curve also appears obvious exothermic peak, mainly exothermic reaction of oxidation decomposition. The residue remaining of4bis 1.8% at 600 ℃.

Fig.1 TG-DTG-DSC curve of menthyl ester
The TG-DTG-DSC curve of4bis shown in Fig.1. The TG curve showed that at 20-215 ℃, a small amount (2.9%) of weight was lost. At 215-345 ℃, the main weight loss process occurred, and the weight loss ratio was 92.7%. The DTG curve showed an obvious peak at 324 ℃, the maximum weight loss rate reached 18.8%/min. The area of the DSC curve also appear obvious exothermic peak, mainly exothermic reaction of oxidation decomposition. The residue remaining of4bwas 0.7% at 600 ℃.
Thus, under a nitrogen atmosphere, only 50% of the weight of menthol[21]is lost at 146 ℃, which is unlike the results obtained under an air atmosphere. When the weight loss rate of4aand4bis 50%, they are 313 ℃ and 310 ℃, respectively. The menthyl esters showed good thermal stability at room temperature with a light fruit aroma. The main pyrolysis product of the menthyl esters[22]under high temperatures is menthol, and therefore these menthyl esters can be used as a spices at low or high temperatures.
3 Conclusion
The route of solvent free acetylation was characterized by simple operation, short time, high yield and mild conditions. Menthyl ester is a kind of latent fragrant compounds with a wide range of temperature applications. Because their molecular structures contain menthol and phenolic acid group, these compounds are physiologically active, making them relevant to medical, food, cosmetics and other fields applications, and further explorations of these menthyl esters and their potential applications are necessary.
