寄生植物锁阳种子萌发方法及愈伤组织、初生吸器诱导研究
2020-03-05陈贵林
岳 鑫 陈贵林
(1. 内蒙古大学生命科学学院,呼和浩特 010021;2. 内蒙古医科大学药学院,呼和浩特 010021)
Cynomorium songaricum Rupr. is an obligate parasitic plant distributed in Mongolia,Central Asia and Northwest China[1]. C.songaricum is used to treat kidney disease,intestinal ailments and impotence.Biomedical and phytochemical evidences show that C.songaricum contains triterpenes that inhibit HIV-1 proteases and Hepatitis C(HCV)[2~4]. It also contains the high-molecular weight polymer procyanidin CSPP-A,which suppresses the growth of methicillin-resistant Staphylococcus aureus[1]. To date,studies assessing the physiology and biotechnology of C.songaricum are scarce,because of its obligate parasitism and lack of perniciousness.
Parasitism is a lifestyle that has been repeatedly found throughout evolution. At least 4 000 plant species are parasitic and obtain some or all nutrients by directly invading other plants[5]. The interaction between an obligate root parasite and the host begins early in the life cycle(i.e.,germination). Parasitic plants sense surrounding host roots and germinate via mechanisms possibly evolving from a conserved germination system employed by non-parasitic species[6]. These plants use secondary metabolites produced by host plant roots as signal molecules to induce germination as well as developmental programs speci fi c for parasitism[7]. Such chemical molecular signals for germination stimulation have been identified,and comprise three classes,including strigolactones,dihydrosorgoleone and other molecules whose structures and activities require detailed investigation[8]. Multiple other molecules induce seed germination in Striga and Orobanche seeds in vitro,including kinetin,abscisic acid,inositol,methionine,and ethylene[9].Revealed germination inducers might affect aminocyclopropanecarboxylate(ACC)oxidase in Striga asiatica seed conditioning,converting ACC into ethylene[10]. These findings indicate many molecules could alter germination through a shared mechanism[11].
From the germinated seed,the development of a particular multicellular organ termed haustorium represents a characteristic property of plant parasites,which help parasitic organisms invade host and generate connections[12-13].The haustorium is triggered by haustorium-inducing factors(HIFs)and redox signals[12]. Currently known HIFs are grouped into four classes,including flavonoids,p-hydroxy acids,quinones and cytokinins[8]. Haustorium signaling is induced by redox cycling of HIFs,with the inducer’s redox potential determining its activity[14].Induced the formation of papillae using a low concentration of KT(<0.1%)from Cuscuta japonica Choisy,with no primordia found[15].Added KT(10 ppm)to the medium to induce a structure similar to the haustorium of the semi-parasitic plant Rhinanthus serotirus[16].Applied BA to induce haustorium formation in Cuscuta chinensis Lam[17]. In addition,reports are available on cytokinin-induced haustorium in other parasitic plants such as Striga sp.[18],and other studies found that cytokinin(KT)plays a decisive role in haustorium formation,while auxin has the opposite function.
The pyrene seeds of C.songaricum are especially difficult to germinate,partly because of a thick,impervious pericarp that contains large amounts of abscisic acid(ABA),which occupies half of the seed’s volume[19]. The hard seed coat has to be fractured to reverse dormancy[20].In addition,embryo development in C.songaricum tends to stop at the stage of the multicellular globular proembryo without formation of a suspensor. Release from dormancy is erratic since different stimulus thresholds are required for inducing germination of individual seeds[21].
Studies assessing C.songaricum,with high medicinal value,have focused on improving its parasitic relationship with its hosts,facilitate artificial cultivation and increase supply. Because haustorium development is a crucial step in this process,this study used different tissue culture methods to induce callus formation from seed explants and subsequently establish Haustorium organogenesis. The present findings may subsequently help clarify the parasitic mechanisms involved in the interaction of C.songaricum with its hosts.
So far,there is no efficient method for seed germination in C.songaricum. In addition,studies assessing the structure and function of C.songaricum,as well as the parasitic relationship between C.songaricum and its host are almost inexistent,which leads to unsuccessful cultivation of C.songaricum.The wild sources of C.songaricum are increasingly endangered by disorderly mining for use in pharmaceutical industries and product development. The World Conservation Union(IUCN)currently defines the endangered level of C.songaricum as vulnerable(VU),which is designated as a Class Ⅱprotected plant in the Washington Convention(CITES)[22].The protocol for haustorium organogenesis from the callus opens up the possibility of evaluating the processes involved in host-parasite relationships,which may help in C.songaricum protection and utilization.
1 Materials and Methods
1.1 Explant preparation
C. songaricum seeds were obtained from Hangjing County,Ordos,China,and preserved in an ultra-cold freezer at -70°C. The pericarp was removed by rubbing the seed with emery paper. The seeds were then soaked in 70% ethanol. The surface was sterilized with 0.1% mercuric chloride solution(6 min),and the seeds underwent five washes with sterilized water. Then surface-sterilized seeds were cultured on B5medium.
1.2 Callus culture medium and conditions
B5medium with a series of concentration gradients of 2,4-dichlorophenoxyacetic acid(2,4-D),kinetin(KT)and gibberellic acid(GA3)was used to induce callus formation(Table 1),with pH adjusted to 6.0 before agar(3%)addition. Each medium was then submitted to autoclave(25 min,120°C). Sterile triangular flasks were added 60 mL of callus induction medium,followed by addition of 50 seeds. Cultures were incubated in the dark at 25±1°C. Three quintuplicate independent assays were performed.Callus induction was evaluated after 40 d of culture by counting calluses.
1.3 Microscopy
Seeds cultured for 0,20,30 and 40 d,respectively,were mounted on microscope slides,and embryos were examined. The embryos were obtained by crushing the seeds with another microscope slide.Then,embryos were transferred to formalin-aceto-alcohol(FAA)stationary liquid medium for 24 h and observed under a cell microscope(Axio Observer A1,ZEISS).
1.4 Haustorium organogenesis media and conditions
B5 medium was added 0.25 mg·L-1KT and various 2,4-D amounts(Table 2)to induce haustorium organogenesis from the callus.For all media,pH was adjusted to 6.0 before agar(3%)addition. Each medium was then submitted to autoclave(25 min,120°C). Sterile triangular flasks were filled with 60 mL haustorium differentiation medium,and five calluses were added to each flask. Incubation was carried out away from light at 25±1°C. Three independent assays consisting of five triangular flasks were performed.
1.5 Scanning electron microscopy(SEM)
Haustorium formed from calluses were submitted to fixation(3% glutaraldehyde)and washing.Sample dehydration was carried out with ethyl alcohol gradient(10-minute intervals)followed by critical-point drying(liquid CO;10 min). Then,the specimens were mounted on aluminum stubs with double-sided tape and underwent platinum(Pt)coating on an ion sputter apparatus(Hitachi E-1010,Japan). An accelerating voltage of 15 kV was employed for analysis by emission SEM on a Hitachi S-4300 field(S-3400N,Hitachi)at different resolutions and magnifications.
1.6 Statistical analysis
The seeds that developed calluses were counted,and haustorium in per callus were identified.The callus induction rate represented the number of explants that formed a callus divided by that of all inoculated explants,multiplied by 100%. The rate of haustorium formation reflected the number of explants that formed a haustorium divided by that of all inoculated callus,multiplied by 100%. The average number of haustorium formed per callus was used for analysis. The data were analyzed with the SPSS software(v19).P<0.05 indicated statistical significance.
2 Results and analysis
2.1 Germination conditions
During seed imbibition,it was hypothesized that GA production occurs[23~24]. Moreover,GA supplemented exogenously decreases the minimum effective exposure time to germination for stimulants employed during conditioning,promoting seed germination in parasitic species[25]. GA3does not participate in the process of radicle breakthrough of the seed testa. Instead,it activates the transcription factor gibberellin- and abscisic acid-regulated Myb(GAMyb),promotes α -amylase synthesis and induces endosperm degradation,all of which facilitate seed germination. In this study,an adequate concentration of GA3,incombination with other plant growth regulators included in the medium,promoted embryo development and germination of C.songaricum seeds.
The tiny embryo of C.songaricum appeared as a multicellular spherical proembryo with no germ cell differentiation,with a radicle or cotyledon found close to the micropylar end(Fig.1A). Embryo cells varied in size,with larger and smaller ones located near the chalazal and micropylar ends,respectively(Fig.1B). The cells were polyhedral in shape,tightly packed and contained large nuclei.
During culture,the embryos initially became differentiated at two poles(Fig.1C). This was followed by unipolar development during the process of germination(Fig.1D). The level of embryonic development and differentiation of mature C.songaricum seeds was similar to that of other parasitic angiosperms. The present findings support these previous observations.
2.2 Callus induction
The embryo broke through the seed coat at the micropylar end after 40 d culture(Fig.2B),and the radicle continued to elongate in subsequent days(Fig.2C). The radicle’s top became intumescent(Fig.2D)and eventually formed the callus(Fig.2E),which was pure white and dense,growing rapidly from the radicle(Fig.2F). After another 20 d,it turned brown(Fig.2G),and embryogenic callus formation occurred(Fig.2H). Embryogenic cells were mostly cylindrical,with large nuclei and starch grains(Fig.2I). The rates of callus formation from C.songaricum seeds were assessed in the dark under various combinations of three hormonal plant growth regulators(Table 1). The highest callus induction rate(13.7%)after a 40 d incubation was produced by the combination of 1,0.5 and 1 mg·mL-1of 2,4-D,KT,and GA3,respectively. This rate was significantly higher than those obtained with other combinations.
The synergistic effects of auxins(2,4-D)and cytokinins assisted C. songaricum germination and promoted callus formation. Studies assessing other holoparasitic or hemiparasitic plants have shown that combined use of auxins and cytokinins promotes callus generation. However,the same auxins or cytokinins play significantly different roles in distinct parasitic plants[26~28]. In this study,optimal callus formation from C.songaricum seeds was obtained with 1 mg·L-12,4-D and 0.5 mg·L-1KT. This study firstly reported in vitro callus generation from C.songaricum seeds.
2.3 Haustorium organogenesis
No haustoriuml hair was observed on the surface of the primary haustorium. Furthermore,the structure was similar to that of the primary haustorium from seed germination. The number of haustorium per callus was determined. The best results were obtained with 1.0 mg·mL-12,4-D,which yieldedsix haustorium per callus by Day 60. The callus broke through the radicle and formed root-like organs of 3 to 4 mm in length(Fig.3A). The tops of these structures were enlarged to form globular shaped organs(primary haustorium;Fig.3A-B). Scanning electron microscopy indicated that the haustoriuml top was composed of a uniform fossa surrounded by a thick ribbon like structure(Fig. 3D-E)with protrusions(Fig.3F),increasing to approximately eight on Day 60 and remaining constant thereafter(Table 2).Interestingly,some of the primary haustorium branched to form adventitious roots of 3 to 4 cm in length(Fig.3C). The tip of each adventitious root formed nascent primary haustorium,which then branched out into adventitious roots.

Fig.1 Development of C.songaricum embryo in different periods of cultivationSeeds cultured for 0,20,30 and 40 d were examined. The embryos were obtained by crushing the seeds with another microscope slide,transferred to the formalin-aceto-alcohol(FAA)stationary liquid medium for 24 h,and observed with bright field illumination(Fig.1A,C and D).The embryo was stained with a fluorochrome and observed with a fluorescence objective lens(Fig.1B)

Fig.2 Callus development in different periods of cultivation in C.songaricumThe embryo broke through the seed coat at the micropylar end after 40 days of culture(Fig.2B). The radicle continued to elongate in the following days(Fig. 2C). The radicle’s top became intumescent(Fig. 2D)and eventually formed a callus(Fig. 2E). The callus was pure white and dense,and grew rapidly from the radicle(Fig.2F). After another 20 d,it became brown(Fig.2G),and embryogenic callus formation occurred(Fig. 2H). Embryogenic cells were mostly cylindrical,with large nuclei and starch grains(Fig.2I)
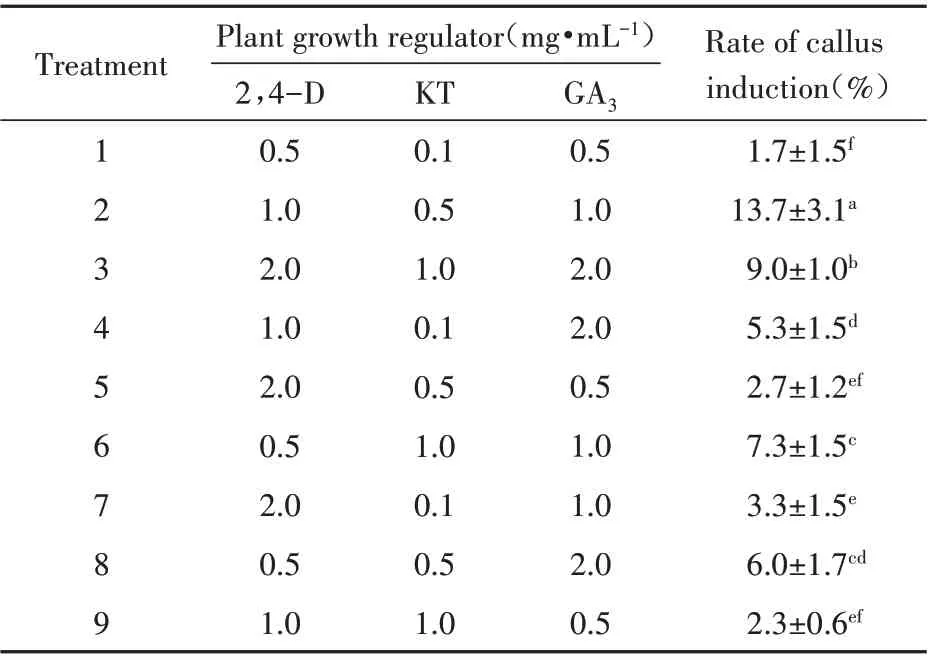
Table 1 Synergistic effects of 2,4-dichlorophenoxyacetic acid(2,4-D),kinesin(KT)and gibberellin(GA3)on callus induction in C.songaricum Rupr. Seeds
With regard to morphological organogenesis of the primary haustorium,the haustorium is initiated by localized cell growth,alongside haustoriuml hair growth in multiple species.Parasitic plant species develop haustorium close to the root tip or along thestem under stimulation by specific substances produced by the respective host[29]. Haustorium have a morphology comparable to that of Rhizobium-induced determinant nodules but a different developmental ontogeny.Cell division starting in the root cortex represents an initial event in nodules’organogenetic process,and nodule primordia comprise actively dividing cells. Meanwhile,haustoriuml swelling is primarily explained by the isodiametric root cell growth. Haustorium development equally differs from lateral root expansion due to the lack of epidermal rupture in the parasite[30].

Table 2 Effect of plant growth regulators on haustorium induction in the callus of C.songaricum seed

Fig.3 The development of C.songaricum haustorium in different periods of cultivation
Furthermore,facultative parasitic organisms,such as Phtheirospermum,develop lateral haustorium in the root transition and elongation regions,and a single root could generate many haustorium[12].However,obligate parasitic organisms,including
Striga,Orobanche and Phelipanche spp. develop only one haustorium at the tip of each of the emerging radical apexes upon germination.
3 Discussion
The haustorium is the channel through which a parasitic plant invades its host tissue to uptake materials it needs. According to the function,the haustorium has two types,one is primary haustorium and the other is secondary haustorium. Depending on the position of the primary haustorium formed on the parasite root,primary haustorium is divided into lateral haustorium and terminal haustorium[31]. During lateral haustorium development,a branch appears in the elongation zone of the main root of the parasite,at the top of which a primary haustorium is formed,which without interference in continuous top elongation and multiple primary haustorium formations[32].On the contrary,there is only one terminal haustorium,the structure of which could terminate root growth. However,the development of haustorium in all types begins with the perception of secretions from host root nearby. The secretions,as known to trigger haustorium development,are called haustorium inducing factors(HIFs),including strigolactones,flavonoids,quinones and cytokinins. HIFs play a crucial role in the formation of primary haustorium by initiating signals leading to the accumulation of reactive oxygen species[33].
The 2,6-dimethoxy-p-benzoquinone(DMBQ)is an efficient HIFs to initiate the formation of haustorium in many kinds of parasite plants without their host.Polyphenols secreted by the host are oxidized to quinones by the H2O2secreted by parasite plants[14,34].However,the DMBQ is not a panacea in triggering the haustorium formation in all species of parasite,which means that the function of HIFs is species-specific. The development of Phelipanche ramose L.haustorium initiated in the presence of cZ/tZ cytokinins and was prevented in the presence of cytokinin receptor[35].
In this study,the cytokinin was considered to be the HIFs of C.songaricum,but the function of cytokinin as a HIF has not been elucidated here. A report assessing haustoriuml anatomy in Cuscuta japonica Choisy exogenously administered cytokinin showed that KT treatment promotes radial elongation in cortical cells and the formation of meristematic tissues,laying a material foundation for cell dedifferentiation and division. When endogenous cytokinin amounts are lower than the effective concentration,the dedifferentiation "switch" of cortical cells cannot be activated,which prevents the formation of the papillary or primordial primordium. At low levels of exogenous KT(<0.1%),although cortical cells may be elongated,they cannot be constituted into the primordial base. This indicates that only when exogenous cytokinin concentration reaches a certain level(the optimal KT concentration in this study was considered to be 0.25 mg·L-1),endogenous cytokinin would reach the effective concentration,activating the "switch" to dedifferentiate cortical cells to form the primordial primordium. So only one concentration of cytokinin was chose to test the cooperation effect between cytokinin and auxin on formation of the C.songaricum haustorium.
These results indicate that auxin is very important in primary haustorium formation from C.songaricum callus in the presence of cytokinin.It was reported that Phtheirospermum japonicum sensed DMBQ and quickly activitied lots of genes to express,including gene YUC3,which involved in auxin synthesis. YUC3 activated about 18 hpis in epidermal cells near the parasitic-host contact point. After knockout of the YUC3 gene,the number of haustorium decreased significantly. The ectopic expression of YUC3 led to the formation of the haustorium-like structure and the increase of auxin. It suggested that local auxin biosynthesis mediated by YUC3 is vital for the haustorium to initiate development[36].In addition,the process of auxin transport is considered to be an essential factor in the haustorium initiation. In the study of the formation of the Triphysaria versicolor haustorium,it was found that the formation of the haustorium was inhibited by using the polar auxin transport inhibitor TIBA,while was recovered by applying exogenous auxin[30]. The above phenomena indicated that auxin transport is a necessary factor for DMBQ to trigger the formation of a haustorium.
From this study,it could be speculated that the cooperation of exogenous auxin and cytokinin plays the most important role in the formation of primary haustorium from the callus in C.songaricum.The synergistic mechanism of cytokinin and auxin on callus differentiation into haustorium needs further study.
All in all,seed germination and the formation of haustorium are the key to the completion of parasitic growth of C.songaricum. Exogenous signal substances can effectively promote the completion of this crucial link.Therefore,the study of exogenous signal substances is of great significance to the parasitic growth mechanism and artificial planting of C.songaricum.
