新型含2,4-二氯苯基的1,2,4-三唑喹唑啉衍生物的合成及抑菌活性
2020-07-15张贵强郭晴晴易君明赵廷渊邢林娜
张贵强, 郭晴晴, 易君明, 赵廷渊, 王 梅, 邢林娜
(兴义民族师范学院 贵州省化学合成及环境污染控制和修复技术特色重点实验室,贵州 兴义 562400)
柑橘溃疡病、烟草青枯病、水稻百叶枯病是常见的农作物疾病,对农作物的质量和产量造成了不利影响,导致了严重的经济损失。现有农药抑菌剂受耐药性影响,防治效果不太理想。开发高效、低毒、环境友好型的新型杀菌剂成为农药领域的研究热点。
2,4-二氯苯基、1,2,4-三唑和喹唑啉作为活性药效基团[1-7],在新农药创制中应用广泛。戊菌唑、糠菌唑、己唑醇、戊菌唑和啶斑肟等农药中含2,4-二氯苯基结构;三唑酮、亚胺唑、唑蚜威等高效低毒的农药中含有1,2,4-三唑结构;喹螨醚、氟喹唑等农药中含有喹唑啉基团。
1,3,4-噻二嗪也是重要的药效基团,具有抗菌、调节植物生长、杀虫和抗病毒等药理活性[8-11]。喹唑啉结构具有良好的农药抑菌活性[12-14]。高元磊等[15]合成的喹唑啉化合物对小麦赤霉病菌和苹果腐烂病菌的抑制率优于对照药剂噁霉灵。杨绪红等[16]合成的喹唑啉化合物对烟草青枯病菌的抑制率优于对照药剂噻菌酮。
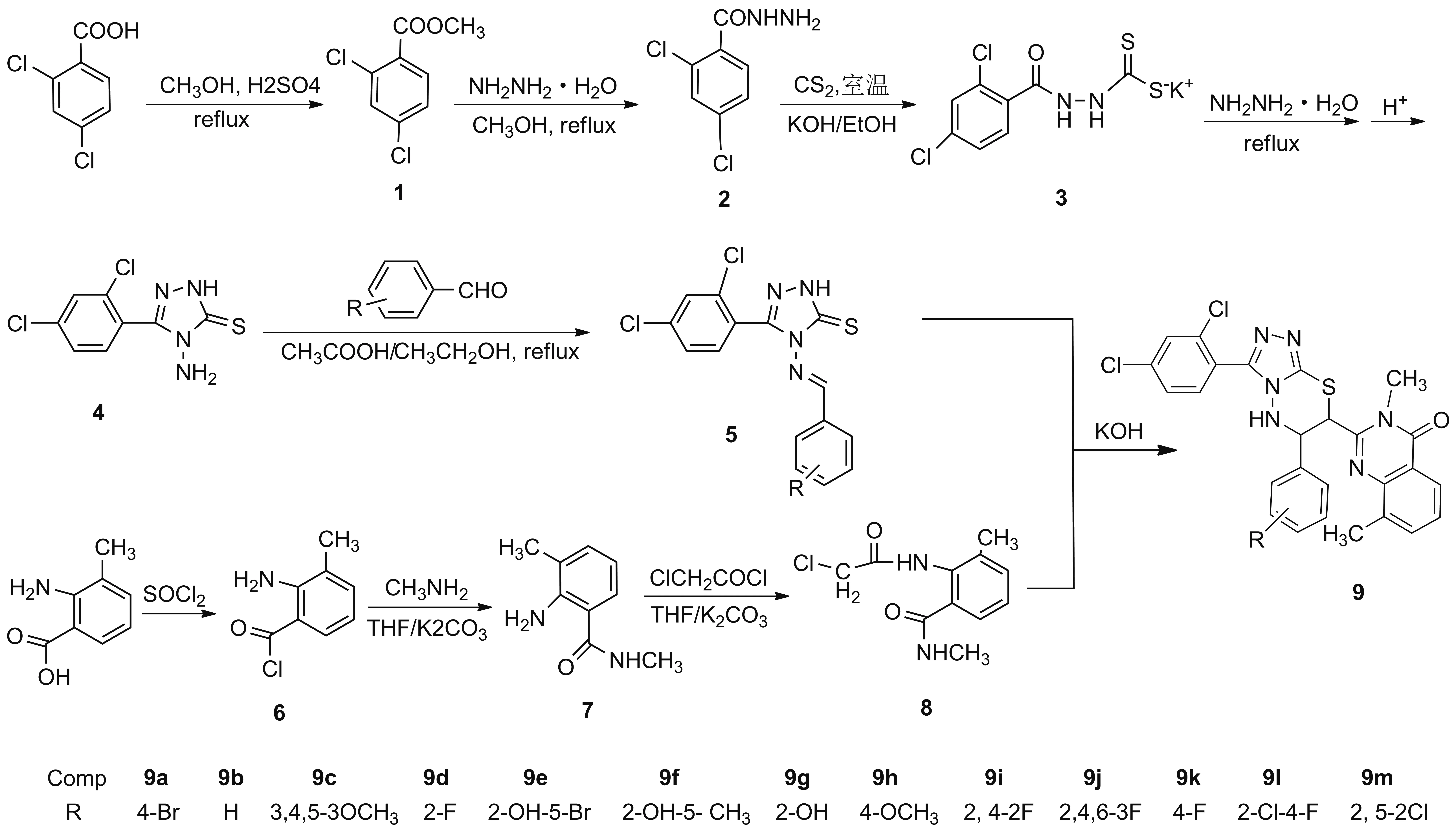
拼接高活性药效基团,常能得到高活性农药制剂。如烯唑醇、亚胺唑、唑啶草酮、氟醚唑,同时含有2,4-二氯苯基和1,2,4-三唑基团;氟喹唑同时含有2,4-二氯苯基、1,2,4-三唑基团和喹唑啉基团。本文根据活性基团拼接原理,将2,4-二氯苯基、1,2,4-三唑、1,3,4-噻二嗪结构引入喹唑啉结构中,设计并合成了13个未见文献报道的含2,4-二氯苯基的1,2,4-三唑1,3,4-噻二嗪喹唑啉化合物(9a~9m, Scheme 1),收率59%~74%,其结构经1H NMR,13C NMR,19F NMR和MS表征。采用浊度法测试目标化合物对柑橘溃疡病菌(Xac)、烟草青枯病菌(Rs)、水稻百叶枯病菌(Xoo)的抑制活性。
1 实验部分
1.1 仪器与试剂
X-5型熔点仪;JEOL-ECX 400 MHz型核磁共振仪(DMSO-d6为溶剂,TMS为内标);TSQ 8000/MSD三级四级杆气相色谱质谱。
化合物1~5[3], 2-(2-氯乙酰氨基)-3-甲基-N-甲基苯甲酰胺(8)[17]按文献方法合成;2,4-二氯苯甲酸,2-氨基-3-甲基苯甲酸,上海阿拉丁试剂有限公司;其余所用试剂均为分析纯。
1.2 9a~9m的合成通法
将中间体52 mmol和1%KOH溶液100 mL加入250 mL反应瓶中,搅拌至澄清;缓慢滴加中间体80.51g(2.1mmol)的乙醇(40 mL)溶液,滴毕,搅拌下于50 ℃反应至终点(TLC检测)。冰浴冷却,抽滤,滤饼用冰乙醇洗涤,经硅胶柱层析[洗脱剂:V(石油醚)/V(乙酸乙酯)=3/1]纯化得9a~9m。
3-(2,4-二氯苯基)-6-(4-溴苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9a): 白色晶体, 产率62%, m.p.164~166 ℃;1H NMR(DMSO-d6,400 MHz)δ: 7.95(d,J=8.0 Hz, 1H, quinazoline-H), 7.86(s, 1H, 2,4-2ClPh-H), 7.64(s, 3H, 2,4-2ClPh-H+NH), 7.53(d,J=8.0 Hz, 2H, 4-BrPh-H), 7.41(t,J=8.0 Hz, 1H, quinazoline-H), 7.34(d,J=8.0 Hz, 2H, 4-BrPh-H), 7.25(d,J=4.0 Hz, 1H, quinazoline-H), 5.59(d,J=4.0 Hz, 1H, SCH), 5.26(t,J=4.0 Hz, 1H , NCH), 3.74(s, 3H, NCH3), 2.34(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ: 161.96, 153.93, 150.12, 144.92, 142.10, 136.78, 136.59, 135.58, 135.52, 134.96, 134.24, 131.98, 129.88, 129.76, 128.20, 127.40, 125.34, 124.36, 121.71, 120.50, 57.78, 30.55, 17.08; MSm/z: 615.0{[M+H]+}。
3-(2,4-二氯苯基)-6-苯基-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9b): 白色晶体, 产率59%, m.p.156~158 ℃;1H NMR(DMSO-d6, 400 MHz)δ: 8.15(d,J=8.0 Hz, 1H, quinazoline-H), 7.62(d,J=4.0 Hz, 1H, 2,4-2ClPh-H), 7.56(d,J=1.6 Hz, 1H, Ph-H), 7.50(d,J=4.0 Hz, 1H, quinazoline-H), 7.43(t,J=8.0 Hz, 1H, quinazoline-H), 7.35(dd,J=4.0 Hz, 4.0 Hz, 1H, Ph-H), 7.27~7.29(m, 4H, Ph-H+NH), 7.26(s, 1H, ArH), 7.20(d,J=8.0 Hz, 1H, Ph-H), 5.44(d,J=4.0 Hz, 1H, SCH), 5.01(t,J=4.0 Hz, 1H, NCH), 3.75(s, 3H, NCH3), 2.60(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ: 161.94, 154.03, 150.14, 144.98, 142.35, 137.31, 136.54, 135.58, 135.20, 134.94, 134.18, 129.89, 129.08, 128.47, 128.17, 127.45, 127.08, 125.43, 124.36, 120.46, 58.67, 41.92, 30.53, 17.06; MSm/z: 536.1{[M+H]+}。
Scheme 1
3-(2,4-二氯苯基)-6-(3,4,5-三甲氧基苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9c): 白色晶体, 产率62%, m.p.196~198 ℃;1H NMR(DMSO-d6,400 MHz)δ: 7.93(d,J=8.0 Hz, 1H, quinazoline-H), 7.85(d,J=1.6 Hz, 1H, 2,4-2ClPh-H), 7.64(d,J=8.0 Hz, 1H, quinazoline-H), 7.63(d,J=1.6 Hz, 1H, 2,4-2ClPh-H), 7.66(s, 1H, 2,4-2ClPh-H), 7.42(t,J=8.0 Hz, 1H, quinazoline-H), 6.69(d,J=8.0 Hz, 2H, methoxyPh-H), 7.12(t,J=4.0 Hz, 1H, NH), 6.69(d,J=5.6 Hz, 1H, SCH), 5.10(d,J=4.0 Hz, 8.0 Hz, 1H, NCH), 3.80(s, 3H, OCH3), 3.74(s, 3H, CH3), 3.70(s, 6H, OCH3), 2.62(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ: 161.89, 153.75, 153.31, 150.21, 145.01, 142.81, 137.63, 136.53, 135.52, 135.21, 134.83, 134.10, 132.71, 129.94, 128.14, 127.38, 125.27, 124.37, 120.46, 105.22, 60.41, 59.92, 42.36, 30.68, 17.06; MSm/z: 626.2{[M+H]+}。
3-(2,4-二氯苯基)-6-(2-氟苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9d): 白色晶体, 产率68%, m.p.176~178 ℃;1H NMR(DMSO-d6,400 MHz)δ: 7.93(d,J=8.0 Hz, 1H, quinazoline-H), 7.82(s, 1H, 2,4-2ClPh-H), 7.62~7.64(m, 3H, 2,4-2ClPh-H+NH), 7.32~7.41(m, 2H, Ph-H), 7.20~7.25(m, 2H, Ph-H), 7.15(d,J=8.0 Hz, 1H, Ph-H), 7.09(d,J=8.0 Hz, 1H, Ph-H), 5.59(d,J=5.2 Hz , 1H, SCH), 5.40(t,J=4.0 Hz, 1H , NCH), 3.73(s, 3H, NCH3), 2.26(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ: 161.95, 161.04, 158.60, 153.28, 149.78, 145.02, 142.62, 136.58, 135.64, 135.17, 134.87, 134.15, 130.87, 130.78, 129.87, 128.46, 128.42, 128.18, 127.37, 125.34, 125.30, 125.27, 125.20, 124.31, 120.49, 116.32, 116.10, 56.51, 53.91, 42.54, 30.45, 30.43, 19.03, 16.74;19F NMR(DMSO-d6, 400 MHz)δ: -115.72, -117.07; MSm/z:554.2{[M+H]+}。
3-(2,4-二氯苯基)-6-(2-羟基-5-溴苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9e): 白色晶体, 产率64%, m.p.201~202 ℃;1H NMR(DMSO-d6, 400 MHz)δ: 7.96(d,J=8.0 Hz, 1H, quinazoline-H), 7.86(d,J=8.0 Hz, 1H, 2,4-2ClPh-H), 7.68(d,J=8.0 Hz, 1H, quinazoline-H), 7.61(d,J=4.0 Hz, 1H, 2,4-2ClPh-H), 7.37~7.41(m, 5H, Ph-H), 6.82(d,J=8.0 Hz, 1H, NH), 6.00(d,J=8.0 Hz , 1H, SCH), 5.49(t,J=4.0 Hz, 8.0 Hz , 1H, NCH), 4.82(s, 1H, OH), 3.42(s, 3H, CH3), 2.39(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ: 167.52, 161.98, 161.61, 157.75, 157.25, 151.82, 150.97, 150.18, 149.06, 144.99, 144.52, 137.17, 136.73, 136.01, 135.53, 135.23, 133.84, 133.59, 133.18, 129.95, 129.50, 128.86, 128.59, 128.03, 127.85, 127.72, 127.60, 126.91, 124.40, 124.35, 120.85, 120.75, 120.26, 112.99, 112.73, 112.63, 87.15, 63.73, 60.23, 38.02, 30.60, 25.96, 19.03, 17.28, 17.18, 16.89, 14.55; MSm/z: 631.3{[M+H]+}。
Scheme 2
3-(2,4-二氯苯基)-6-(2-羟基-5-甲基苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9f): 白色晶体, 产率63%, m.p.186~188 ℃;1H NMR(DMSO-d6,400 MHz)δ: 14.11(s, 1H, OH), 7.96(d,J=8.0 Hz, 1H, quinazoline-H), 7.91(d,J=4.0 Hz, 1H, 2,4-2ClPh-H), 7.64(d,J=8.0 Hz, 1H, quinazoline-H), 7.58(dd,J=4.0 Hz, 4.0 Hz, 1H, Ph-H), 7.43(t,J=8.0 Hz, 1H, Ph-H), 7.31(d,J=8.0 Hz, 1H, Ph-H), 7.13(d,J=4.0 Hz, 1H, Ph-H), 7.00(d,J=8.0 Hz, 1H, Ph-H), 6.67(d,J=8.0 Hz, 1H, NH), 6.58(s, 1H, Ph-H), 6.15(t,J=4.0 Hz, 8.0 Hz, 1H, SCH), 5.90(d,J=4.0 Hz, 1H, SCH), 3.62(s, 3H, CH3), 2.34(s, 3H, CH3), 2.19(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ: 167.56, 162.05, 156.18, 151.34, 149.11, 144.59, 137.03, 136.01, 135.35, 135.21, 133.93, 130.91, 130.54, 129.94, 128.01, 127.64, 126.14, 125.92, 124.33, 124.23, 120.74, 110.04, 86.66, 63.57, 30.60, 20.85, 15.93; MSm/z: 565.1{[M+H]+}。
3-(2,4-二氯苯基)-6-(2-羟基苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9g): 白色晶体, 产率65%, m.p.206~208 ℃;1H NMR(DMSO-d6, 400 MHz)δ: 14.14(s, 1H, OH), 7.96(d,J=8.0 Hz, 1H, quinazoline-H), 7.86(d,J=4.0 Hz, 1H, 2,4-2ClPh-H), 7.63(d,J=8.0 Hz, 1H, quinazoline-H), 7.54(dd,J=4.0 Hz, 4.0 Hz, 1H, Ph-H), 7.44(t,J=8.0 Hz, 1H, ArH), 7.17~7.35(m, 4H, Ph-H), 6.99(s, 1H, Ph-H), 6.92(t,J=8.0 Hz, 1H, Ph-H), 6.80(d,J=8.0 Hz, 1H, ArH), 6.29(t,J=4.0 Hz, 1H, SCH), 5.83(s, 1H, SCH), 3.61(s, 3H, CH3), 2.33(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ: 167.52, 162.02, 158.43, 151.13, 149.11, 144.54, 136.99, 136.03, 135.30, 135.24, 135.20, 133.71, 130.63, 129.89, 127.89, 127.65, 125.87, 125.81, 124.33, 124.15, 121.81, 120.75, 110.53, 86.52, 63.11, 60.23, 30.56, 21.23, 16.87, 14.56; MSm/z: 551.1{[M]+}。
3-(2,4-二氯苯基)-6-(4-甲氧基苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9h): 白色晶体, 产率56%, m.p.216~218 ℃;1H NMR(DMSO-d6,400 MHz)δ: 7.94(d,J=8.0 Hz, 1H, quinazoline-H), 7.86(s, 1H, 2,4-2ClPh-H), 7.63~7.71(m, 3H, Ph-H+NH), 7.43(t,J=8.0 Hz, 1H, quinazoline-H), 7.29(d,J=8.0 Hz, 2H, 4-OCH3Ph-H), 7.12~7.17(m, 1H, Ph-H), 6.85(d,J=8.0 Hz, 2H, 4-OCH3Ph-H), 5.55(d,J=4.0 Hz, 1H, SCH), 5.14(t,J=4.0 Hz, 1H, NCH), 3.59~3.83(m, 6H, CH3+OCH3), 2.38(s, 3H, CH3);13C NMR(125 MHz, DMSO-d6)δ:161.91, 159.56, 159.28, 154.07, 150.24, 145.00, 142.43, 136.52, 135.58, 135.22, 134.95, 134.20, 129.88, 129.03, 128.77, 128.38, 128.17, 127.94, 127.37, 125.45, 124.37, 120.44, 115.26, 114.42, 60.56, 58.52, 55.53, 42.03, 30.55, 17.11; MSm/z: 566.1{[M+H]+}。
3-(2,4-二氯苯基)-6-(2,4-二氟苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9i): 白色晶体, 产率66%, m.p.191~193 ℃;1H NMR(DMSO-d6,400 MHz)δ: 7.93(d,J=8.0 Hz, 1H, quinazoline-H), 7.84(d,J=8.0 Hz, 1H, 2,4-2ClPh-H),7.62~7.64(m, 3H, Ph-H+NH), 7.40(t,J=8.0 Hz, 1H, quinazoline-H), 7.27~7.34(m, 2H, Ph-H), 7.04~7.12(m, 2H, Ph-H), 5.57(d,J=4.0 Hz , 1H, SCH), 5.36(t,J=4.0 Hz、8.0 Hz, 1H, NCH), 3.71(s, 2H, CH3), 2.27(s, 2H, CH3);19F NMR(DMSO-d6, 400 MHz)δ: -111.02, -109.02, -109.63, -111.18, -112.56, -113.02;13C NMR(125 MHz, DMSO-d6)δ: 161.72, 158.26, 153.19, 152.03, 149.82, 149.02, 147.04, 145.09, 144.99, 142.53, 136.91, 136.60, 135.34, 135.21, 135.06, 134.26, 134.19, 134.16, 130.09, 129.87, 128.30, 128.19, 126.93, 125.23, 124.36, 120.50, 120.24, 105.64, 30.51, 30.46, 17.05, 16.77; MSm/z: 571.1{[M+H]+}。
3-(2,4-二氯苯基)-6-(2,4,6-三氟苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9j): 白色晶体, 产率64%, m.p.182~184 ℃;1H NMR(DMSO-d6, 400 MHz)δ: 7.93(d,J=8.0 Hz, 1H, quinazoline-H), 7.84(d,J=8.0 Hz, 1H, 2,4-2ClPh-H), 7.62~7.64(m, 3H, Ph-H +NH), 7.41(t,J=8.0 Hz, 1H, quinazoline-H), 7.27~7.34(m, 1H, Ph-H), 7.04~7.12(m, 2H, Ph-H), 5.61(t,J=8.0 Hz, 1H, NCH), 5.55(d,J=4.0 Hz, 1H, SCH), 3.64(s, 3H, CH3), 2.33(s, 3H, CH3);19F NMR(DMSO-d6,400 MHz)δ: -106.50, -108.78;13C NMR(125 MHz, DMSO-d6)δ: 161.75, 152.31, 150.25, 144.77, 143.32, 136.60, 135.66, 135.40, 134.73, 134.18, 129.95, 128.78, 127.70, 125.05, 124.43, 120.47, 102.16, 101.87, 54.59, 43.46, 30.62, 16.74; MSm/z: 589.1{[M+H]+}。
3-(2,4-二氯苯基)-6-(4-氟苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9k): 白色晶体, 产率62%, m.p.189~191 ℃;1H NMR(DMSO-d6, 400 MHz)δ: 7.95(d,J=8.0 Hz, 1H, quinazoline-H), 7.86(s, 1H, 2,4-2ClPh-H), 7.63-7.67(m, 3H, Ph-H), 7.40~7.45(m, 3H, Ph-H), 7.22(d,J=8.0 Hz, 1H, quinazoline-H), 7.17(t,J=8.0 Hz, 1H, quinazoline-H), 5.58(d,J=8.0 Hz, 1H, SCH), 5.24( t,J=8.0 Hz, 4.0 Hz, 1H, NCH), 3.72(s, 3H, CH3), 2.35(s, 3H, CH3);19F NMR(DMSO-d6,400 MHz)δ: -114.06;13C NMR(125 MHz, DMSO-d6)δ: 163.29, 161.94, 160.85, 153.98, 150.16, 144.94, 142.23, 136.57, 135.58, 135.22, 134.96, 134.22, 133.56, 133.53, 129.89, 129.73, 129.66, 128.18, 127.39, 125.37, 124.37, 120.47, 116.02, 115.80, 57.99, 55.39, 41.75, 30.56, 17.10; MSm/z: 553.1{[M+H]+}。
3-(2,4-二氯苯基)-6-(2-氯-4-氟苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9l): 白色晶体, 产率61%, m.p.187~189 ℃;1H NMR(DMSO-d6, 400 MHz)δ: 7.91(d,J=8.0 Hz, 1H, quinazoline-H), 7.83(s, 1H, 2,4-2ClPh-H), 7.56~7.63(m, 4H, Ph-H+NH), 7.45(dd,J=4.0 Hz, 4.0 Hz, 1H, Ph-H), 7.40(t,J=4.0 Hz, 1H, Ph-H), 7.16~7.23(m, 2H, Ph-H), 5.74(d,J=8.0 Hz, 1H, SCH), 5.48(t,J=8.0 Hz, 1H, NCH), 3.72(s, 3H, CH3), 2.34(s, 3H, CH3);19F NMR(DMSO-d6, 400 MHz)δ: -110.23, -110.88;13C NMR(125 MHz, DMSO-d6)δ: 163.11, 161.73, 161.36, 160.63, 153.07, 153.00, 150.36, 149.21, 145.02, 144.88, 143.02, 142.68, 136.55, 136.14, 136.07, 135.56, 135.31, 135.14, 134.92, 134.87, 134.76, 134.53, 134.22, 131.60, 130.17, 130.08, 129.85, 128.05, 127.88, 127.56, 127.39, 125.32, 125.09, 124.38, 120.33, 119.89, 117.84, 117.59, 115.34, 115.13, 58.20, 57.81, 42.50, 31.00, 30.00, 17.12, 16.79; MSm/z: 587.1{[M+H]+}。
3-(2,4-二氯苯基)-6-(2,5-二氯苯基)-7-[3,8-二甲基喹唑啉-4(3H)-酮]-5H-1,2,4-三唑[3,4-b][1,3,4]噻二嗪(9m): 白色晶体, 产率61%, m.p.182~184 ℃;1H NMR(DMSO-d6,400 MHz)δ: 7.91(d,J=8.0 Hz, 1H, quinazoline-H), 7.83(d,J=2.0 Hz, 1H, Ph-H), 7.56~7.63(m, 4H, Ph-H), 7.50(d,J=8.0 Hz, 1H, Ph-H), 7.42(d,J=4.0 Hz, 1H, Ph-H), 7.40(s, 1H, Ph-H), 7.21(d,J=8.0 Hz, 1H, Ph-H), 5.68(d,J=8.0 Hz, 1H, SCH), 5.48(t,J=8.0 Hz, 1H, SCH), 3.72(s, 3H, CH3), 2.34(s, 3H, CH3).13C NMR(125 MHz, DMSO-d6)δ: 161.83, 153.38, 150.25, 144.85, 143.31, 137.14, 136.62, 135.57, 135.33, 134.86, 134.16, 132.58, 132.23, 131.99, 130.46, 129.92, 128.63, 128.10, 127.58, 125.02, 124.39, 120.35, 58.24, 42.61, 31.10, 17.13; MSm/z: 605.1{[M+H]+}。
1.3 抑菌活性测试
供试药剂浓度为100 μg/mL和50 μg/mL。采用浊度法测试目标化合物对柑橘溃疡病菌、烟草青枯病菌、水稻百叶枯病菌的抑制活性[13]。
2 结果与讨论
2.1 反应机理
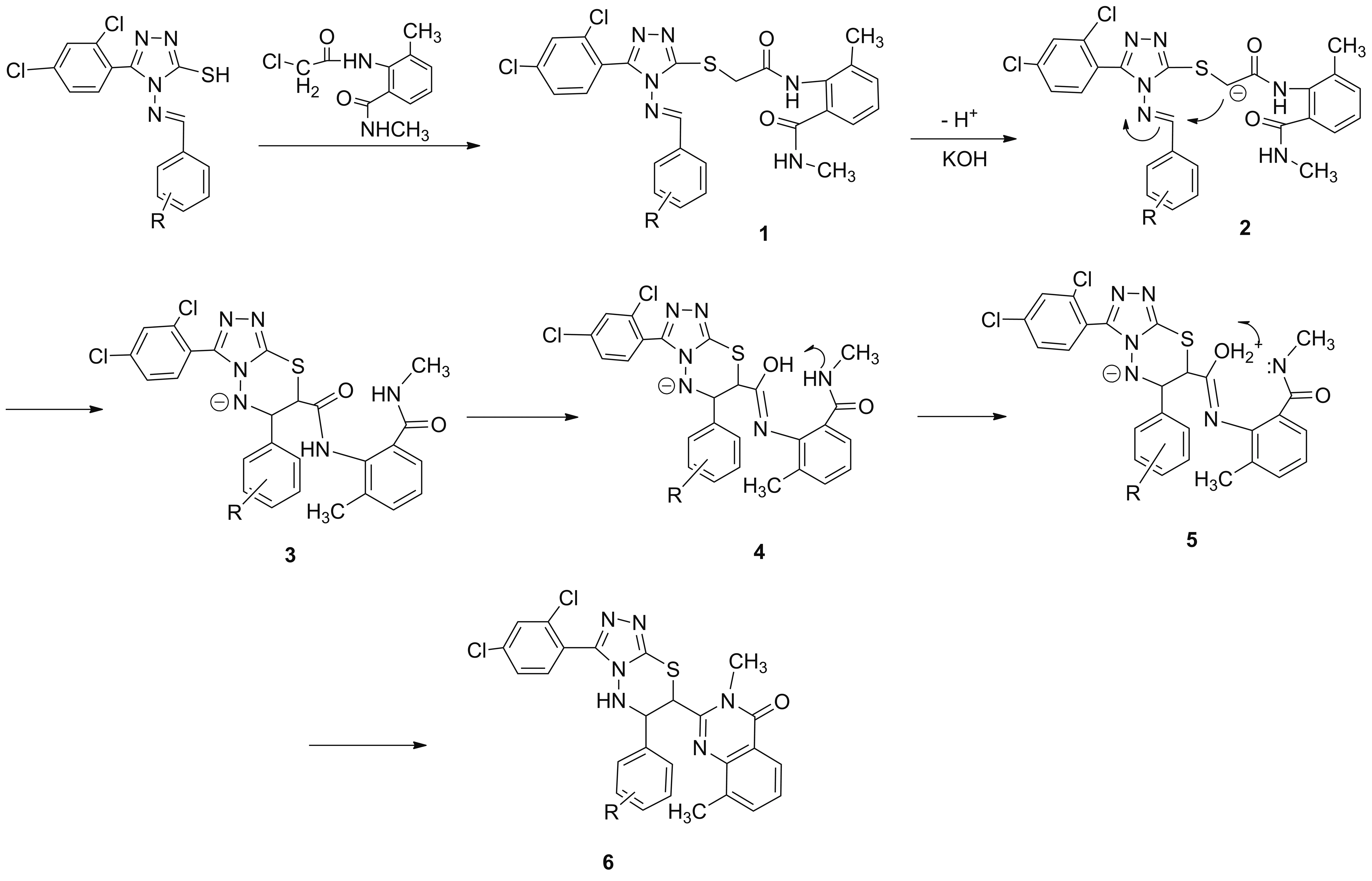
TLC跟踪结果显示,中间体1生成后,随即就有中间体3出现,最终全部转化为6。由此,分析可能的反应机理见Scheme 2:首先生成了硫醚,由于C=O的强吸电子作用,使CH2变得活泼,在KOH碱性下,CH2生成碳负离子,作为亲核试剂与C=N双键发生分子内亲核加成反应得中间体3;在碱性条件下,受1,2,4-三唑[3,4-b][1,3,4]噻二嗪环的吸电子诱导效应影响,酰胺键烯醇化,然后发生分子内闭环脱去H2O后生成喹唑啉酮。
2.2 表征
以化合物9c(Chart 1)的为例,分析了1H NMR数据。δ7.93处特征峰为1-H的吸收峰,受2-H耦合作用影响,裂分为双重峰。δ7.64处特征峰为3-H的吸收峰,受2-H耦合作用影响,裂分为双重峰。δ7.42处特征峰为2-H的吸收峰,1-H和3-H耦合作用影响,裂分为三重峰。δ7.85处特征峰为8-H的吸收峰,受9-H耦合作用影响,裂分为两重峰。δ7.63处特征峰为9-H的吸收峰,受8-H耦合作用影响,裂分为双重峰。δ7.66处特征峰为10-H的吸收峰,为单峰。δ6.69处特征峰为4-H和5-H的吸收峰,化学环境相同,为单峰。δ 7.12处特征峰为N—H的吸收峰,受6-H耦合作用影响,裂分为两重峰。δ6.69处特征吸收峰为S—C—H吸收峰,受6-H耦合作用的影响,裂分为两重峰。δ5.10处特征吸收峰为6-H吸收峰,受7-H耦合作用的影响,裂分为三重峰。
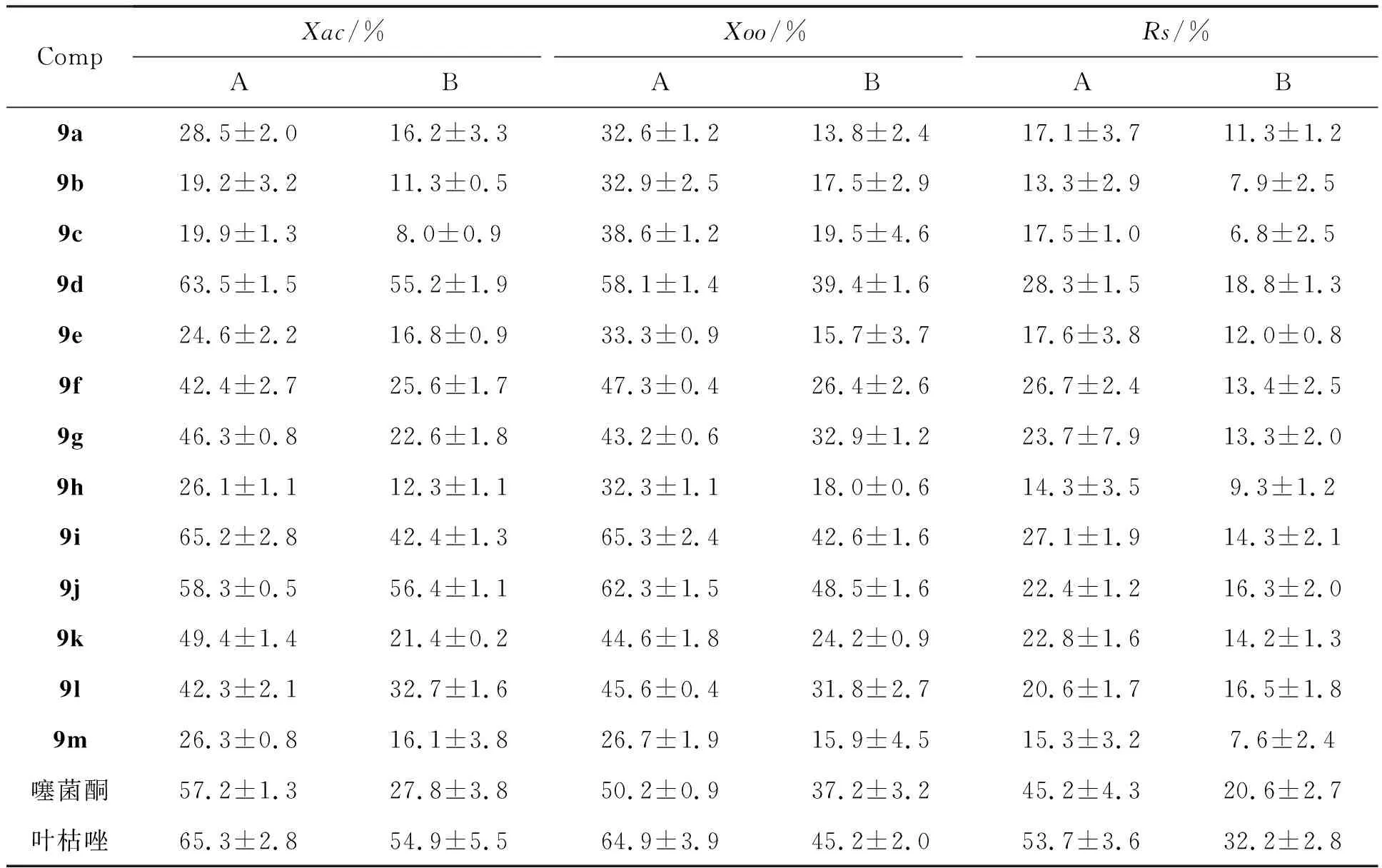
表1 9a~9m对植物病菌的抑制率*
*A: 100 μg/mL, B: 50μg/mL。
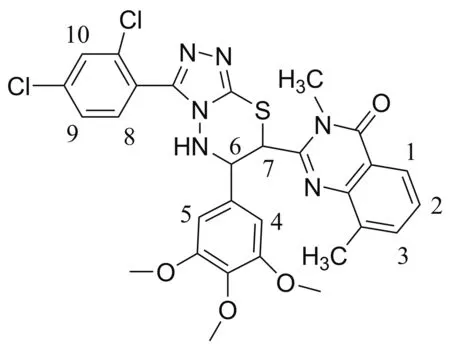
Chart 1
2.2 抑菌活性
表1为目标化合物对水稻白叶枯病菌(Xoo)、柑橘溃疡病菌(Xac)和烟草青枯病菌(Rs)的抑制活性。从表1可以看出,目标化合物对植物病菌的抑制作用与取代基性质有关。化合物9d、9i、9j、9k、9l的结构中含有氟原子,活性相对较高,可能是氟原子的电子效应,使目标化合物分子的电荷分布产生变化,提高了电子云密度,进而增强了此类化合物在细菌体中的吸收、传递速度和穿透力。当R为羟基取代时,化合物活性相对较好,原因可能是羟基的引入提高了化合物的溶解性,使目标化合物更容易与受体结合。当浓度为100 μg/mL时,化合物9d、9i、9j对柑橘溃疡病菌的抑制率分别为63.5%、 65.2%、 58.3%,与对照药剂噻菌酮(57.2%)和叶枯唑(65.3%)相当。当浓度为50 μg/mL时,化合物9j对柑橘溃疡病菌的抑制率为56.4 %,与对照药剂叶枯唑(54.9%)相当。当浓度为100 μg/mL时,化合物9i、9j对水稻百叶枯病菌的抑制率分别为65.3%和62.3%,和对照药剂噻菌酮(50.2%)、叶枯唑(64.9%)相当。当浓度为50 μg/mL时,化合物9j对水稻百叶枯病菌的抑制率为48.5%,和对照药剂噻菌酮(37.2%)和叶枯唑(45.2%)相当。
合成了13个新型的含2,4-二氯苯基的1,2,4-三唑1,3,4-噻二嗪喹唑啉酮化合物(9a~9m),并采用浊度法测试了生物活性。结果表明,化合物9k和9j对柑橘溃疡病菌和水稻百叶枯病菌的抑制率和对照药剂相当,具备进一步开发的价值。
