Characterization and com parison of innate immune GST genes in two Sinonovacula constrictapopulations from Vietnam and China
2020-06-30NGUYENDanghaiNIUDonghongXIEShumeiLIJiale
NGUYEN Danghai,NIU Donghong,XIE Shumei,LIJiale
(1.Key Laboratory of Genetic Resources for Freshwater Aquaculture and Fisheries,Ministry of Agriculture and Rural Affairs,ShanghaiOcean University,Shanghai 201306,China;2.Research Institute for Aquaculture No.1,Dinh Bang,Tu Son,BacNinh 999100,Vietnam)
Abstract:Razor clams Sinonovacula constricta are seriously affected by bacterial pathogens,which causes high losses in hatcheries and in natural beds.Glutathione S-transferases(GSTs)are enzymes that catalyze xenobiotic metabolism in the phase IIdetoxification process,and they can be used as biomarkers to assess the presence of organic and inorganic contaminants in aquatic environments.However,very little is known about the function of GST genes in bivalves.In this study,we first cloned and expressed Sc-GST gene from S.constricta.The full-length cDNA of Sc-GST contained 1 052 base pairs(bp),including 700 bp of the 5′-untranslated region(UTR),280 bp of the 3′-UTR,and a 678 bp open reading frame that encoded a 225 amino acid polypeptide.We then used real-time qPCR to examine the Sc-GST mRNA tissue distribution pattern in S.constricta from China and time-course of expression in the hemolymph of S.constricta from both China and Vietnam in response to the pathogen Vibrio anguillarum.The Sc-GST gene was predominantly expressed in the immune tissues,including hemocytes,mantle,gill,and liver.In particular,Sc-GST gene expression in S.constricta from Vietnam significantly elevated at12 h after injection and increased compared to the values from those of China during 12-72 h of the experiment.One explanation for these different responses is that the bacteria used in the experimentwere obtained from China,and the S.constricta also from China have probably already adapted to this pathogen,and thus,showed a less severe response to infection.Results of this study suggest that the Sc-GST gene might play important roles in modulating the immune response of S.constricta.
Keywords:Sinonovacula constricta;glutathione S-transferases;immune response;mRNA expression
Razor clamSinonovacula constricta(Lamarck 1818)is one of the fourmajor cultured clam species in China,along withCrassostrea gigas,Ruditapes philippinarum, andTegillarca granosa[1]. It is widely distributed in intertidal zones and estuarine waters along theWest Pacific coast.In recent years,S.constrictahas become exceedingly susceptible to degradation of germplasm resources and pollution due to high-density aquaculture and pathogenic infection[2].
AlthoughS.constrictahas been cultured for hundreds of years in China,in Vietnam they are only collected from natural beds. TRANet al.(2015)reported that the general genetic variation ofS.constrictain China was distinct from that of Vietnamese clams[1]. This suggests that wild VietnameseS.constrictamight be a better germplasm resource than cultured clams in China.
Glutathione S-transferases(GSTs),also known as ligandins,comprise a family of eukaryotic and prokaryotic phase IImetabolic isozymes.GSTs are antioxidants and part of a superfamily of enzymes that havemultiple functions,such as detoxification,targeting for transmembrane transport,protection of tissues from oxidative damage,ligand binding,and/or non-enzymatic binding for intracellular transport[2]. GSTs can metabolize a variety of xenobiotics as well as by-products of oxidative stress,such as reactive oxygen species,thereby facilitating the detoxification of various environmental stressors in prokaryotes and eukaryotes[2-3].For these reasons,GSTs can be used as a biomarker in the evaluation of toxic effects in various organisms[3-5]. In the aquatic environment,GSTs have been used as biomarkers in animals such as fish,shellfish,and worms[4,6-8].Most previous studies[6-8]measured the activity of total GSTs as the biochemical end point.Recently,the transcriptional response of individualGSTgenes has been examined,and results indicate that these genes have potential as biomarkers in aquatic toxicological assessments[9-11].
The goal of the present study is to compare GSTs from two dissimilar groups ofS.constrictaat the gene level.TheGSTgene was cloned and used as a biomarker to compare the hemocytes ofS.constrictafrom cultured (China) and wild(Vietnam) environment. Additionally, the expression profile ofGSTgenes in response to bacteria was assessed in the two groups.
1 M aterials and methods
1.1 Clam rearing and sam p ling
All experimental clams were obtained from Yuejingyang Farm, Ninghai City, Zhejiang Province,China and Cat Ba Island,Haiphong,Vietnam.The clams used in the experiment had a mean body weight of 9.2 g and amean body length of 5.5 cm.Those from China were kept at22-24℃in 27‰ salinity water,and those from Vietnam were kept at25-27℃ in 30‰ salinity water.The clams were acclimated to the laboratory conditions for 1 week in a circulating water system at 25℃,and they were fed twice weekly with algae(Chlorella vulgaris)purchased from SHANGHAIZZBIO CO.,LTD., China. Various tissues, including hemolymph,mantle,gonad,foot,siphon,gill,and liver,were collected from three clams from both China and Vietnam populations for full-length cDNA cloning and tissue expression analysis.All animal handling and experiments were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Shanghai Ocean University.
All sampleswere flash frozen in liquid nitrogen during collection,followed by storage at-80℃until RNA extraction.The total RNAs were isolated using TRIZOLReagent(Invitrogen,USA)following themanufacturer’s protocol.All RNA sampleswere treated with RNase-free DNase(Takara,Japan),quantified on a Nanodrop 2000C spectrophotometer(Thermo Scientific,USA),and stored at-80℃.RNA samples with a 260/280 ratio between 1.90 and 2.10 and a 260/230 ratio between 2.00 and 2.50 were considered satisfactory and used in this study.
First-strand cDNA synthesiswas performed in a volume of 20μL with 1μg of total RNA and the PrimeScript TMRT reagent kit(Takara,Japan)according to themanufacturer’s protocol.All cDNA sampleswere stored at-20℃.
1.2 Cloning the full-length cDNA of Sc-GST
The cDNA library ofS.constricta[12]was searched using theGSTgene from zebrafish(Danio rerio)as the query.The unique sequences obtained were analyzed using BlastX to confirm that the expressed sequence tags were related to theGSTgene.The chz_0054_C09 and chz_0023_H02 clones in the library contained the full 3′terminal and partial 5′terminal cDNA of Sc-GST;the 5′terminal cDNA of chz_0014_F04 was not complete.To confirm the transcript identities and sequence accuracy,re-sequencing was performed using the Sanger technology on the ABI3730 platform(Applied Biosystems,USA).To complete the cDNA sequence,the 5′terminal sequence was obtained using the 5′-Full RACE kit(Takara),using a 5′RACE primer(Supplementary Table 1)according to the manufacturers’ instructions.In addition,theSc-GSTgene open reading frame(ORF)was identified using the Open Reading Frame Finder(http://www.ncbi.nlm.nih.gov/gorf/gorf.htm l), and the signal peptide was predicted by the SignalP4.1 Server(http://www.cbs.dtu.dk/services/SignalP/).
The total volume of the PCR reaction forSc-GSTwas 25.0μL.The reaction mixture contained 1.0μL of cDNA template,0.5μL of sense primer,0.5μL of anti-sense primer,12.5μL ofTaq-Mix,and 10.5 μL of PCR-grade water.The PCR program used for short sequence verification ofSc-GSTwas:1 cycle of 94℃/2 min;35 cycles of 94℃/30 s,59℃/30 s,72℃/1 min;and 1 cycle of 72℃/10 min.The PCR products were ligated into pGEM-T easy vector(Promega,USA),transformed into competentEscherichia coliDH5αcells,plated onto LB-agar Petri dishes,and incubated overnight at37℃.Positive clones containing the insert with the expected size were identified by colony PCR.Six of the positive clones for each gene were picked and sequenced on an ABI PRISM 3730XL Automated Sequencer,using BigDye terminator v 4.10(Applied Biosystems).
The 3′RACE PCR was performed in a 50.0 μL reaction volume containing 8.0μL of 1 ×cDNA Dilution Buffer-II,4.0μL of 10×LA PCR Buffer-II(Mg2+free),3.0μL of MgCl2,0.25μL of Takara LATaq,2.0μL of cDNA,2.0μL of gene specific outer primer,2.0μL of 3′RACE outer primer(Universal),and 28.75μL of PCR-grade water.The PCR conditions for the both outer and inner 3′RACE were 1 cycle of 94℃/2 min;30 cycles of94℃/30 s,55℃/30 s,72℃/2 min;and 1 cycle of 72℃/10 min.The PCR products were cloned and sequenced as described above.
Gene-specific primers were designed from partial gene sequences using the Primer Premier 5 program and were used with the adaptor primers UPM and NUP to amplify the genes fromS.constrictacDNA (Table 1). The full-length sequence,5′-untranslated regions(5′-UTRs),and 3′-untranslated regions(3′-UTRs)for theSc-GSTgene were obtained using the SMARTerTMRACE cDNA Amplification kit(Clontech Laboratories,Inc., USA) to synthesize first stand cDNA according to the manufacturer’s instructions.The PCR conditionswere 94℃/1 min,25 cycles of 94℃/30 s,68℃/30 s,72℃/2 min,and 72℃/10 min.The PCR products were gel-purified using the Wizard®SV Gel and PCR Clean-Up System(Promega)before being cloned into the pMD 19-T vector(Takara).The pMD 19-T vector with the full-length gene was transformed intoE.coliDH5α competent cells,plated on LB-agar Petri dishes,and incubated overnight at 37℃.Positive colonies containing inserts of the expected sizewere screened by colony PCR.Three of the positive clones were picked for commercial sequencing(Sangon Biotech,China).
1.3 Bioinform atics analysis
The nucleotide and amino acid sequence identity analyses were performed using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi).The ORF ofSc-GSTcDNA was predicted using the ORF Finder(http://www.ncbi.nlm.nih.gov/projects/gorf/). The deduced amino acid sequence was analyzed with the Expert Protein Analysis System(http://www.expasy.org/),and the predicted transmembrane domain was determined using the TMHMM Server v.2.0(http://www.cbs.dtu.dk/services/TMHMM/). The putative furin cleavage site was identified using the ProP 1.0 Server(http://www.cbs.dtu.dk/services/ProP/).The needle program (http://www.ebi.ac.uk/Tools/emboss/align/)was used to determine the identities between different sequences.Multiple sequence alignments were performed using the CLUSTALW 2.1 program[13].The phylogenetic tree was constructed based on the deduced full-length amino acid sequences using the neighbor-joining algorithm in MEGA version 4.1,and analysis reliability was assessed by 1 000 bootstrap replicates[14].
1.4 Bacterial challenge experiment
For the bacterial challenge experiment,the four treatment groups(50 clams each group)were as follows:1)China control group(foot injected with 50μL phosphate-buffered saline(PBS);2)ChinaVibrio anguillarum-challenged group,foot injected with 50μL of pathogen suspension(1 x 108cells·μL-1),OD600=0.4,obtained from the Aquatic Pathogen Collection Centre of the Ministry of Agriculture and Rural Affairs,Shanghai,China);3)Vietnam control group(50μL PBS);4)VietnamV.anguillarum-challenged group(50μL pathogen suspension,1×108cells· μL-1).Injectionswere performed with a 26 gauge needle.All groupswere cultured under similar conditions in a circulating water system at25℃.
To determineSc-GSTgene expression in healthy tissues,samples of gill,mantle,gonad,foot,siphon,liver,and hemolymph(four pools of six individuals each)were isolated and flash-frozen in liquid nitrogen.Tissues were homogenized in liquid nitrogen using amortar and pestle then stored at-80℃ until RNA extraction.Similarly,the hemocytes,the most important tissue for immunity[15](four pools of six individuals each),were sampled at0,4,8,12,24,48,72 h afterV.anguillaruminfection for RNA extraction.Correspondingly,uninfected control samples were taken at each time point.
1.5 Real-time quantitative PCR(qRT-PCR)analysis
To examine expression of the related immune geneSc-GST,qRT-PCR analysis was conducted on seven tissues(hemolymph,mantle,foot,siphon,gill,liver,and gonad)from healthy clams from the China population.To compare the gene expression inS.constrictafrom China and Vietnam,qRT-PCR analysiswas also conducted on hemolymph collected from the four groups in the bacterial challenge experiment.Primerswere designed based on theSc-GSTcDNA sequence obtained in this study(Table 1).A 10-fold dilution series of cDNAs containing target gene fragmentswas used to construct standard curves.The amplification efficiency was measured from the regression slope of the standard curve.Only the primer pairs that had a single peak in melting curve analysis and displayed amplification efficiency close to the theoretical 100%were retained for further use.
The total volume of reaction mixture was 20.0 μl and contained 10.0μL of 2×SYBR Green SupermixTaq(Takara,Japan),0.6μL of sense primer,0.6μL of anti-sense primer,2.0μL of cDNA sample,and 6.8μL of nuclease-free water.The reaction consisting of the same reaction mixture without template was used as the negative control.qRT-PCR was performed in triplicate for each sample on a CFX96TMReal-Time-System(BIORAD,USA).ForSc-GST,the PCR parameters were as follows:1 cycle of 95℃/3 min;35 cycles of 95℃/15 s,50℃/15 s;and dissociation curve analysis of 5 s per step from 65℃to 95℃to verify that a single productwas amplified.
All statistical analyses were based on theSc-GSTexpression levels normalized to the 18S rRNA level ( forward primer: 5′TCGGTTCTATTGCGTTGGTTTT 3′;reverse primer:5′CAGTTGGCATCGTTTATGGTCA 3′).
1.6 Statistical analysis
The triplicate fluorescence intensities of the control and treatment products,as measured by crossing-point(Ct)values,were compared and converted into fold differences by the relative quantification method using the Relative Expression Software Tool 384 v.1 (REST)[16], assuming 100%efficiency.Expression differences between the control and treatment groups were assessed for statistical significance(P<0.05) using a randomization test in the REST software.
2 Results
2.1 Cloning and characterization of Sc-GST
Specific primers for immune-related geneswere designed and used for RACE-PCR to obtain 5′-and 3′-cDNA ends.The full-length cDNA ofSc-GST(GenBank accession no.KP993504)was composed of 1 052 base pairs(bp),including 700 bp of the 5′-UTR and 280 bp of the 3′-UTR,which contained the poly(A)tail.The 678 bp ORF encoded a 225 amino acid polypeptide.
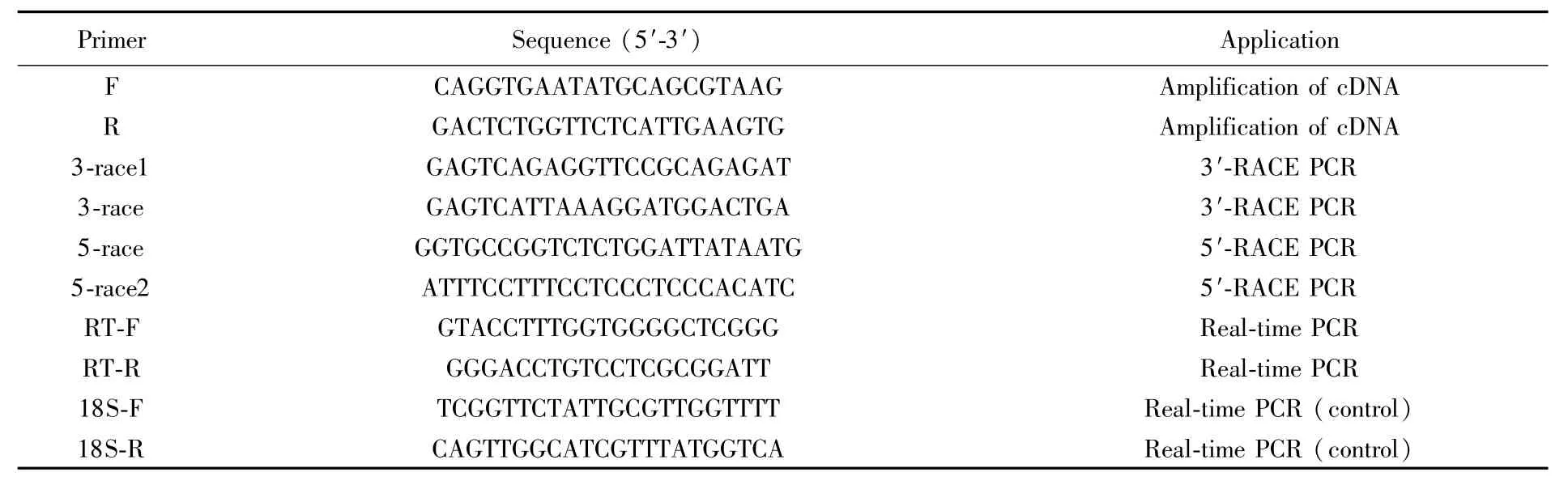
Tab.1 O ligonucleotide primers used to am plify Sc-GST gene

Fig.1 Am ino acid sequence alignm ent of severalmollusc species Sc-GST w ith GSTs using ClustalW 2.1 program
2.2 Phylogenetic analysis of Sc-GST sequence
To study the evolutionary relationship ofSc-GSTwith other known proteins,amino acid sequences corresponding to representative orthologs from several species were aligned with theSc-GSTgene using the ClustalW 2.1 software.The deduced amino acid sequence ofSc-GSTshowed high homology with the sequences of other organisms in the GenBank database,such as the Pacific oyster(C.gigas)(53%similarity),Manila clam(R.philippinarum)(48%similarity),and disk abalone(Haliotis discus discus)(47%similarity)(Fig.1).Phylogenetic analysis was conducted to identify the GST-related sequences inS.constricta,which showed thatSc-GSTclusters with other ortholog proteins, with the highest similarity toR.philippinarum(Fig.2).
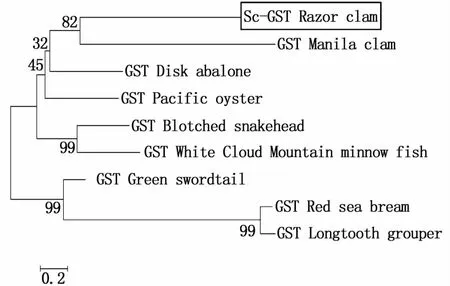
Fig.2 Phylogenetic tree show ing the relationship of Sc-GST am ino acid sequence w ith other identified GST sequences
2.3 Expression of Sc-GST in healthy S.constricta from China
The expression levels ofSc-GSTgene,as determined by qRT-PCR and normalized to 18S rRNA,varied among different tissues.The highest expression levels were observed in gill,and the lowest expression level occurred in siphon(Fig.3).
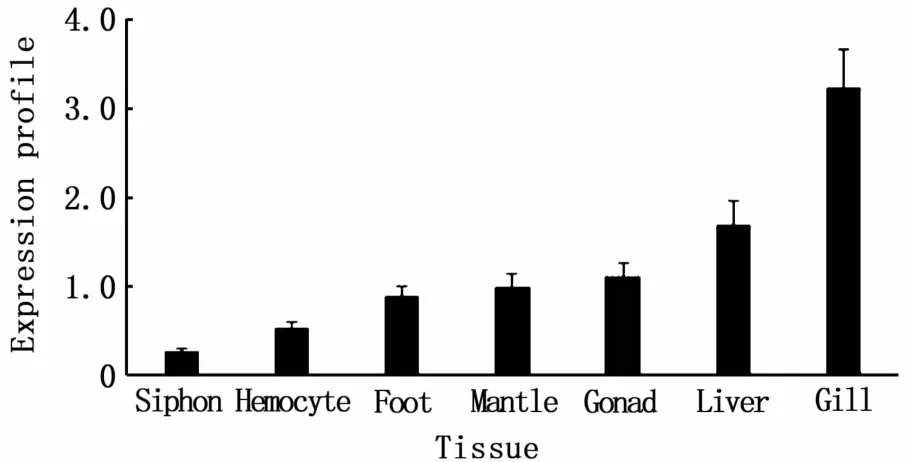
Fig.3 Tissue expression profile of Sc-GST gene
2.4 Com parison of relative expression level of Sc-GST after bacterial challenge in S.constricta from Vietnamese and Chinese populations
The expression pattern ofSc-GSTwas assessed in hemolymph of twoS.constrictapopulations at 0,4,8,12,24,48,72 h post-injection withV.anguillarum,using qRT-PCR.For theGSTgene and18Sgene,only one peak at the corresponding melting temperaturewas observed in the dissociation curve analysis,indicating that the PCR was specifically amplified(data not shown).Relative expressionswere determined as fold changes relative to the PBS-injected control samples.After infection withV.anguillarum,Sc-GSTexpression was upregulated or down-regulated in a group-specific or time-specific manner.
The effect ofV.anguillarumonS.constricta.hemolymph was detected at 12 h post-injection in both populations(Fig.4).Expression ofSc-GSTmRNA in both groups stayed nearly at the baseline level for the first4 h post-challenge,although clams from Vietnam exhibited lower expression compared to clams from China at these two time points.Expression quickly increased by 12 h and remained at a high level until peaking at the end of the experiment at72 h.
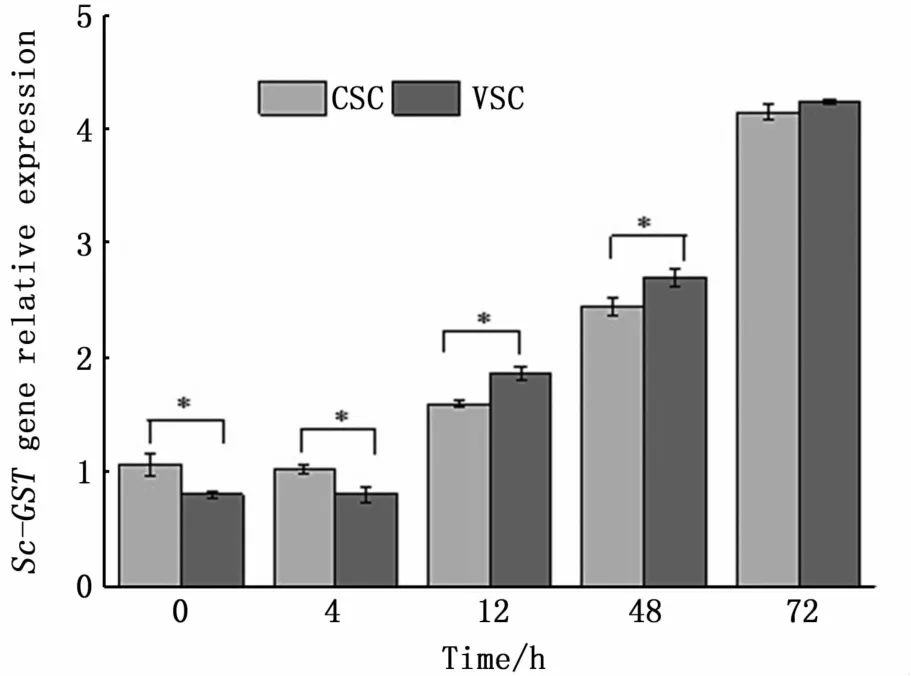
Fig.4 Quantitative expression profiles of Sc-GST gene in hemolym ph from Vietnamese(VSC)and Chinese(CSC)S.constricta at different tim e points after challenge w ith V.anguillarum
3 Discussion
GSTs are well-known multifunctional enzymes involved in the detoxification of many toxicants,including herbicides, insecticides, and drugs.Therefore,gene expression and/or activity of GSTs frequently is utilized as a biomarker of exposure to xenobiotics in environmental studies.Furthermore,GST variants have been identified in a range of species.For instance,41 GSTs from the fruit flyDrosophila melanogaster[17]and 37 GSTs from the mosquitoAnopheles gambiae[18]have been reported.Many GSTs have been identified and characterized in aquatic species,including molluscs such as the clamAtactodea striata[19], squidOmmastrephessloani pacificus[20], andR.philippinarum[21-22].Additionally,themu class GSTs have been reported in certainmarine species,including whiteleg shrimpLitopenaeus vannamei[23], rock shellThais clavigera[24],H.discus discus[25], andR.philippinarum[22].Most of these studies focused on biochemical characterization and expression profiles after pathogenic and toxic chemical exposures.However,a limited number ofGSTisoforms from molluscs have been identified,particularly inS.constricta. In this study, we identified and functionally characterized aGSTgene fromS.constricta.
TheSc-GSTgene ofS.constrictawas cloned using RACE-PCR.Sc-GSTwas most similar to theGSTofC.gigas(Cgc-GST)with 53%similarity.In theGSTfamily,there are at least 14 subclass members with different primary structures,enzyme properties, and physiological functions[26].According to different functions,GSTgenes would be expressed differently in tissues.Mu class GSTs,as one of the most important components of the mammalian GST system,displayed ubiquitous and high level expression in all inner organs of humans and mice,illustrating their pivotal role in defense against xenobiotics and endogenetic oxidative stress[27-28].
We used qRT-PCR analysis to monitor expression in several tissues of the normalS.constricta.The highest expression ofSc-GSTwas detected in gill tissue,which was openly exposed to waterborne xenobiotics, followed by liver.Similarly,GSTswere highly expressed in gill tissue ofR.philippinarum[21],H.discus discus[25],andT.clavigera[24].Other studies analysingGSTσ[22]andGSTθ[29]fromR.philippinarumalso demonstrated strongmRNA expression levels in gill.These findings clearly indicate that gill tissue is important in terms ofSc-GSTexpression.Because gills are constantly exposed to the external aquatic environment,high expression ofSc-GSTin gills indicates the contribution of this tissue to detoxification ofwaterborne xenobiotics.
GSTs can detoxify bacterialmetabolites,which can be present in infected biological systems.In this study,we challengedS.constrictawith live bacteria(V.anguillarum).The expression profiles ofSc-GSTrevealed stimulant-specific elevated levels in the early phase of exposure thatwasmaintained into the late phases.These responsive profiles support the hypothesis that GSTs play an immune role inS.constricta,in addition to their conventional function as a xenobiotic detoxifier.The up-regulated transcription ofSc-GSTcould correlate with the induced production of reactive oxygen species from the respiratory burst process during phagocytosis.These results hint thatS.constrictaGSTsmight play leading roles in clam immunity under hostpathological conditions.
In this study,immune-relatedSc-GSTgene transcripts were highly up-regulated after bacterial injections in both populations.V.anguillaruminfection suppressed the immune response at later time points.In hemolymph,the expression level of Sc-mRNA in hemolymph tended to increase continuously from 12 h to 72 h after bacterial challenge.It has been suggested thatGSTgenes might play an important immune role against bacterial pathogens.In this study,expression ofSc-GSTdiffered significantly between the two groups ofS.constrictaat all time points except 72 h.Sc-GSTgene expression ofS.constrictafrom Vietnam was significantly elevated relative to the Chinese population at12 h and 48 h post-exposure,and the values increased until the experiment finished at 72 h.A possible explanation for this difference is that the bacteria used in the experiment were obtained from China,thus likely theS.constrictafrom China had been already adapted to this pathogen,and therefore,had a lower level ofSc-GSTexpression in hemolymph.
In conclusion,this is the first study to clone and characterize fromS.constricta.Sc-GSTwas differentially expressed in different tissues from normalS.constrictasampled from Vietnam and China.We successfully cloned the full-length cDNA ofSc-GST,analyzed the structure of the protein,examined the phylogenetic relationship ofS.constrictabased onGST,and demonstrated the expression profiles in normal tissues and in tissues from clams exposed toV.anguillaruminfection.Of the sampling locations analyzed,Vietnam is characterized by having high water temperature and relatively low inbreeding incidence,whereas China has lower water temperature,but a higher incidence of inbreeding.In terms of gene expression,S.constrictafrom Vietnam weremore seriously affected byV.anguillaruminfection than clams from China.The results of this study indicated that the immunerelatedSc-GSTgene was significantly up-regulated after bacterial infection,which suggested its potential role in the innate immune response.The increased expression ofSc-GSTinduced byV.anguillaruminfection confirms its role in antibacterial processes inS.constricta.Further studies should focus on understanding themechanism ofS.constricta’s innate immune response to these pathogens in both Vietnam and China.
Acknowledgements
We thank International Science Editing(http://www.internationalscienceediting.com )for editing thismanuscript.
