Characterization of the parent and hydroxylated polycyclic aromatic hydrocarbons in the soil of the Fildes Peninsula, Antarctica
2020-03-31LIRuijingGAOYunzeGAOHuiJINShuaichenCAOShengkaiYEJiandongNAGuangshui
LI Ruijing, GAO Yunze,2, GAO Hui, JIN Shuaichen, CAO Shengkai, YE Jiandong,2 & NA Guangshui,3*
Characterization of the parent and hydroxylated polycyclic aromatic hydrocarbons in the soil of the Fildes Peninsula, Antarctica
LI Ruijing1, GAO Yunze1,2, GAO Hui1, JIN Shuaichen1, CAO Shengkai1, YE Jiandong1,2& NA Guangshui1,3*
1National Marine Environmental Monitoring Center, Dalian 116023, China;2College of Marine Ecology and Environment, Shanghai Ocean University, Shanghai 201306, China;3Hainan Tropical Ocean University, Sanya 572022, China
Polycyclic aromatic hydrocarbons (PAHs) and hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) were investigated in the soil of the Fildes Peninsula, Antarctica. Various analytes were detected, and the concentration of OH-PAHs was 0.300–1.847 ng·g−1dry weight, with the dominant components being danthron and 1-hydroxy-phenanthrene. The relationship between soil total organic matter (TOM), OH-PAHs, and the parent PAHs in the soil was studied. No significant correlation was detected between the spatial distribution of OH-PAHs and the occurrence of PAHs, whereas a positive correlation with TOM was found.
Fildes Peninsula, soil, polycyclic aromatic hydrocarbons, hydroxylated polycyclic aromatic hydrocarbons, total organic matter
1 Introduction
Due to the teratogenicity, carcinogenicity, and mutagenicity of polycyclic aromatic hydrocarbons (PAHs), recent studies have focused on their distribution characteristics and treatment methods in the environment. However, in a complex ecosystem, PAHs may be transformed into various derivatives by physical, chemical, and biological actions during long-range transport, including alkyl-PAHs, nitro-PAHs, and oxygenated-PAHs (Walgraeve et al., 2010). Unfortunately, with the increase in hydrophilia, oxygenated- PAHs exhibit stronger biological toxicity and carcinogenicity, which potentially causes great harm to the ecosystem (Lundstedt et al., 2007). Evidence from bacteria to humans indicates that various derivatives of PAHs display more severe genotoxicity than the untransformed parent PAHs (Wang et al., 2009).
Oxygenated-PAHs include aromatic-ketones, aromatic- aldehydes, and quinone compounds, which have been found in various environmental media, including water, soil, animal tissues, and even in baby food (Rey-Salgueiro et al., 2009; Layshock et al., 2010; Bandowe et al., 2014). Of these, hydroxylated-polycyclic aromatic hydrocarbons (OH-PAHs), such as 1-hydroxypyrene, have been widely used as biomarkers for measuring environmental exposure to PAHs. For example, studies have indicated that the concentration of OH-PAHs in fish bile is positively correlated with PAH pollution in the environment (Johnson- Restrepo et al., 2008). The atmosphere is generally considered to be the main occurrence site of OH-PAHs, and in the process of atmospheric transmission and diffusion, PAHs will react with hydroxyl radicals in atmospheric particles (Albinet et al., 2008). Furthermore, OH-PAHs are speculated to be the final product of PAHs after biological and chemical processes in the natural environment and are stored in the environment for a long time (Walgraeve et al., 2010). It is evident that the environmental behavior of OH-PAHs is attracting increasing attention. However, few studies have reported on OH-PAHs in the Antarctic region.
The Antarctic region is a cold-climate ecosystem that is covered in perennial ice and snow and it is characterized by slow biological metabolism. It is also one of the most significant and sensitive regions to global climate and environmental changes, and it is rarely disturbed by human activities. Due to the above unique environmental characteristics, the Antarctic region constitutes an ideal region for tracking the environmental ecological behavior of pollutants and the global geochemical transport process. The distribution characteristics of OH-PAHs in this region are affected by many factors. Due to the direct transformation relationship between PAHs and OH-PAHs, PAHs will be an important factor affecting for distribution characteristics of OH-PAHs. In addition, as an important medium in the environment, soil is also an aggregation site for various pollutants in the atmosphere. Thus, the total organic matter (TOM) of the soil also constitutes an important factor affecting the local distribution of OH- PAHs. Previous studies have discussed the mechanisms of the derivative reaction of PAHs in the environment; for example, PAHs are degraded by fungi and bacteria in the mangrove soil environment, and this process of transforming from PAHs to OH-PAHs is considered as the main process of PAH clearance in the environment (Chan et al., 2006; Luan et al., 2006; Li et al., 2009). However, several lines of evidence indicate that the degradation of PAHs and the increase in OH-PAHs are associated with increased biotoxicity in the environment, and the accumulation of this biotoxicity will greatly hinder the biodegradation process. To improve our understanding of PAH biodegradation by this natural process, a better comprehension of the metabolic processes derived from PAHs in the soil is required, especially OH-PAHs, which may be the final product. We thus studied 16 species of PAHs and 11 derivatives of OH-PAHs in the soils of the Antarctic Fildes Peninsula. Their spatial distribution, composition, ratio, and correlation with corresponding maternal PAHs were discussed in this research, and the relationship between the occurrence of OH-PAHs and PAHs and soil TOM was explored and analyzed.
2 Experimental section
2.1 Study sites and sampling methodology
Soil samples were collected at different sites of the Fildes Peninsula during the 30th Chinese National Antarctic Research Expedition in 2014 (January–March). The Fildes Peninsula is located in the southwest of King George Island at 62°08'48"–62°14'02"S, 58°40'59"–59°01'50"W. It has a total area of about 15 km2, with a typical oceanic climate. Due to the unique geographical location and environmental characteristics, the surface plants are relatively simple and mainly include lichen, algae, and bryophytes. The ecological environment in this region has a low resistance to the intrusion of external pollutants.
In order to explore the environmental behaviors of PAHs and OH-PAHs in this region, 14 soil samples (Figure 1) were collected in 2014 (January–March). The soil samples constituted surface soil after vegetation clearing and were collected at a depth of < 5 cm, wrapped in aluminum foil, and stored at −20 ℃.
2.2 Material and reagents
The PAH compounds (Wellington, America) measured in this study were as follows: naphthalene (Nap), acenaphthylene (Ace), acenaphthene (Acp), fluorene (Fl), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benzo[]anthracene (BaA), chrysene (Chr), benzo[]fluoranthene (BbF), benzo[]fluoranthene (BkF), benzo[]pyrene (BaP), indeno[]pyrene (InP), dibenz[]anthracene (DbA), and benzo[]perylene (BghiP). The OH-PAH follows: 2-Naphthol (2-OH-Nap), 2- Hydroxyfluorene (2-OH-Flu), 2-Hydroxy-9-fluorenone (2- OH-9-Flu), 2-Hydroxy-phenanthrene (2-OH-Phe), 3- Hydroxy- phenanthrene (3-OH-Phe), 1-Hydroxy-phenanthrene (1-OH- Phe), 9-Phenanthrol (9-OH-Phe), 4-Hydroxy- phenanthrene (4-OH-Phe), 1-Hydroxypyrene (1-OH-Pyr), 2-Hydroxy-9, 10-anthraquinone (2-OH-9,10-AQ), Danthron (1,8-DH-9, 10-AQ). The n-hexane, dichloromethane, and acetone (Sigma-Aldrich, USA) used in this experiment were all chromatography grade. All of the glassware was cleaned with Milli-Q water and acetone and then baked at 250 ℃ for 10 h.
2.3 Extraction and analysis
For the PAH pretreatment, the soil samples were thoroughly mixed, freeze-dried to constant weight, and ground using an 80-mesh sieve. Five grams of sample was placed into a 50 mL glass centrifuge tube and mixed with 1 mL of the PAH alternative internal standard naphthalene-d8, acenaphthene-d10, Philippines-d10, bend-d12, nd-d12 (100 ng·mL−1). In brief, soil samples were extracted with ultrasonicated using 50 mL of acetone and n-hexane for 30 min, twice. The extracts were combined and concentrated to 3–5 mL with a turbo evaporator. The concentrate was then poured onto a silica gel alumina column (packed bottom-up with 1.0 g anhydrous sodium sulfate, 2 g activated silica gel (presoaked in hexane), 4 g neutral aluminum oxide, and 1.0 g anhydrous sodium sulfate), purified, and eluted with 60 mL n-hexane/dichloromethane (1:1, v:v) and 60 mL of dichloromethane successively. The extract was further evaporated and in preparation for the gas chromatography- tandem mass spectrometry (GC-MS/MS) analysis.

Figure 1 Sampling sites in Fildes Peninsula, Antarctica.
For the OH-PAH pretreatment, the extraction and enrichment process is the same as the PAH process described above. The concentrate was poured into the Bond Elut SI solid phase extraction column (Agilent, USA) for purification. The extract was further evaporated and in preparation for the ultra-high-performance-tandem mass spectrometry (UPLC-MS/MS) analysis.
2.3.1 PAH instrumental analysis method
Electron impact ion source (EI) was used with an energy of 70 eVandquadrupole, ion source, and transmission line temperatures of 150 ℃, 200 ℃, and 275 ℃ respectively. The carrier gas was methane, the flow rate was 40 mL·min−1, and selected ion monitoring (SIM) detection was implemented.
2.3.2 OH-PAH instrumental analysis method
The chromatographic conditions were as follows: An ACQUITY UPLC ® C18 column (1.7 μm, 2.1 × 50 mm2) was used for the chromatographic separation. The mobile phase was acetonitrile (0.02%) with an ammonia gradient elution. The flow rate was 0.3 mL·min−1, the sample injection volume was 5 μL, and the column temperature was 30 ℃. The mobile phase consisted of 40% acetonitrile for 0–3 min; increasing from 40% to 55% over 3–4 min and remaining at 55% for 4–7 min; decreasing from 55% to 40% over 7–8 min; and then maintained at 40% for 8–11 min.
The mass spectrometry conditions were as follows: The compound was detected by electrospray ionization (ESI) in the negative ion detection mode using a selective reaction monitoring (SRM) mode. The electrospray voltage was 4 kV; the ion transport capillary temperature was 350 ℃; the sheath gas was 23 arb; and the auxiliary gas was 12 arb.
Eleven OH-PAHs were quantitatively calculated by using the internal standard method, among which 2-/3- OH-Phe and 1-/9-OH-Phe were combined quantitatively.
2.4 Quality assurance and control
During the entire analytical procedure, the quality and sample control should be strictly controlled. The recovery rate was calculated using matrix labeling. Blank samples were combined with standard solutions of different concentrations, and three parallel tests were performed to correct the accuracy. The detection limits of the PAHs and OH-PAHs were based on the standard of three times the signal-to-noise ratio (SNR), and the detection limits of the PAHs and OH-PAHs ranged between 0.01–0.11 ng·g−1and 0.01–0.23 SNR ng·g−1, respectively. The recovery rate of the PAHs in the soil was 73.81% (Nap)–112.74% (BbF), and the detection limits were 0.03 ng·g−1(Nap) and 0.29 ng·g−1(InP). The recovery rate of the OH-PAHs was 57.6% (3-oh-phe)–91.07% (2-oh-nap), and the detection limit was 0.05–0.78 ng·g−1. The quality assurance and control samples were pretreated, analyzed, and tested simultaneously with actual samples to ensure the uniformity of the errors during the entire process. SPSS19.0 statistical software was used for all of the data analysis, and linear correlation analysis was performed. Statistical significance was determined at< 0.05.
3 Results and discussion
3.1 Spatial distribution of PAHs and OH-PAHs in surface soils
Sixteen species of PAHs were detected across the soil samples from the Fildes Peninsula, Antarctica. Their spatial distribution is shown in Figure 2. The ∑16PAHs concentration ranged from 21.831 to 77.114 ng·g−1dry weight (dw), with the highest concentration at SC3 (77.114 ng·g−1dw) and the lowest at SG2 (21.831 ng·g−1dw). The PAHs in the soil samples at SC1, SC3, SD4, SE3, and SF1 were significantly higher than at the other sampling points. Geographically, these sites are relatively close to human facilities in Antarctic, and thus these soils are subject to various human activities, including fuel combustion and oil leakage pollution from the research stations (Martins et al., 2010). SC1, SD4, and SE3 are close to the Antarctic station. The activities of the scientific research station and the fuel combustion may have a significant impact on the PAH concentration in the soil. SF1 is the closest site to the Antarctic oil depot, and its soil may be contaminated by micro-leaks and oil volatilization from storage tanks. SG2 possessed the lowest PAH concentration in this study and is located in the northeast at some distance from the area of human activity.
Several ratios of paired PAH isomers have been widely employed in previous studies for distinguishing the emission sources of PAHs in various environmental matrices, including marine sediments (Yunker et al., 1999, 2002). Flu/(Flu+Pyr) is divided into three ranges, including < 0.4, 0.4–0.5, and > 0.5, which respectively represent that the sample may be polluted by a petroleum source, petroleum combustion source, and a biomass combustion source. Similarly, BaA/(BaA+Chr) is also divided into three intervals of < 0.2, 0.2–0.4, and > 0.4, which also indicates three pollution sources of petroleum, petroleum combustion, and biomass combustion. Based on the above, we calculated the ratios of these two groups of PAHs in this study, which were used to evaluate the possible pollution sources of each sampling site. As shown in Figure 3, petroleum combustion was the main pollution source of the 16 PAHs in the soil. From an energy structure perspective, there is little biomass combustion in Antarctica, such as wood and coal. Therefore, the biomass combustion indicator in the figure is likely to originate from external long-distance transportation.
As shown in Figure 4, we also analyzed the OH-PAHs in the soil. In general, the spatial distribution of the OH-PAHs in the soil differed from that of the PAHs. The OH-PAH concentration was much lower than that of the PAHs in this region, which may be related to their mutual transformation. According to previous studies, PAHs in soil can be transformed to OH-PAHs by photo- and bio- transformation and degradation (Ge et al., 2016). But considering of the lower temperature and oligotrophy in polar areas, bio-transformation is weak. Thus photo- degradation can be the main reason of PAHs transformation and degradation into OH-PAHs in surface soil in polar regions. In this study, the average concentration of OH- PAHs in the soil was 1.003 ± 0.379 ng·g−1dw. SE4 had the lowest total OH-PAH concentration of 0.300 ng·g−1dw, while SI1 had the maximum concentration of soil OH-PAHs at 1.847 ng·g−1dw. Compared with other studies on OH-PAHs in the middle and low latitudes, such as a study on oxygen-containing polycyclic aromatic hydrocarbons in the particulate phase in Haihe in China (Qiao et al., 2014), a study on the surface soil in Uzbekistan (Bandowe et al., 2010), and a study on the surface soil in Bratislava in Slovakia (Bandowe et al., 2011), the concentration of OH-PAHs in this study is very low.
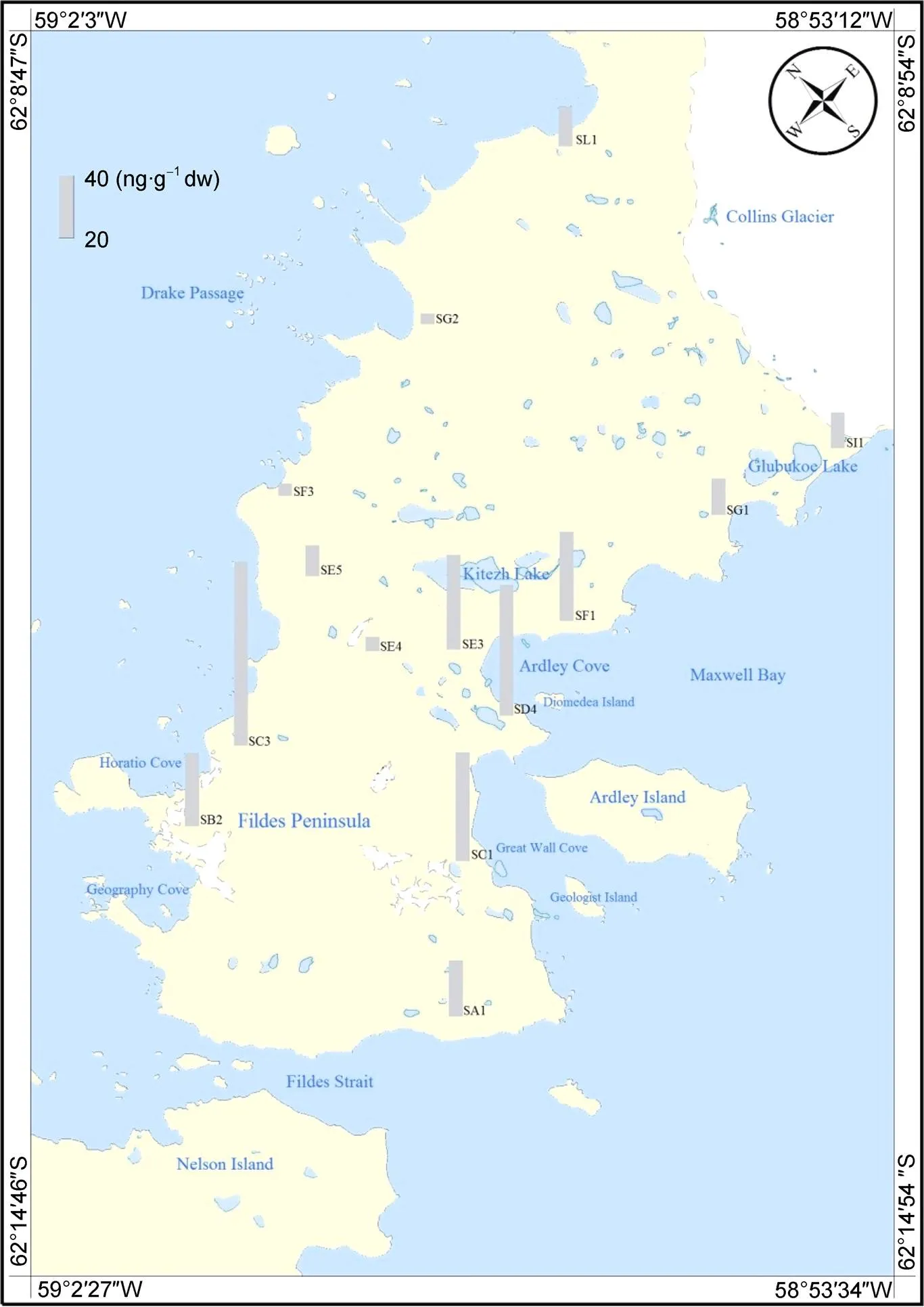
Figure 2 Spatial distribution of PAHs in the soil.
Figure 3 Ratios of Flu/(Flu+Pyr) versus BaA/(BaA+Chr) in the soil samples.

Figure 4 Spatial distribution of OH-PAHs in the soil.
3.2 Composition characteristics and influencing factors of OH-PAHs
The composition characteristics of the OH-PAHs are shown in Figure 5. In general, 1, 8-DH-9,10-AC accounted for the largest proportion in most samples, while 2-OH-9, 10-AQ, and 2-OH-9-Flu accounted for the smallest proportion in all samples. It is worth noting that 1-OH-Pyr had a relatively high proportion in the soil of the four sampling sites of SD4, SF3, SG1, and SG2, and its proportion in the soil at SD4 was as high as 55.56%. As a component with strong genetic toxicity in OH-PAHs, 1-OH-Pyr has a significant harmful effect on organisms in the environment. Although the overall level of OH-PAHs in this area is quite low, such a high concentration of 1-OH-Pyr requires consideration. 1-OH-Pyr is also commonly used as a biomarker to indicate the severity of the PAH pollution faced by organisms under various environmental conditions, and related studies have been conducted on its concentration in human urine, generally within the order of 1–10 ng·mL−1(Guo et al., 2013). In this study, the concentration of OH-PAHs has reached the corresponding concentration in organisms at this level, suggesting that OH-PAH pollution may potentially harm local organisms.
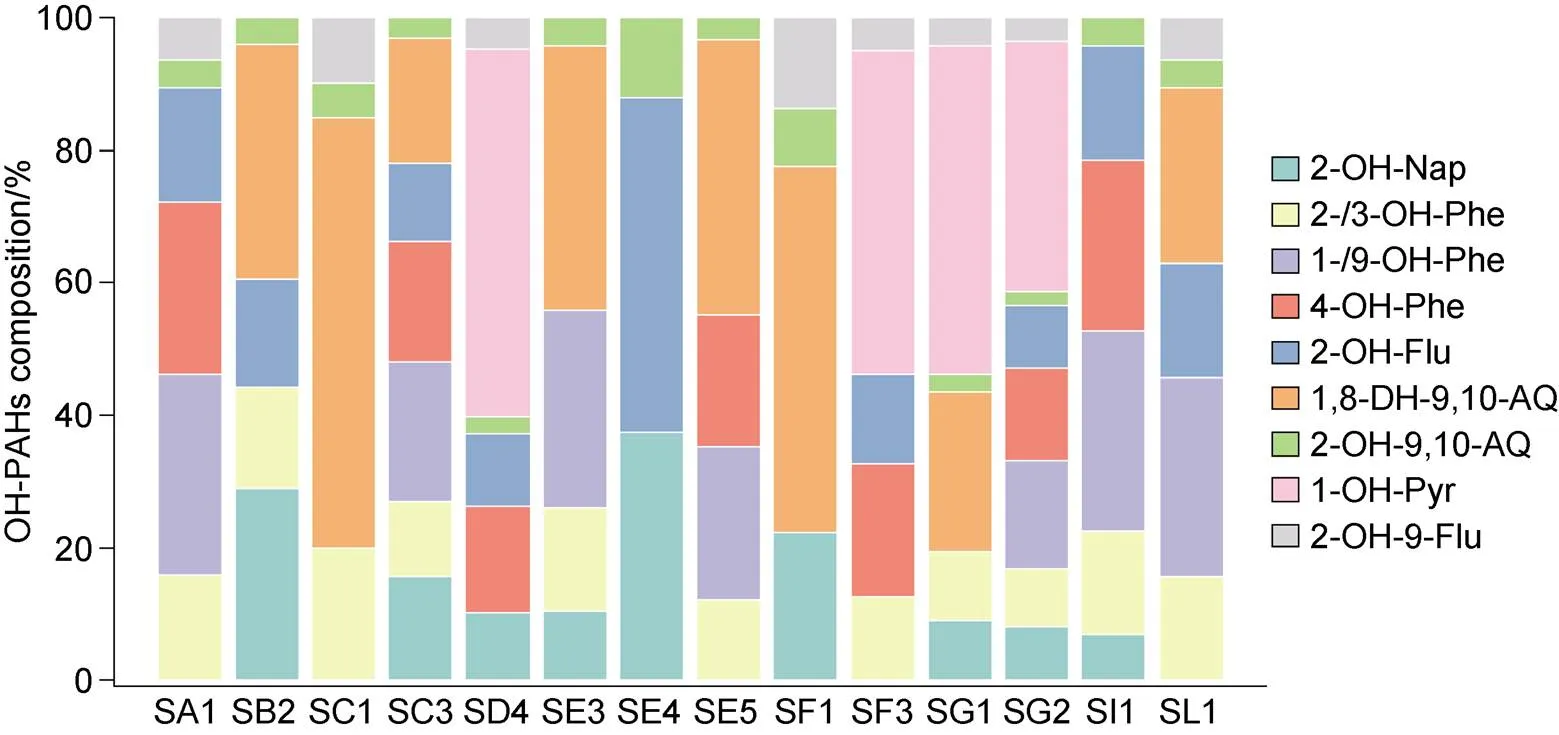
Figure 5 Compositional profile of specific hydroxylated PAH to total hydroxylated PAHs in the soil.
In order to explore the influence of PAHs on the composition characteristics of the OH-PAHs, we investigated the change trend of the contribution rate of each component of the OH-PAHs with the total amount of PAHs. Most of the OH-PAH components did not exhibit a significant trend with the change in PAH concentration, and their distributionin this region didn’t show significant differences. In the conversion process of PAHs and OH-PAHs, hydroxyl radicals (during the day) and nitro groups (during the night) are considered to play a primary role, and the concentration of ozone is also an important factor in the reaction process (Vione et al., 2004). Due to its unique geographical location, the ozone layer above the Antarctic is thin. In addition, the ecosystem is relatively simple, and the human activity history is short. Thus, the active groups in the atmosphere are also relatively thin, which may lead to the inefficient conversion of local PAHs into OH-PAHs. It is also possible that a considerable proportion of PAHs has been converted into OH-PAHs and have landed on the Antarctic continent during the process of long-distance transmission, thus reflecting the present distribution of PAHs and OH- PAHs.
Soil TOM is an important physical and chemical index of the soil environment that affects the occurrence and distribution characteristics of hydrophobic organic pollutants in the soil to a large extent (Cabrerizo et al., 2012; Zhu et al., 2014). In this study, the maximum soil TOM was 20.21%, which was measured at sampling point SD4. The lowest TOM of only 0.80% was detected at SE4. The effect of organic matter content on the behavior of heavy metals, polybrominated diphenyl ethers (PBDEs), and PAHs in the soil and sediment environment has been reflected in many studies, including the effect on the migration of organic matter in the soil, the bioavailability, and other aspects (Bayen, 2012; Zhu et al., 2014). In this study, we found the concentration of PAHs and OH-PAHs increased with TOM in soil (as shown in Figure 6). This indicates that TOM may be an influencing factor on the distribution characteristics of PAHs and OH-PAHs in this region. However, the concentrations of PAHs and OH-PAHs have no significant correlation with TOM (>0.05) respectively, which indicates that there are other factors influencing the spatial distribution characteristics. This may be related to local human activities, such as the operation of scientific research stations or the continuous development of Antarctic tourism, which may affect the distribution of local OH-PAHs in the soil (Martins et al., 2010).
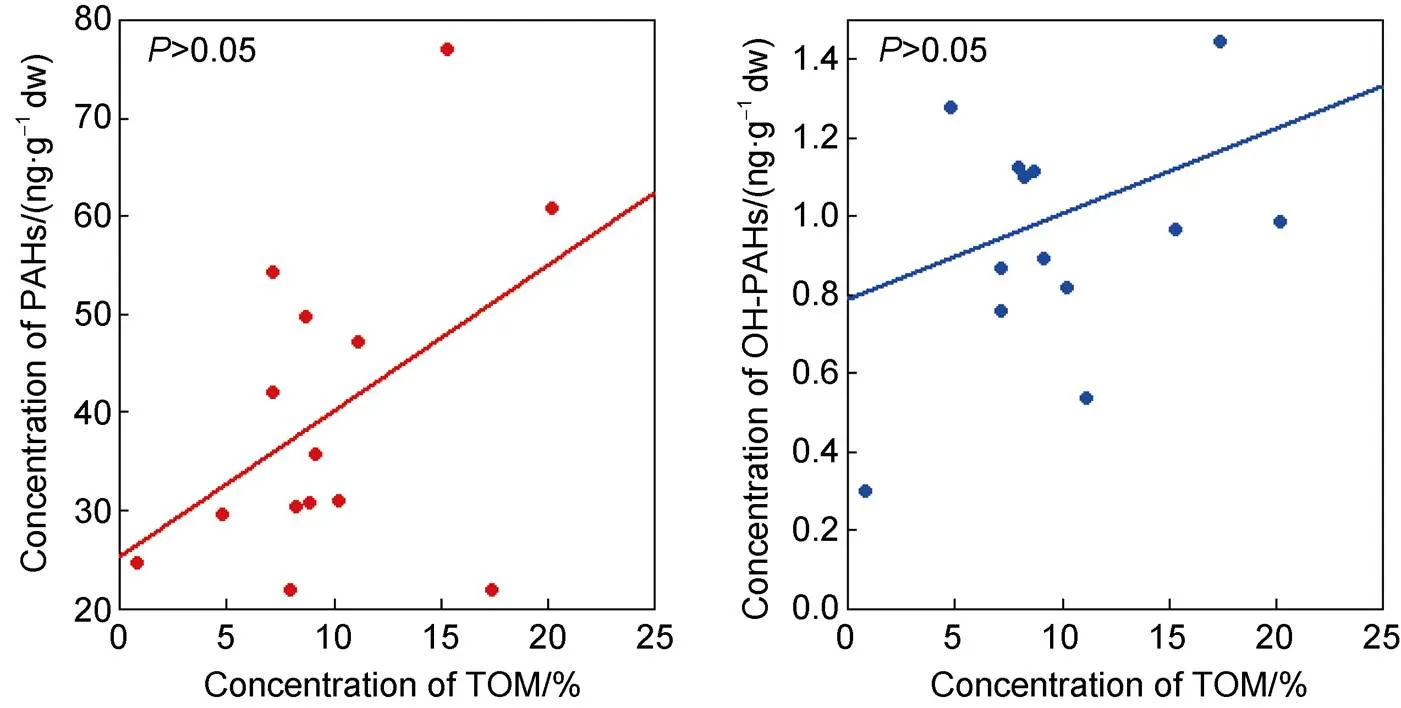
Figure 6 Concentration of PAHs and hydroxylated PAHs vs TOM in the soil.
3.3 OH-PAH and PAH dependence in the environment
The concentration ratio of OH-PAHs and their corresponding maternal PAHs in the soil is shown in Table 1. The ratio of ∑OH-PAHs to ∑PAHs ranged from 0.011 (SF1) to 0.066 (SG2), and the proportion of OH-PAHs was high in regions with a low degree of PAH pollution. This may be related to the local conversion of OH-PAHs into PAHs. In addition, Antarctica is a cold and remote region, and its pollutant status is affected by global long-range transportation. PAHs are converted into OH-PAHs during long-range transmission converts, and eventually settle in the polar region (Walgraeve et al., 2010), which will also affect the content of OH-PAHs in this area.
In remote polar regions, for some derivatives of PAHs, such as OH-PAHs, the contribution of various physical and chemical processes to atmospheric transport and deposition is more noteworthy than local combustion. In each OH-PAH and its corresponding maternal PAH, 1,8-DH- 9,10-AQ, 1-OH-Pyr/Pyr, and 2-OH-Flu/Flu were about an order of magnitude higher than the ratio of other OH-PAHs to PAHs. This phenomenon of 2-OH-Flu/Flu is also found in shellfish tissues, sediments, and urban dust (Layshock et al., 2010). However, the mechanism behind this remains unclear.
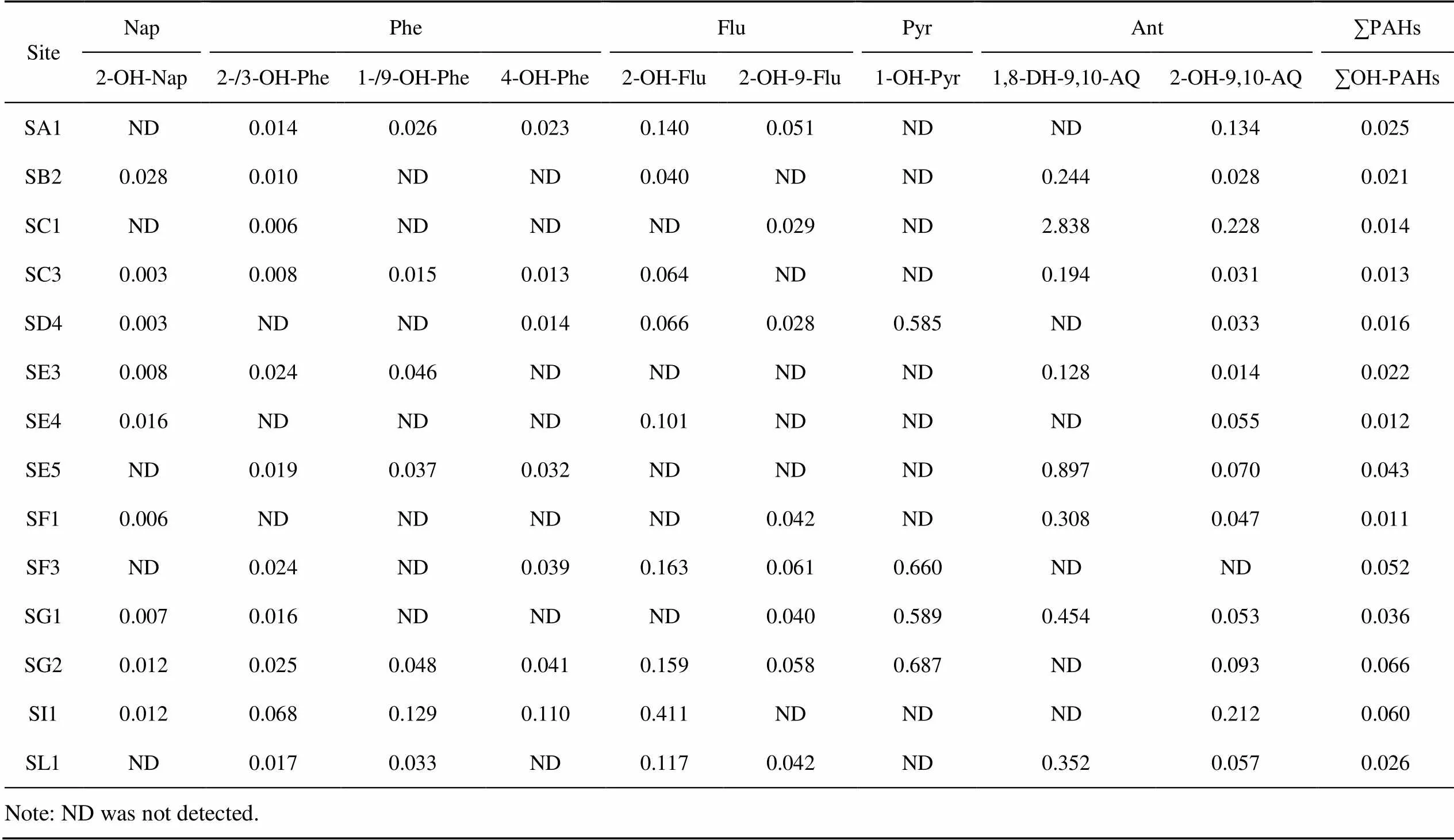
Table 1 Concentration ratios of selected hydroxylated PAHs to their corresponding parent PAHs
The ratio of PAH derivative to its parent PAH has been studied in many fields. Previous studies have shown that the ratio of 9,10-AQ to Ant in the soil can be used to track the source form of the pollution. When 9,10-AQ/Ant<1, we believe that they may originate from nearby point source pollution (McKinney et al., 1999). When 9,10-AQ/Ant>1, it indicates that the settlement is affected by long-distance transmission, because the photochemical reaction in the atmospheric transmission process can better promote the Ant to be converted into 9,10-AQ. In our study, we analyzed two hydroxyl derivatives of 9,10-AQ, 2-OH-9,10-AQ and 1,8-DH- 9,10-AQ, neither the ratio of 2-OH-9,10-AQ/Ant nor the ratio of 1,8-DH-9,10-AQ/Ant has more than 1. Although we have not conducted research on 9,10-AQ, such research on its two hydroxyl groups can still provide relevant inspiration for us to judge the source of pollution (it may also indicate the existence of local point source pollution, or the thought activity, or biological metabolic activity), laying a foundation for further research.
4 Conclusions
This is the first time that OH-PAHs have been studied in Antarctic soil. In this study, a total of nine OH-PAHs in the soil of the Fildes Peninsula in Antarctica were evaluated, and 16 parental PAHs were further discussed. In the soil of this region, 1,8-DH-9,10-AQ, and 1-OH-Pyr are the main contributors, and the concentration of all of the OH-PAHs is far lower than the PAHs. The OH-PAHs in the soil of this region are derived from complex sources. In addition to the transformation of PAHs in the process of long-distance migration, the data show that they are also affected by local sources, which may be derived from the accumulation of PAHs in the soil through biochemical processes. At the same time, TOM also affects the distribution of OH-PAHs and PAHs in the soil of this region to some extent, but it did not constitute the only influencing factor. The formation of OH-PAHs is a highly complex environmental process, and the occurrence and residual forms of OH-PAHs and PAHs in the environment are also affected by a variety of environmental factors. Very little is known regarding the formation process, particularly in this pristine area. Therefore, to clarify the complex transformation process and occurrence of PAH derivatives and PAHs in the environment, additional research is required. As Antarctica is subjected to minimal human disturbance, it constitutes an ideal research area for elucidating these processes.
Acknowledgements The research was supported by the National Natural Science Foundation of China (Grant nos. 41976222, 41406088 and 21377032), Open Fund for the Key Laboratory of Ocean-Atmospheric Chemistry and Global Change, State Oceanic Administration, China (Grant no. GCMAC1812), Open Fund for the State Key Laboratory of Environmental Chemistry and Ecotoxicology, China (Grant no. KF2018-05), National Key Research and Development Project of China (Grant no. 2019YF0901104). We also thank Chinese Arctic and Antarctic Administration and Polar Research Institute of China for the arrangements during the 30th Chinese National Antarctic Research Expedition in 2014.
Albinet A, Leoz-Garziandia E, Budzinski H, et al. 2008. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys Part 2: Particle size distribution. Atmos Environ, 42(1): 55-64, doi:10.1016/j.atmosenv. 2007.10.008.
Bandowe B A M, Lueso M G, Wilcke W. 2014. Oxygenated polycyclic aromatic hydrocarbons and azaarenes in urban soils: a comparison of a tropical city (Bangkok) with two temperate cities (Bratislava and Gothenburg). Chemosphere, 107: 407-414, doi:10.1016/j.chemosphere. 2014.01.017.
Bandowe B A M, Shukurov N, Kersten M, et al. 2010. Polycyclic aromatic hydrocarbons (PAHs) and their oxygen-containing derivatives (OPAHs) in soils from the Angren industrial area, Uzbekistan. Environ Pollut, 158(9): 2888-2899, doi:10.1016/j.envpol.2010.06.012.
Bandowe B A M, Sobocka J, Wilcke W. 2011. Oxygen-containing polycyclic aromatic hydrocarbons (OPAHs) in urban soils of Bratislava, Slovakia: Patterns, relation to PAHs and vertical distribution. Environ Pollut, 159(2): 539-549, doi:10.1016/j.envpol. 2010.10.011.
Bayen S. 2012. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: a review. Environ Int, 48: 84-101, doi:10.1016/j.envint.2012.07.008.
Cabrerizo A, Dachs J, Barceló D, et al. 2012. Influence of organic matter content and human activities on the occurrence of organic pollutants in Antarctic soils, lichens, grass, and mosses. Environ Sci Technol, 46(3): 1396-1405, doi:10.1021/es203425b.
Chan S M N, Luan T, Wong M H, et al. 2006. Removal and biodegradation of polycyclic aromatic hydrocarbons by. Environ Toxicol Chem, 25(7): 1772-1779, doi:10.1897/05-354r.1.
Ge L, Li J, Na G, et al. 2016. Photochemical degradation of hydroxy PAHs in ice: Implications for the polar areas. Chemosphere, 155: 375-379, doi:10.1016/j.chemosphere.2016.04.087.
Guo Y, Senthilkumar K, Alomirah H, et al. 2013. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol, 47(6): 2932-2938, doi:10.1021/es3052262.
Johnson-Restrepo B, Olivero-Verbel J, Lu S J, et al. 2008. Polycyclic aromatic hydrocarbons and their hydroxylated metabolites in fish bile and sediments from coastal waters of Colombia. Environ Pollut, 151(3): 452-459, doi:10.1016/j.envpol.2007.04.011.
Layshock J A, Wilson G, Anderson K A. 2010. Ketone and quinone-substituted polycyclic aromatic hydrocarbons in mussel tissue, sediment, urban dust, and diesel particulate matrices. Environ Toxicol Chem, 29(11): 2450-2460, doi:10.1002/etc.301.
Li C H, Zhou H W, Wong Y S, et al. 2009. Vertical distribution and anaerobic biodegradation of polycyclic aromatic hydrocarbons in mangrove sediments in Hong Kong, South China. Sci Total Environ, 407(21): 5772-5779, doi:10.1016/j.scitotenv.2009.07.034.
Luan T G, Yu K S H, Zhong Y, et al. 2006. Study of metabolites from the degradation of polycyclic aromatic hydrocarbons (PAHs) by bacterial consortium enriched from mangrove sediments. Chemosphere, 65(11): 2289-2296, doi:10.1016/j.chemosphere.2006.05.013.
Lundstedt S, White P A, Lemieux C L, et al. 2007. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH- contaminated sites. AMBIO: A J Hum Environ, 36(6): 475-485, doi:10.1579/0044-7447(2007)36[475:sfatho]2.0.co;2.
Martins C C, Bícego M C, Rose N L, et al. 2010. Historical record of polycyclic aromatic hydrocarbons (PAHs) and spheroidal carbonaceous particles (SCPs) in marine sediment cores from Admiralty Bay, King George Island, Antarctica. Environ Pollut, 158(1): 192-200, doi:10.1016/j.envpol.2009.07.025.
McKinney R A, Pruell R J, Burgess R M. 1999. Ratio of the concentration of anthraquinone to anthracene in coastal marine sediments. Chemosphere, 38(10): 2415-2430.
Qiao M, Qi W, Liu H, et al. 2014. Oxygenated, nitrated, methyl and parent polycyclic aromatic hydrocarbons in rivers of Haihe River System, China: Occurrence, possible formation, and source and fate in a water-shortage area. Sci Total Environ, 481: 178-185, doi:10.1016/j.scitotenv.2014.02.050.
Rey-Salgueiro L, Martínez-Carballo E, García-Falcón M S, et al. 2009. Occurrence of polycyclic aromatic hydrocarbons and their hydroxylated metabolites in infant foods. Food Chem, 115(3): 814-819, doi:10.1016/j.foodchem.2008.12.095.
Vione D, Barra S, de Gennaro G, et al. 2004. Polycyclic aromatic hydrocarbons in the atmosphere: monitoring, sources, sinks and fate. II: sinks and fate. Ann Di Chimica, 94(4): 257-268, doi:10.1002/adic. 200490031.
Walgraeve C, Demeestere K, Dewulf J, et al. 2010. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos Environ, 44(15): 1831-1846, doi:10.1016/j.atmosenv.2009.12.004.
Wang L R, Wang Y, Chen J W, et al. 2009. A structure-based investigation on the binding interaction of hydroxylated polycyclic aromatic hydrocarbons with DNA. Toxicology, 262(3): 250-257, doi:10.1016/ j.tox.2009.06.015.
Yunker M B, Backus S M, Pannatier E G, et al. 2002. Sources and significance of alkane and PAH hydrocarbons in Canadian Arctic rivers. Estuar Coast Shelf S, 55(1): 1-31, doi:10.1006/ecss.2001.0880.
Yunker M B, Macdonald R W, Goyette D, et al. 1999. Natural and anthropogenic inputs of hydrocarbons to the Strait of Georgia. Sci Total Environ, 225(3): 181-209, doi:10.1016/s0048-9697(98)00362-3.
Zhu H W, Wang Y, Wang X W, et al. 2014. Distribution and accumulation of polybrominated diphenyl ethers (PBDEs) in Hong Kong mangrove sediments. Sci Total Environ, 468-469: 130-139, doi:10.1016/j. scitotenv.2013.08.021.
30 October 2019;
2 January 2020;
6 March 2020
10.13679/j.advps.2019.0037
: Li R J, Gao Y Z, Gao H, et al. Characterization of the parent and hydroxylated polycyclic aromatic hydrocarbons in the soil of the Fildes Peninsula, Antarctica. Adv Polar Sci, 2020, 31(1): 64-73,
10.13679/j.advps.2019.0037
, E-mail: gsna@nmemc.org.cn
杂志排行
Advances in Polar Science的其它文章
- New category, Editorial Opinion, attracts more attention from the international polar community
- Laboratory experimental study of water drag force exerted on ridge keel
- Multi-sensor data merging of sea ice concentration and thickness
- Features of sea ice motion observed with ice buoys from the central Arctic Ocean to Fram Strait
- An assessment of the impacts of diesel power plants on air quality in Antarctica
- Atmospheric responses over Asia to sea ice loss in the Barents and Kara seas in mid–late winter and early spring: a perspective revealed from CMIP5 data
