新型TOPK抑制剂的设计、合成及抗肿瘤活性研究
2020-04-22黄珍方宇希黄志勇李荣东
黄珍 方宇希 黄志勇 李荣东
〔摘要〕 目的 将TOPK抑制剂OTS514的母核噻吩并[2,3-c]喹啉酮环替换成喹唑啉,探究含有喹唑啉的新型TOPK抑制剂的抗肿瘤细胞增殖活性。方法 以OTS514作为先导化合物设计并合成一系列4-氨基喹唑啉衍生物。采用MTT法测试目标化合物对肺癌细胞(A549)和乳腺癌细胞(MDA-MB-231)的抗增殖活性。结果 合成了16个未见报道的新化合物,其结构经1H NMR和高分辨MS确证。手性因子对抗肿瘤活性影响不明显,暴露的氨基能增强化合物的抗肿瘤活性。体外抗肿瘤实验表明,化合物12a-12f的活性与OTS514相当。结论 新骨架的TOPK抑制剂具有和OTS514相当的抗肿瘤活性,为进一步探索含有喹唑啉药效团的TOPK抑制剂研究打下了基础。
〔关键词〕 TOPK抑制剂;抗肿瘤药;喹唑啉;合成
〔中图分类号〕R914.5 〔文献标志码〕A 〔文章编号〕doi:10.3969/j.issn.1674-070X.2020.03.010
〔Abstract〕 Objective To investigate the anti-tumor cell proliferation activity of a novel TOPK inhibitor containing quinazoline, the parental thiophene [2,3-c] quinolinone ring of TOPK inhibitor OTS514 was replaced by quinazoline. Methods A series of 4-aminoquinazoline derivatives were designed and synthesized with OTS514 as the lead compound. MTT method was used to test the antiproliferative activity of the target compound on lung cancer cells (A549) and breast cancer cells (MDA-MB-231). Results Sixteen new compounds were synthesized and their structures were confirmed by 1H NMR and high-resolution MS. The effect of chiral factors on antitumor activity was not obvious, and the exposed amino group can enhance the antitumor activity of the compounds. The antitumor activity of compound 12a-12f in vitro was similar to that of OTS514. Conclusion The new skeleton TOPK inhibitor has the same antitumor activity as OTS 514, which lays a foundation for further study of TOPK inhibitors containing quinazoline pharmacophore.
〔Keywords〕 TOPK inhibitors; antineoplastic agents; quinazoline; synthesis
惡性肿瘤严重威胁着人类的生命健康。目前,它们已超越其他疾病,成为变色的“头号杀手”[1]。与传统抗肿瘤药物相比,靶向抗肿瘤药物具有提高疗效、提高药物选择性和患者依从性等优点,已成为抗肿瘤药物的重要研究方向[2-3]。TOPK,也称为PBK或PDZ结合激酶,是MAPKK蛋白家族的成员[4-5]。它是一种丝氨酸苏氨酸丝裂原激活蛋白激酶,在肺癌、乳腺癌、结直肠癌、淋巴瘤、白血病、黑素瘤、胆管癌和胶质瘤等多种人类癌症中高度表达[6-14]。TOPK可能是药物开发的一个有前途的分子靶点,它涉及多种细胞功能,包括肿瘤发展、细胞生长、凋亡和炎症[15-18]。 根据TOPK抑制剂的作用机理和构效关系,发现TOPK抑制剂的3环芳香环结构是其抗肿瘤活性的关键,侧链的改变可能改变药物的选择性,如:HI-TOPK-032[19]可有效抑制PBK/TOPK激酶的活性,对细胞外信号调节激酶1、氨基末端激酶和p38 蛋白激酶(p38 kinase activities)等激酶影响较弱;HI-TOPK-032可通过减少ERK-RSK磷酸化达到抑制贴壁依赖型和非依赖型结肠癌细胞生长。但是现有的PBK/TOPK抑制剂存在一定的缺陷:(1)分子中的3个或者4个芳香性结构片段具有较强的脂溶性,因此其理化性质在成药性上存在一定的技术缺陷。(2)该类药物具有血液毒性反应,虽然把药物包裹在脂质体中能够有效避免毒性反应,但是仍存在用药安全性问题。
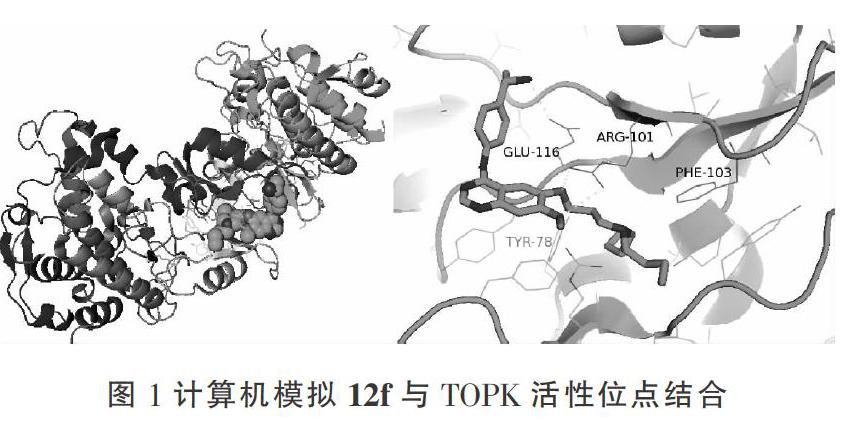
考虑到7-羟基-苯并咪唑类化合物和噻吩并[2,3-c]喹诺酮类化合物具有抑制TOPK激酶活性,采用骨架跃迁原理,对其进行结构改造。将噻吩并[2,3-c]喹诺酮环替换成喹唑啉环。通过计算机模拟化合物12f与TOPK的活性位点对接,发现化合物12f与TOPK的ARG-101和GLU-116形成氢键,从而表现出对TOPK的抑制作用。见图1。
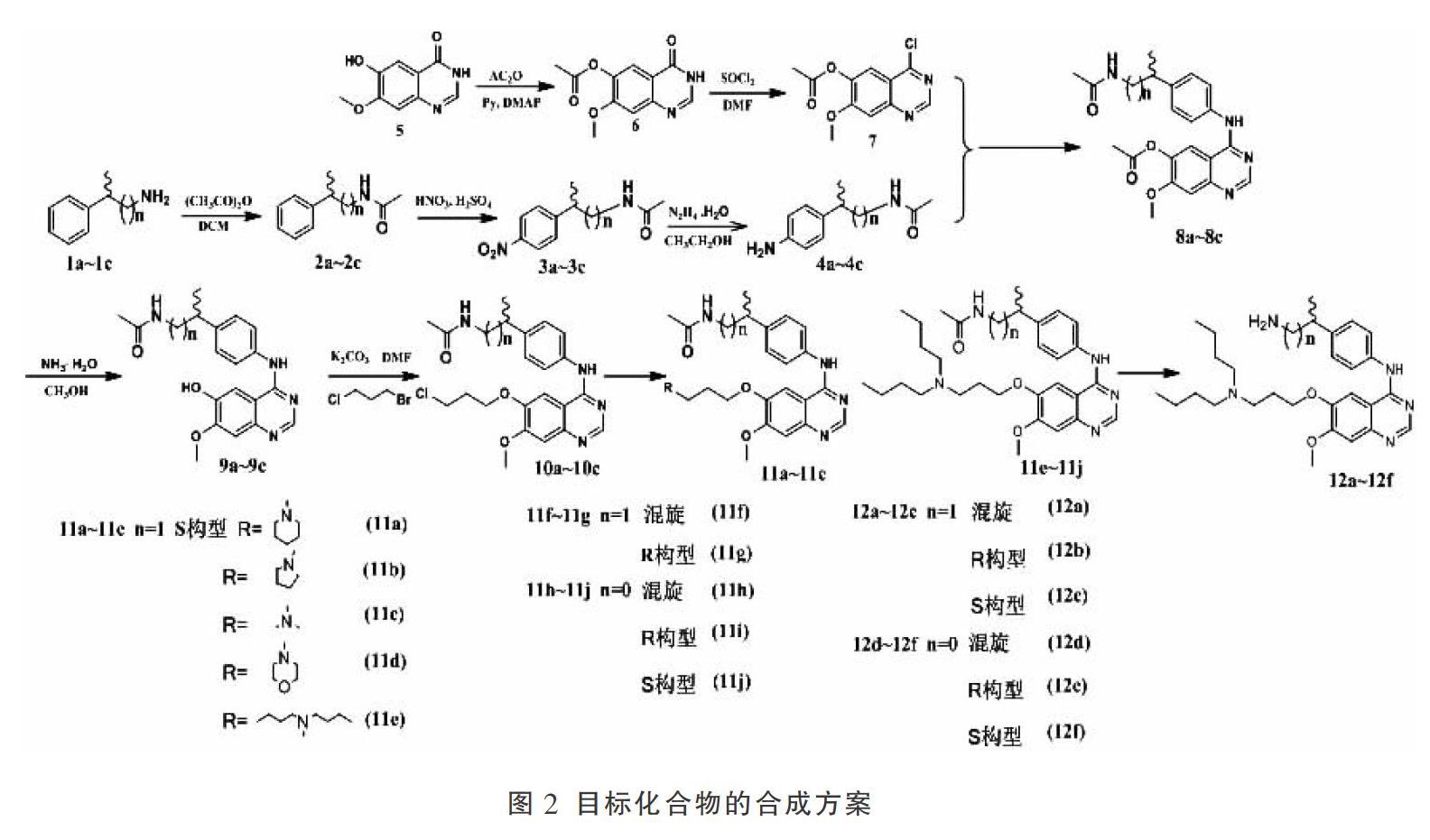
目标化合物11a-11j和12a-12f合成方案见图2。以2-苯基-1-丙胺的混旋、R、S构型和1-苯基乙胺的混旋、R、S构型为原料合成关键中间体4;以6-羟基-7-甲氧基-4-酮喹唑啉为起始原料经过乙酰化和氯化得到中间体7。将中间体4和7对接得到化合物8,在其6位上修饰得到化合物11a-11j。将化合物11e-11j再进一步脱去乙酰基得到目标化合物12a-12f。
1 仪器与试药
YRT-3型熔点仪(天津海益达科技有限公司);RE-2000B型旋转蒸发仪(巩义市予华仪器有限公司);ZF7三用紫外分析仪(巩义市予华仪器有限公司);Water Xevo G2-XS TOf质谱仪(美国沃特世公司);核磁共振波谱仪AVANCE III HD 600(德国布鲁克公司);Multiskan MK3多功能酶标仪(Thermo公司);A549人肺癌细胞株(上海酶研生物科技有限公司);MDA-MB-231人乳腺癌细胞株(上海酶研生物科技有限公司);吉非替尼(Solarbio公司)。
实验所有试剂均为市售得分析纯或化学纯,所有试剂使用前都未作处理。
2 方法与结果
2.1 目标化合物的合成
2.1.1 N-(2-苯基丙基)乙酰胺(2)的制备 2-苯基-1-丙胺(13.5 g,0.1 mol)溶于二氯甲烷中(54 mL),在0 ℃滴加乙酸酐(8.6 mL,0.092 mol)的二氯甲烷(70 mL)溶液,搅拌反应30 min,再室温搅拌反应8 h,TLC检测反应完全后,停止反应。向反应液中加入二氯甲烷(45 mL)和2% NaOH溶液(50 mL),分出有机相,水(100 mL×2)洗涤,无水硫酸钠干燥,减压回收二氯甲烷,得淡黄色油状液体12.15 g,收率: 68.9%。HRMS(ESI)Calcd for C11H14N2O3[M+H]+177.1145, found 177.1136。
2.1.2 N-[2-(4-硝基苯基)丙基]乙酰胺(3)的制备 N-(2-苯基丙基)乙酰胺(9.0 g,0.051 mol)-5 ℃滴加混酸11 mL(98%硫酸∶68%硝酸=6∶5),滴加完毕后继续反应1 h,室温搅拌反应12 h,TLC检测反应完全后,停止反应。倒入冰水中,二氯甲烷 (40 mL×3)提取,分出有机相,无水硫酸钠干燥,减压蒸除二氯甲烷,得到黄色固体产物8.0 g,收率:70.9%,m.p.83.5~84.3 ℃。HRMS(ESI)Calcd for C11H14N2O3[M+H]+222.1054, found 222.1056。
2.1.3 N-[2-(4-氨基苯基)丙基]乙酰胺(4)的制备 N-[2-(4-硝基苯基)丙基]乙酰胺(11.1 g,0.05 mol)、无水乙醇(92 mL)、活性炭(0.92 g,0.078 mol)和六水合三氯化铁(0.23 g,0.001 mol)88 ℃搅拌30 min,滴加80%水合肼(7.6 mL,0.13 mol),继续回流反应6 h,趁热抽滤,减压蒸除无水乙醇,二氯甲烷 (40 mL×3)提取,无水硫酸钠干燥,减压蒸除二氯甲烷,得粗产物柱色谱纯化(乙酸乙酯∶石油醚=4∶1),得7.0 g黄色油状物,收率:73.1%。HRMS(ESI)Calcd for C11H16N2O[M+H]+193.1296, found 193.1305。
2.1.4 7-甲氧基-6-乙酰氧基-4-氯喹唑啉(7)的制备 6-羟基-7-甲氧基-4-酮喹唑啉(16.7 g,0.1 mol)、乙酸酐(125 mL,1.3 mol)和吡啶(16.7 mL,0.22 mol)100 ℃搅拌30 min,加入4-二甲氨基吡啶(0.75 g,0.006 mol),继续搅拌6 h,停止反应,减压蒸除乙酸酐,加入大量冰水搅拌,抽滤,烘干,得黄白色固体。将其加至氯化亚砜(158 mL,2.1 mol)中,80 ℃搅拌,再缓慢滴加N,N-二甲基甲酰胺(6.2 mL,0.08 mol)。6 h后停止反应。减压回收氯化亚砜,加入少量甲苯搅拌,再减压回收甲苯。将反应物倒入冰水中,抽滤,得灰白色固体17.9 g,收率: 82.3%[24]。
2.1.5 4-[4-(2-乙酰胺基-1-甲基乙基)苯氨基]-7-甲氧基-6-乙酰氧基喹唑啉(8)的制备 N-[2-(4-氨基苯基)丙基]乙酰胺(9.5 g,0.05 mol)、异丙醇 (59.5 mL)和7-甲氧基-6-乙酰氧基-4-氯喹唑啉(11.2 g,0.043 mol)90 ℃加热反应4 h,TLC检测反应完全后,停止反应,抽滤,滤饼用少量异丙醇洗涤,烘干,得淡黄色固体15.2 g,收率:75.3%,m.p.>200 ℃。HRMS(ESI)Calcd for C22H24N4O4[M+H]+ 409.1847, found 409.1856。
2.1.6 4-[4-(2-乙酰胺基-1-甲基乙基)苯氨基]-6-羟基-7-甲氧基喹唑啉(9)的制备 4-[4-(2-乙酰胺基-1-甲基乙基)苯氨基]-7-甲氧基-6-乙酰氧基喹唑啉(8.4 g,0.02 mol)和甲醇(60 mL)升温至70 ℃搅拌,加入氨水(5.8 mL,0.15 mol),溶液由浑浊变澄清,继续反应4 h,TLC检测反应完全后,停止反应。减压回收甲醇,得黄色油状物。加入丙酮(15 mL),升温至65 ℃回流,出现黄白色固体,停止反应,过滤,得黄白色固体6.7 g,收率:88.9%,m.p.183.2~188.6 ℃。HRMS(ESI)Calcd for C20H22N4O3[M+H]+367.1638, found 367.1643。
2.1.7 4-[4-(2-乙酰胺基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-氯丙氧基)喹唑啉(10)的制備4-[4-(2-乙酰胺基-1-甲基乙基)苯氨基]-6-羟基-7-甲氧基喹唑啉(7.4 g,0.02 mol)、N,N-二甲基甲酰胺(74 mL)、碳酸钾(4.2 g,0.03 mol)和少量碘化钾,75 ℃搅拌20 min,加入1,3-溴氯丙烷(2.2 mL,0.023 mol),继续反应5 h,TLC检测反应完全后,停止反应。加水100 mL,搅拌有固体析出,抽滤得粗产品,丙酮重结晶得到黄色固体7.4 g,收率:83.0%,m.p.175.3~176.2 ℃。HRMS(ESI)Calcd for C23H27ClN4O3[M+H]+443.1820, found 443.1826。
2.1.8 目標化合物11f的合成 4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-6-羟基-7-甲氧基喹唑啉(3.6 g,0.01 mol)、N,N-二甲基甲酰胺(45 mL)、碳酸钾(2.0 g,0.014 mol)和少量碘化钾95 ℃搅拌,再缓慢加入N-(3-氯丙基)-二丁基胺(2.72 mL,0.011 mol),继续反应6 h,TLC检测反应完全后,停止反应。加水150 mL,搅拌有固体析出,抽滤得粗产品。在室温下,将粗产品溶于丙酮,缓慢滴加盐酸,搅拌有固体出现,抽滤,得淡黄色固体4.2 g,收率:77.8%。
目标化合物11a-11j的合成方法参照上述方法。
2.1.9 目标化合物12a的合成 4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(1 g,0.002 mol)和1N HCl(5 mL)依次加入到100 mL的圆底烧瓶中。升温至95 ℃回流反应48 h,HPLC监控检测反应完全后,停止反应。减压蒸馏,用少量无水乙醇多次洗涤,继续减压蒸除无水乙醇,真空干燥得黄白色固体0.7 g,收率:77.8%。
目标化合物12a-12f的合成方法参照上述方法。
2.2 目标化合物的图谱数据
2.2.1 (S)-4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(1-六氢哌啶基)丙氧基)喹唑啉(11a) 淡黄色固体,收率 58.0%,m.p.160.2~163.3 ℃。1H NMR (600 MHz, MeOD) δ 8.35 (s, 1H), 7.78 (s, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.28 (d, J = 8.5 Hz, 2H), 7.15 (s, 1H), 4.30 (t, J = 5.7 Hz, 2H), 3.99 (s, 3H), 3.37-3.32 (m, 2H), 3.19-3.06 (m, 6H), 3.00-2.96 (m, 1H), 2.30-2.26 (m, 2H), 1.90 (s, 3H), 1.86-1.79 (m, 4H), 1.27 (d, 3H), 1.20-1.04 (m, 1H), 0.96-0.76 (m, 1H). HRMS(ESI)Calcd for C28H37N5O3[M+H]+492.2939, found 429.2930。
2.2.2 (S)-4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(1-四氢吡咯基)丙氧基)喹唑啉(11b) 淡黄色固体,收率57.0%,m.p.153.1~154.3 ℃。1H NMR(600 MHz, MeOD) δ 8.42 (s, 1H), 7.90 (s, 1H), 7.66 (d, J = 8.3 Hz, 2H), 7.30 (d, J= 8.5 Hz, 2H), 7.18 (s, 1H), 4.39 (t,J = 5.4 Hz, 2H), 4.03(s, 3H), 3.51 (t, J = 7.1 Hz, 2H), 3.39-3.33 (m, 2H), 3.23-3.18 (m, 4H), 3.01-2.97 (m, 1H), 2.41-2.34 (m, 2H), 1.90 (s, 3H), 1.33-1.31(m, 4H), 1.28 (d, J = 4.1 Hz, 3H)。HRMS(ESI)Calcd for C27H35N5O3[M+H]+478.2813, found 478.2805。
2.2.3 (S)-4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二甲胺基)丙氧基)喹唑啉(11c) 淡黄色固体,收率:65.0%,m.p.137.2~139.6 ℃。1H NMR (600 MHz, MeOD) δ 8.34 (s, 1H), 7.71 (s, 1H), 7.62 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 8.4 Hz, 2H), 7.11 (s, 1H), 4.23 (t, J = 6.0 Hz, 2H), 3.98 (s, 3H), 3.41-3.32 (m, 2H), 3.00-2.95 (m, 1H), 2.79 (t, J =7.5Hz, 2H), 2.47 (s, 6H), 2.17-2.11 (m, 2H), 1.90 (s, 3H), 1.28 (d, J = 7.0 Hz, 3H)。HRMS(ESI)Calcd for C25H33N5O3[M+H]+ 452.2653, found 452.2660。
2.2.4 (S)-4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(1-吗啉基)丙氧基)喹唑啉(11d) 白色固体,收率:70.2%,m.p.168.4~170.0 ℃。1H NMR (600 MHz, MeOD) δ 8.65(s, 1H), 8.17 (s, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.36 (d, J = 8.3 Hz, 2H), 7.27 (s, 1H), 4.45 (t, J = 5.4 Hz, 2H), 4.10 (d, 2H), 4.08 (s, 3H), 3.89 (t, J = 11.9 Hz,3H), 3.66 (d, J = 12.4 Hz, 2H), 3.48 (t, J = 7.4 Hz, 2H), 3.37 (d, J = 7.4 Hz, 2H), 3.24 (td, J = 12.3, 3.4 Hz,4H), 3.07-3.00 (m, 1H), 2.47-2.41 (m, 2H), 1.93 (s, 3H), 1.30 (d, J = 7.0 Hz, 3H)。HRMS(ESI)Calcd for C27H35N5O4[M+H]+494.2709, found 494.2703。
2.2.5 (S)-4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(11e) 灰白色固体,收率: 76.0%,m.p.155.4~156.8 ℃。 1H NMR (600 MHz, MeOD) δ 8.66 (s, 1H), 8.20 (s, 1H), 7.70 (d, J = 8.1 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 7.28 (s, 1H), 4.43 (t, J = 5.2 Hz, 2H), 4.09 (s, 3H), 3.47 (t, J = 7.7 Hz,2H), 3.40 (d, J = 6.8 Hz, 2H), 3.25 (t, J = 8.3 Hz, 4H), 3.07-3.02 (m, 1H), 2.42-2.31 (m, 2H), 1.96 (s, 3H), 1.83-1.70 (m, 4H), 1.51-1.41 (m, 4H), 1.30 (d, J = 7.0 Hz, 3H), 1.03 (t, J = 7.4 Hz, 6H)。HRMS(ESI)Calcd for C31H45N5O3 [M+H]+536.3588, found 536.3597。
2.2.6 4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(11f) 灰白色固體,收率: 77.8%,m.p.155.4~157.9 ℃。1H NMR (600 MHz, MeOD) δ 8.36 (s, 1H), 7.77 (s, 1H), 7.63 (d, J = 8.2 Hz, 3H), 7.28 (d, J = 8.2 Hz, 3H), 7.14 (s, 1H), 4.28 (t, J = 5.5 Hz, 2H), 3.99 (s, 3H), 3.45-3.32 (m, 2H),3.09 (t, J = 6.4 Hz, 4H), 3.00-2.95 (m, 1H), 2.92-2.78 (m, 4H), 2.21-2.13 (m, 2H), 1.90 (s, 3H), 1.65-1.56 (m, 4H), 1.42-1.35 (m, 4H), 1.28 (d, J = 6.9 Hz, 3H), 0.97 (t, J = 7.4 Hz, 6H)。HRMS(ESI)Calcd for C31H45N5O3[M+H]+536.3611, found 536.3618。
2.2.7 (R)-4-[4-(2-乙酰氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(11g) 灰白色固体,收率:75.5%,m.p.148.0~149.0 ℃。1H NMR (600 MHz, MeOD) δ 8.66 (s, 1H), 8.19 (s, 1H), 7.69 (d, J = 8.0 Hz, 2H), 7.63 (d, J = 8.4 Hz, 2H), 7.27 (s, 1H), 4.43 (t, J = 4.9 Hz, 2H), 4.09 (s, 3H),3.48 (t, J = 6.8 Hz, 2H),3.38 (d, J = 6.8 Hz, 2H), 3.25 (t, J = 8.3 Hz, 4H), 3.05-3.01 (m, 1H), 2.40-2.36 (m, 2H),1.92 (s, 3H), 1.81-1.74(m, 4H), 1.49-1.44(m, 4H),1.30 (d, J = 7.0 Hz, 3H), 1.03 (t, J = 7.4 Hz, 6H)。HRMS(ESI)Calcd for C31H45N5O3 [M+H]+536.3614, found 536.3615。
2.2.8 4-[4-(1-乙酰氨基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(11h) 灰白色固体,收率: 60.0%,m.p.165.4~167.7 ℃。1H NMR (600 MHz, MeOD) δ 8.37 (s, 1H), 7.79 (s, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.37 (d, J = 8.4 Hz, 2H), 7.17 (s, 1H), 5.04-5.01 (m, 1H), 4.30 (t, J = 5.6 Hz, 2H), 4.00 (s, 3H), 3.15 (t, J = 15.5 Hz, 2H), 2.93 (t, J = 6.9 Hz,4H), 2.23-2.18 (m, 2H), 1.98 (s, 3H), 1.67-1.62 (m, 4H), 1.47 (d, J = 7.0 Hz, 3H), 1.42-1.38 (m, 4H), 0.98 (t, J = 7.4 Hz, 6H)。HRMS(ESI)Calcd for C30H43N5O3[M+H]+ 522.3316, found 522.3323。
2.2.9 (R)-4-[4-(1-乙酰氨基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(11i) 灰白色固体,收率:76.0%,m.p.155.4~157.9 ℃。1H NMR (600 MHz, MeOD) δ 8.36 (s, 1H), 7.74 (s, 1H), 7.66 (d, J = 8.3 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 7.15 (s, 1H), 5.04-5.00 (m, 1H), 4.24 (t, J = 5.7 Hz, 2H), 3.99 (s, 3H), 2.86 (t, J = 7.1 Hz, 2H), 2.62 (t, J = 20.9, 13.1 Hz, 4H), 2.08 (dd, J = 13.3, 6.9 Hz, 2H), 1.98 (s, 3H), 1.55-1.50 (m, 4H), 1.47 (d, J = 7.0 Hz, 3H), 1.34 (dd, J = 14.9, 7.5 Hz, 4H), 0.93 (t, J = 7.4 Hz, 6H)。HRMS(ESI) Calcd for C30H43N5O3[M+H]+522.3442, found 522.3448。
2.2.10 (S)-4-[4-(1-乙酰氨基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(11j) 灰白色固体,收率:77.0%,m.p.158.4~160.9 ℃。1H NMR (600 MHz, MeOD) δ 8.66 (s, 1H), 8.16 (s, 1H), 7.70 (d, J = 8.4 Hz, 2H), 7.43 (d, J = 8.5 Hz, 2H), 7.26 (s, 1H), 5.05-5.02 (m, 1H), 4.42 (t, J = 4.8 Hz, 2H), 4.09 (s, 3H), 3.47 (t, J = 7.7 Hz, 2H), 3.25 (t, J = 8.3 Hz, 4H), 2.39-2.35 (m, 2H), 1.99 (s, 3H), 1.80-1.75 (m, 4H), 1.49-1.45 (m, 7H), 1.03 (t, J = 7.4 Hz, 6H)。HRMS(ESI)Calcd for C30H43N5O3[M+H]+522.3419, found 522.3426。
2.2.11 4-[4-(2-氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(12a) 黄白色固体,收率:77.8%,m.p.120.5~123.1 ℃。1H NMR (600 MHz, MeOD) δ 8.67 (s, 1H),8.27 (s, 1H), 7.78 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.6 Hz, 2H), 7.29 (s, 1H), 4.45 (t, J = 5.4 Hz, 2H), 4.09 (s, 3H), 3.48 (t, J = 7.7 Hz,2H), 3.27-3.22 (m, 5H), 3.21-3.17 (m, 2H), 2.41-2.36 (m, 2H), 1.83-1.69 (m, 6H), 1.50-1.43 (m, 4H), 1.40 (d, J = 6.5 Hz, 3H), 1.03 (t, J = 7.3, 2.7 Hz, 6H)。HRMS(ESI)Calcd for C29H47N5O2[M+H]+494.3487, found 494.3496。
2.2.12 (R)-4-[4-(2-氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(12b) 黄白色固体,收率:73.8%,m.p.121.4~122.9 ℃。1H NMR(600 MHz, MeOD) δ 8.67 (s, 1H), 8.27 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 7.45 (d, J = 7.9 Hz, 2H), 7.30 (s, 1H), 4.45 (t, J = 5.0 Hz, 2H), 4.10 (s, 3H), 3.47 (t, J = 7.6 Hz, 2H), 3.27-3.22 (m, 5H), 3.21-3.17 (m, 2H), 2.41-2.36 (m, 2H), 1.82-1.71 (m, 6H), 1.48-1.45 (m, 4H), 1.40 (d, J = 6.5 Hz, 3H), 1.03 (t, J = 7.3 Hz, 6H)。HRMS(ESI)Calcd for C29H47N5O2[M+H]+494.3380, found 494.3376。
2.2.13 (S)-4-[4-(2-氨基-1-甲基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(12c) 黄白色固体,收率:70.0%,m.p.119.9~120.1 ℃。1H NMR(600 MHz, MeOD) δ 8.68 (s, 1H), 8.27 (s, 1H), 7.79 (d, J = 8.1 Hz, 2H), 7.50 (d, J = 18.7 Hz, 2H), 7.29 (s, 1H), 4.31 (t, J = 5.2 Hz, 2H), 4.10 (s, 3H), 3.51-3.45 (m, 4H), 3.45-3.39 (m, 2H), 3.26-3.24 (m, 5H), 2.38-2.32 (m, 2H), 1.80-1.75 (m, 6H), 1.47-1.46 (m, 4H), 1.40 (d, J = 6.5 Hz, 3H), 1.03 (t, J = 7.2 Hz, 6H)。HRMS(ESI)Calcd for C29H47N5O2[M+H]+494.3473, found 494.3476。
2.2.14 4-[4-(1-氨基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(12d) 黃白色固体,收率:66.7%,m.p.126.7~127.9 ℃。1H NMR (600 MHz, MeOD) δ 8.71 (s, 1H), 8.29 (s, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.75-7.67 (m, 2H), 7.60 (d, J = 8.4 Hz, 2H), 7.32 (s, 1H), 5.08-5.02 (m, 1H), 4.46 (t, J = 5.3 Hz, 2H), 4.09 (s, 3H), 3.47 (t, J = 5.9 Hz, 2H), 3.24 (t, J = 5.4 Hz, 4H), 1.80-1.76 (m, 6H), 1.69 (d, J = 6.9 Hz, 3H), 1.03 (t, J = 7.3 Hz, 10H)。HRMS(ESI)Calcd for C28H41N5O2[M+H]+ 480.3230, found 480.3228。
2.2.15 (R)-4-[4-(1-氨基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(12e) 黄白色固体,收率:72.8%,m.p.127.4~130.6 ℃。1H NMR (600 MHz, MeOD) δ 8.71 (s, 1H), 8.29 (s, 1H), 7.92 (d, J = 8.5 Hz, 2H), 7.73-7.66 (m, 2H), 7.59 (d, J = 8.4 Hz, 2H), 7.30 (s, 1H), 5.08-5.01 (m, 1H), 4.46 (t, J = 5.4 Hz, 2H), 4.10 (s, 3H), 3.48 (t,J = 7.9, 7.3 Hz, 2H), 3.25 (t, J = 8.8, 7.9 Hz, 4H), 1.80-1.76 (m, 6H), 1.69 (d, J = 6.9 Hz, 3H), 1.03 (t, J = 7.4 Hz, 10H)。HRMS(ESI)Calcd for C28H41N5O2[M+H]+480.3315, found 480.3301。
2.2.16 (S)-4-[4-(1-氨基乙基)苯氨基]-7-甲氧基-6-(3-(二丁胺基)丙氧基)喹唑啉(12f) 黄白色固体,收率: 76.5%。m.p.127.8~129.5 ℃。1H NMR (600 MHz, MeOD) δ8.71 (s, 1H), 8.29 (s, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.76-7.63 (m, 2H), 7.60 (d, J = 8.3 Hz, 2H), 7.31 (s, 1H), 5.06-4.99 (m, 1H), 4.46 (t, J = 5.1 Hz, 2H), 4.10 (s, 3H), 3.47 (t, J = 15.4, 8.0 Hz, 2H), 3.24 (t, J = 6.6 Hz, 4H), 1.85-1.72 (m, 6H), 1.69 (d, J = 6.9 Hz, 3H), 1.48-1.44 (m, 4H), 1.03 (t, J = 7.4 Hz, 6H)。HRMS(ESI)Calcd for C28H41N5O2[M+H]+480.31 92, found 480.3199。
2.3 体外抗肿瘤活性实验
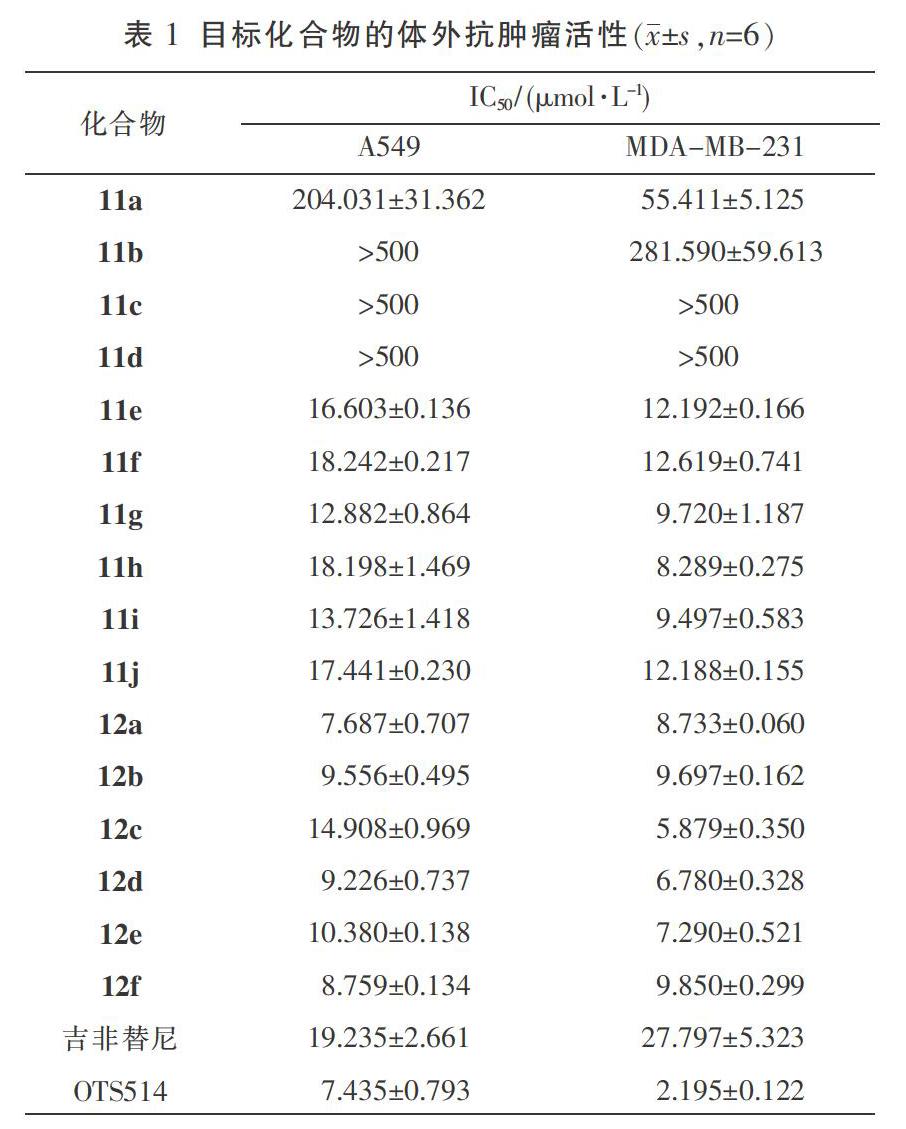
采用MTT比色法测定目标化合物11a-11J及12a-12f对肺癌细胞A549和乳腺癌细胞MDA-MB-231的体外抗肿瘤活性,以OTS514和吉非替尼作为阳性对照药。将对数生长期的A549和 MDA-MB-231细胞接种于96孔板中(8×104个/mL),置温度37 ℃,5% CO2条件下的培养箱中贴壁培养24 h,弃去原培养基。设置空白对照组、阳性对照组和药物组,96孔板内分别加入用0.2% DMSO稀释的梯度浓度药物(0.5、1、5、10、25、50 μmol/L) 100 μL,设6个复孔,置于37 ℃,5% CO2培养箱中培养48 h(A549细胞)和72 h(MDA-MB-231细胞)后,弃去原培养基,每孔加入MTT(0.5 mg/mL)100 μL置于培养箱中继续培养4 h后,出现紫色结晶,弃去MTT溶液,每孔加入150 μL DMSO,置于摇床10 min,用酶标仪在490 nm波长下测其OD值,通过Graphpad prism软件计算化合物的IC50值。结果见表1。
3 讨论
本实验运用局部修饰、电子等排等新药设计原理,将OTS514的母核噻吩并[2,3-c]喹啉酮环替换成喹唑啉环,对其骨架环的4-位和6-位上的取代基进行了重新的结构修饰,成功设计并合成16个目标化合物,经1H NMR、MS进行结构表征。
通过MTT实验结果发现目标化合物对两种肿瘤细胞都有不同程度的抑制作用,6-位取代基分别为3-六氢哌啶丙氧基、3-四氢吡咯丙氧基、3-二甲胺丙氧基、3-吗啉丙氧基和3-二丁胺丙氧基,在五种取代基团中以二丁基胺的效果最佳,可能是二丁基胺中的碳链与生物大分子触碰机率变大使两者之间的范德华力变大,也有疏水键的作用,使两个非极性区域间结合更加牢固,抗肿瘤效果更佳。通过细胞实验发现在4位的手性側链对抗肿瘤活性影响不大,手性侧链中的碳链的长短对活性影响也不是很明显;在化合物11的基础上,将乙酰基脱去得到化合物12a-12f,发现暴露的氨基能增强化合物的抗肿瘤活性,实验证明了计算机模拟的结果,暴露的氨基能与TOPK的GLU-116氨基酸形成氢键,使化合物与TOPK结合更加牢固。MTT实验结果证明了化合物12a-12f的抗肿瘤活性与阳性对照药OTS514相当,且OTS514抗肿瘤活性优于吉非替尼。为进一步探索含有喹唑啉药效团的TOPK抑制剂研究打下了基础。
4 结论
设计、合成了一系列含喹唑啉的TOPK抑制剂,通过将噻吩并[2,3-c]喹诺酮环替换成喹唑啉环,保留对TOPK的抑制特点增加其成药性,通过结构修饰得到16个化合物,并通过1H NMR、MS对其结构确证。通过体外抗肿瘤活性实验,发现目标化合物12a-12f具有与阳性对照药OTS514相当的抗肿瘤活性,为开发新结构的TOPK抑制剂提供了思路。
参考文献
[1] SIEGEL R, MA J, ZOU Z, et al. Cancer statistics, 2014[J]. CA: A Cancer Journal for Clinicians, 2014, 64(1):9-29.
[2] CAPPELLI A, PERICOT MOHR GL, GIULIANI G, et al.Further studies on imidazo[4,5-blpyridine AT1 angiotensin II receptor antagonists.Effects of the transformation of the 4-phenylquinoline backbone into 4-phenylisoquinolinone or 1-phenylindene scaffolds[J]. Journal of Medicinal Chemistry,2006,49(22):6451-6464.
[3] NAKAMURA T. Proton pump inhibitors: Tenatoprazole (TU-199)[J]. Nippon rinsho. Japanese Journal of Clinical Medicine, 2002, 60 (Suppl) 2:650-654.
[4] ABE, Y. Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells[J]. Journal of Biological Chemistry, 2000, 275(28):21525-21531.
[5] GAUDET S, BRANTON D, LUE R A. Characterization of PDZ-binding kinase, a mitotic kinase[J]. Proceedings of the National Academy of Sciences, 2000, 97(10):5167-5172.
[6] SHIH M C, CHEN J Y, WU Y C, et al. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer[J]. Oncogene, 2012, 31(19):2389-2400.
[7] WEI D C, YEH Y C, HUNG J J, et al. Overexpression of T-LAK cell-originated protein kinase predicts poor prognosis in patients with stage I lung adenocarcinoma[J]. Cancer Science, 2012, 103(4):731-738.
[8] PARK J H, LIN M L, NISHIDATE T, et al. PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer[J]. Cancer Research, 2006, 66(18):9186-9195.
[9] ZHU F, ZYKOVA T A, KANG B S, et al. Bidirectional Signals Transduced by TOPK-ERK Interaction Increase Tumorigenesis of HCT116 Colorectal Cancer Cells[J]. Gastroenterology, 2007, 133(1):219-231.
[10] SIMONS-EVELYN M, BAILEY-DELL K, TORETSKY J A, et al. PBK/TOPK Is a Novel Mitotic Kinase Which Is Upregulated in Burkitt\"s Lymphoma and Other Highly Proliferative Malignant Cells[J]. Blood Cells Molecules & Diseases, 2001, 27(5):825-829.
[11] PARKJ H, JEONG Y J, WON H K, et al. Activation of TOPK by lipopolysaccharide promotes induction of inducible nitric oxide synthase through NF-κB activity in leukemia cells[J]. Cellular Signalling, 2014, 26(5):849-856.
[12] NANDI A, TIDWELL M, KARP J, et al. Protein expression of PDZ-binding kinase is up-regulated in hematologic malignancies and strongly down-regulated during terminal differentiation of HL-60 leukemic cells[J]. Blood Cells Molecules & Diseases, 2004, 32(1):240-245.
[13] JOELM, MUGHALA A, GRIEGZ, et al. Targeting PBK/TOPK dec?鄄reases growth and survival of glioma initiating cells in vitro and attenuates tumor growth in vivo[J]. Molecular Cancer, 2015, 14(1):121.
[14] PARK J H, NISHIDATE T, NAKAMURA Y, et al. Critical roles of T-LAK cell-originated protein kinase in cytokinesis[J]. Cancer Science, 2010, 101(2):403-411.
[15] V AYLLON, O'CONNOR R. PBK/TOPK promotes tumour cell proliferation through p38 MAPK activity and regulation of the DNA damage response[J]. Oncogene, 2007, 26(24):3451-3461.
[16] HU F, GARTENHAUS R B, EICHBERG D, et al. PBK/TOPK interacts with the DBD domain of tumor suppressor p53 and modulates expression of transcriptional targets including p21[J]. Oncogene, 2010, 29(40):5464-5474.
[17] ZYKOVA T A, ZHU F, LU C, et al. Lymphokine-Activated Killer T-Cell-Originated Protein Kinase Phosphorylation of Histone H2AX Prevents Arsenite-Induced Apoptosis in RPMI7951 Melanoma Cells[J]. Clinical Cancer Research, 2006, 12(23):6884-6893.
[18] ZYKOVA T A, ZHU F, VAKORINA T I, et al. T-LAK Cell-originated Protein Kinase (TOPK) Phosphorylation of Prx1 at Ser-32 Prevents UVB-induced Apoptosis in RPMI7951 Melanoma Cells through the Regulation of Prx1 Peroxidase Activity[J]. Journal of Biological Chemistry, 2010, 285(38):29138-29146.
[19] KIM D J, LI Y, REDDY K, et al. Novel TOPK Inhibitor HI-TOPK-032 Effectively Suppresses Colon Cancer Growth[J]. Cancer Research, 2012, 72(12):3060-3068.
[20] 宋樹勇.PBK/TOPK蛋白酶抑制剂喹唑啉衍生物的合成[D].湛江:广东医学院,2016.
[21] MIYAMOTO T. Novel molecule targets cytokinesis[J]. Cancer Discovery, 2015, 5(1):OF8.
[22] JINXUAN L, JING-YI C, YA-LIN D, et al. Structure-Based Design, Synthesis, Biological Evaluation, and Molecular Docking of Novel PDE10 Inhibitors With Antioxidant Activities[J]. Fronties in Chemustry, 2018, 6:167-178.
