Statins decrease the risk of acute pancreatitis after endoscopic ultrasound fine-needle aspiration of pancreatic cysts
2020-03-03AntonioFacciorussoVincenzoRosarioBuccinoValentinaDelPreteMatteoAntoninoAntonellaContaldoNicolaMuscatiello
Antonio Facciorusso , Vincenzo Rosario Buccino , Valentina Del Prete , Matteo Antonino , Antonella Contaldo, Nicola Muscatiello
Gastroenterology Unit, Department of Medical Sciences, University of Foggia, AOU Ospedali Riuniti, Viale Pinto 1, 71100 Foggia, Italy
Keywords: Endoscopic ultrasound Fine-needle aspiration Pancreas Lesion
ABSTRACT Background: Basic and clinical studies suggest that statins may prevent and even ameliorate acute pan- creatitis. The present study was to evaluate whether statin decreases the risk of acute pancreatitis in patients undergoing endoscopic ultrasound-guided fine-needle aspiration of pancreatic cysts. Methods: Out of 456 patients with pancreatic cysts referred to our center between 2006 and 2018, 365 were finally included in analyses: 86 were treated with statins and 279 were not at the time of endo- scopic ultrasound fine-needle aspiration. We compared the acute pancreatitis incidence between the two groups, and we also compared other complications such as bleeding and infections. Results: Median age was 64 years [interquartile range (IQR) 62-69] and median cyst size was 24 mm (IQR, 21-29). The most frequent histology was intraductal papillary mucinous neoplasm (45.3% and 42.3% in the two groups, respectively; P = 0.98). All 13 patients experiencing post-endoscopic ultrasound acute pancreatitis were from the control group (4.7%), of which 3 were classified as severe pancreatitis. None of statin users developed post-procedural acute pancreatitis (odds ratio: 0.15; 95% confidence interval: 0.03-0.98; P = 0.03). No difference was registered with regard to severe pancreatitis and other complications. Conclusions: Statins exert a beneficial role in preventing acute pancreatitis in patients with pancreatic cysts undergoing endoscopic ultrasound-guided fine-needle aspiration. If confirmed in prospective trials, our findings may pave the way to an extensive use of statins as prophylactic agents in pancreatic inter- ventional endoscopy.
Introduction
Due to the high prevalence of pancreatic cystic lesions (PCLs) and the risk of malignant progression of certain subtypes, in par- ticular mucinous cystic neoplasms (MCN) and intraductal papillary mucinous neoplasms (IPMN), it is mandatory to define an accurate diagnostic algorithm in these patients [1] .
Cross-sectional imaging and endoscopic ultrasound (EUS) rep- resent the first-line diagnostic option in these patients, how- ever EUS-guided fine-needle aspiration (FNA) is frequently needed to draw reliable conclusions about the malignant nature of the lesion [2 , 3] .
Complete emptying of the PCL is usually performed by using 19 G or 22 G FNA needles with the least number of passes [4] . Albeit EUS-guided FNA of cysts is generally considered a relatively safe procedure, retrospective studies have shown that it may cause complications such as acute pancreatitis (AP), bleeding or infec- tions [5 , 6] . In particular, there is a theoretical risk of inducing pan- creatitis with FNA of IPMNs as there may be a direct communica- tion between the aspirated cyst and the main pancreatic duct [7] .
Three-hydroxy-3-methyl-glutaryl-coenzyme A (HMG Co-A) re- ductase inhibitors (statins) are effective and commonly used worldwide as a treatment for dyslipidemia, and increasing evi- dence shows that statins also have anti-inflammatory effects [8 , 9] . In spite of preliminary discording results, several recent studies demonstrated that the use of statins may actually be a protective factor against AP [10-14] . Furthermore, based on promising results from animal studies [15 , 16] , statins are prophylactic agents of post- endoscopic retrograde cholangiopancreatography (ERCP) pancreati- tis [17 , 18] .
Therefore, the relationship between use of statins and risk of pancreatitis should be re-examined considering this potential ben- eficial effect.
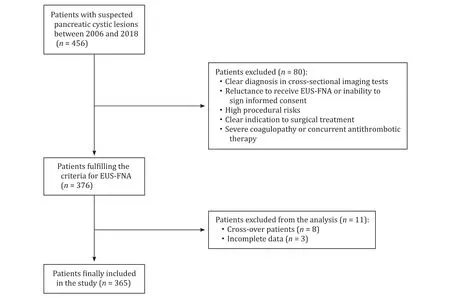
Fig. 1. Study flow chart. Out of the initial 456 patients with pancreatic cystic lesions, 365 subjects were finally included. EUS-FNA: endoscopic ultrasound-guided fine-needle aspiration.
The present study was to evaluate whether statin use decreases the risk of acute pancreatitis in patients undergoing EUS-FNA of PCLs.
Methods
Patients
We reviewed data of our series of 456 patients with sus- pected PCLs evaluated by means of EUS between January 2006 and July 2018. All patients underwent contrast-enhanced cross- sectional imaging (CT-scan or magnetic resonance cholangiopan- creatography) before EUS in order to assess the eventual connec- tion of the cyst with the pancreatic duct. The study was approved by the local Institutional Review Board and patients provided writ- ten informed consent to perform the procedure.
Indications for EUS-FNA were: (1) uncertain PCL charac- terization in cross-sectional imaging; (2) age ≥ 18 years; and (3) patient’s consent. Patients were excluded in the case of: (1) unequivocal diagnosis in first-line diagnostic tests; (2) unwill- ingness to undergo EUS-FNA; (3) high procedural risks; (4) indi- cation to surgery; and (5) coagulopathy (international normalized ratio > 1.5, platelets < 50,0 0 0 ×109/L); ongoing antithrombotic treatment.
EUS-FNA was performed by a 20-year experienced gastroen- terologist (MN) who performed more than 150 EUS-FNA of PCLs before the study period began. Eight cross-over patients from a hypolipidemic drug-class to another were excluded from the anal- ysis in order to avoid misclassification due to class switching. Final flow chart of patients actually recruited in the study is reported in Fig. 1 . The study population included two groups of patients: 86 patients in statin therapy due to dyslipidemia at the time of EUS- FNA (statin users group), and 279 subjects who underwent EUS- FNA while not being in hypolipidemic therapy (control group). In statin users group, 36 patients (41.9%) used simvastatin, 17 (19.8%) atorvastatin, 16 (18.6%) lovastatin, 10 (11.6%) rosuvastatin, and 7 (8.1%) pravastatin.
EUS technique
The EUS technique adopted at our center was described else- where [19-21] .
Briefly, the patient was deeply sedated by a board-certified anesthesiologist with propofol (Diprivan®, AstraZeneca, London, UK) and the procedure was performed with a Pentax FG-36UA ultrasound endoscope (Pentax Europe, Ltd., Hamburg, Germany) using a curved-array transducer. The probe was located adjacent to the gastric wall and then slowly advanced up to duodenum when necessary. Emptying of the cyst was performed through a 22 G needle (EchoTip Ultra, Cook Medical Inc., Bloomington, Indi- ana, USA) with a central stylet (immediately removed after the procedure). A transduodenal approach was adopted in the case of PCLs of the pancreatic head and uncinate process, while the trans- gastric approach was used for PCLs of the body or tail. A single needle pass was performed whenever possible and all PCLs were aspirated to complete emptying. Procedures were performed ei- ther during the hospitalization or in day hospital. In both cases the monitoring protocol was the same.
Follow-up and outcomes
All clinical outcomes and complications were assessed by clini- cians blinded to the hypolipidemic therapy used by the patient.
AP was defined as upper abdominal pain (with or without nau- sea and/or vomiting) accompanied by at least three-fold elevation of serum amylase or lipase within 24 h of the procedure. An expe- rienced gastrointestinal nurse called all patients 24-72 h after the procedure to follow-up on any potential complications.
All adverse events were classified according to Common Termi- nology Criteria for Adverse Events (CTCAE) 4.0 [22] . An adverse event was classified as severe when requiring hospitalization, or resulting in death or disability.
Primary outcome was the incidence of AP after EUS-FNA. Sec- ondary variables included other complications of the procedure (i.e., bleeding or infection).
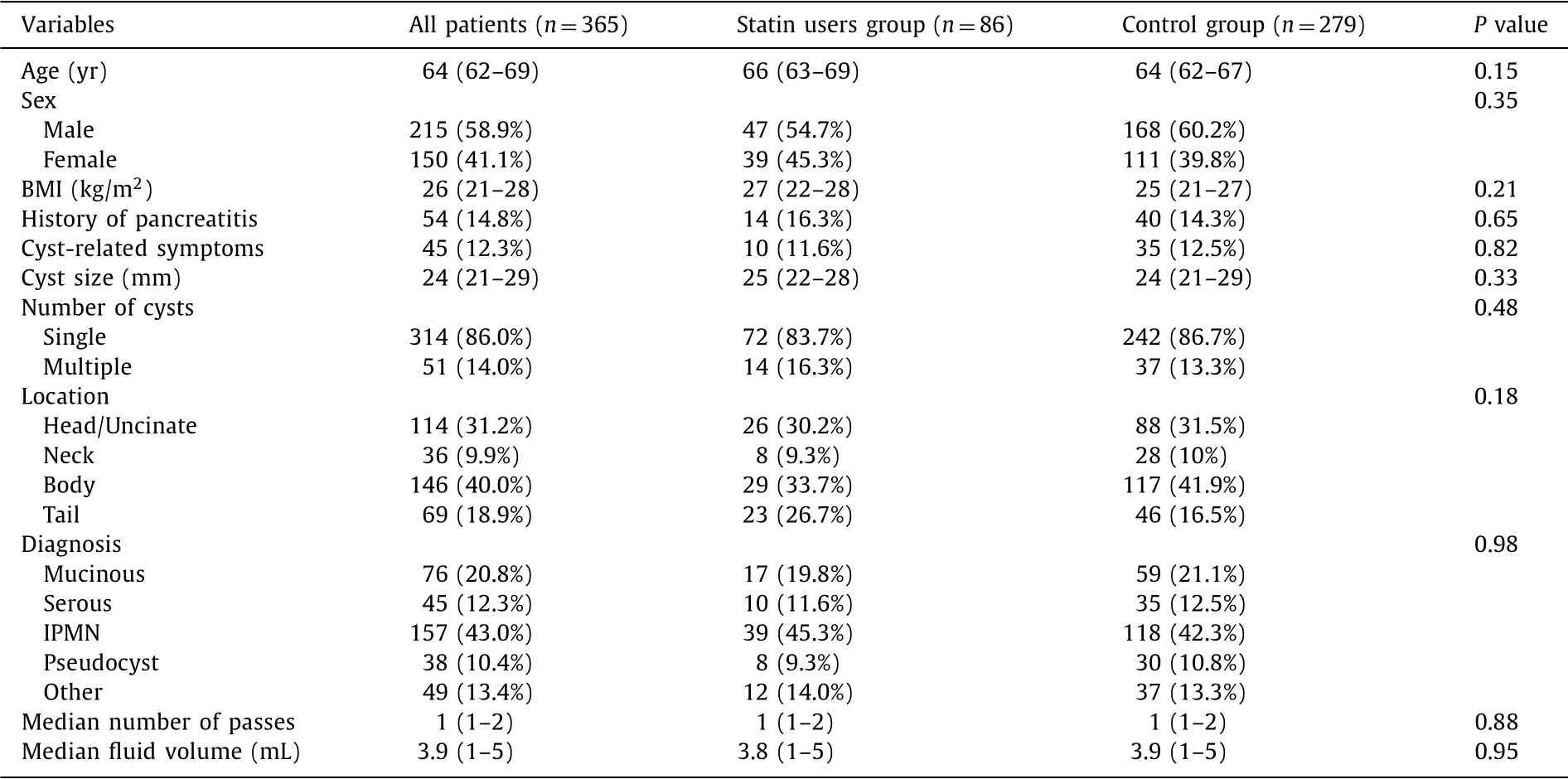
Table 1 Baseline patients’ characteristics.
Cyst classification was performed using cyst fluid analysis, sur- gical histology (when available), and imaging studies. Cytology fea- tures consistent with IPMN featured highly cellular cells arranged predominantly in tall papillary groups distributed in a background of abundant mucin. MCN featured variable columnar to cuboidal cells with cytoplasmic mucin which were typical for its cellular cytomorphology, along with positive staining with mucicarmine stain. Cytology in patients with serous cystadenomas showed small clusters of uniform cuboidal epithelial cells with finely granular cy- toplasm and round nuclei. Patients with pseudocysts revealed neu- trophils and/or histiocytes with some epithelial clusters.
Statistical analysis
Categorical variables were expressed in absolute number and percentage, and comparisons were performed with the Chi-square analysis. Continuous variables were reported as median and in- terquartile range (IQR) and comparisons were computed by the Mann-Whitney test since normality assumption could not be con- firmed for all variables.
The inferential analysis for AP and other complications was conducted using the univariate and multivariate logistic regression model to estimate odds ratio (OR) and 95% confidence interval (CI). Statistically significant variables from the univariate analysis were included into the multivariate model.
All analyses were 2-tailed and the threshold of significance was assessed at P < 0.05. R Statistical Software (Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analysis.
Results
Patients
The baseline characteristics of 365 patients who underwent EUS-FNA of PCLs are reported in Table 1 .
Median age was 64 years (IQR, 62-69) and most patients were male (58.9%), without difference between groups. Median BMI was 26 kg/m2(IQR, 21-28) and history of previous pancreatitis was reg- istered in 54 patients (14.8%), with no difference between the two groups ( P = 0.65).
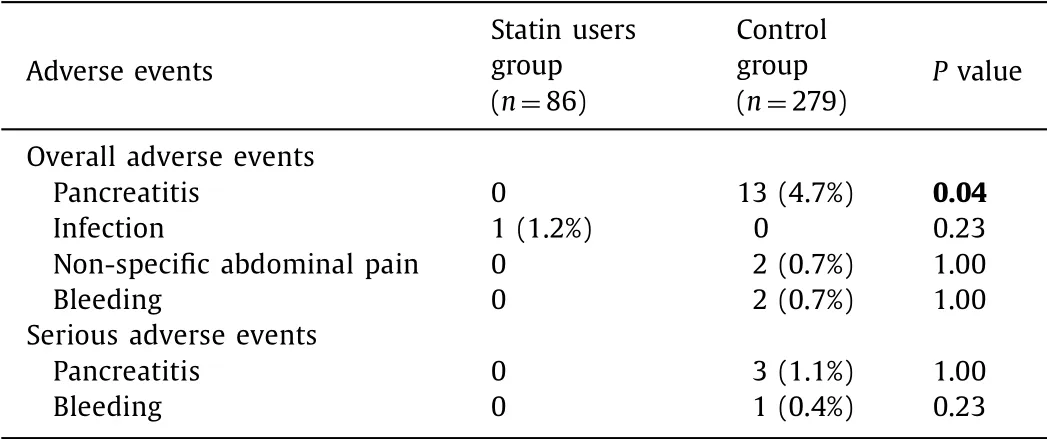
Table 2 Overall and serious adverse events.
Median cyst size was 24 mm (IQR, 21-29) and cyst-related symptoms were present in 11.6% of the statin users group and 12.5% in the control group ( P = 0.82).
Cysts were more frequently single (86.0%) and observed in the pancreatic body (40.0%).
Most frequent histology was IPMN (45.3% in the statin users group and 42.3% in the control group, P = 0.98). Median number of passes was 1 (1-2) in both groups. A direct communication be- tween the cyst and the pancreatic duct was observed in 195 pa- tients (53.4%), of which 49 (57.0%) in the statin users group and 146 (52.3%) in the control group.
Acute pancreatitis
A detailed list of overall and serious complications is reported in Table 2 .
Thirteen patients in the control group (4.7%) experienced post- FNA AP, of which (means of 13, not 365) 3 were classified as severe pancreatitis. As reported in Table 2 , none post-FNA AP was found in the statin users group ( P = 0.04).
Patients with serious abdominal pain were hospitalized, treated with analgesics and antibiotics, and underwent CT-scan to assess the severity of AP, while mild cases rapidly recovered after 6 h of fasting and single IV administration of proton pump inhibitors.

Table 3 Univariate/multivariate analysis of prognostic factors for occurrence of pancreatitis.
In univariate logistic regression analysis, previous history of pancreatitis, cyst histology, and non-use of statins were predictors of AP incidence ( Table 3 ). All of these 3 variables were confirmed as significant parameters in the multivariate analysis ( Table 3 ). In particular, statin use resulted significant protective effect against AP occurrence ( OR = 0.15; 95% CI: 0.03-0.98; P = 0.03).
Obviously, as none of statin users experienced AP, no differ- ence according to drug used (within the statin class) was observed ( P = 1.00).
Other complications
Complications other than AP were rarely reported and homoge- neously distributed between the two study groups. Cyst mild in- fection was observed in 1 patient (1.1%) in the statin users group while non-specific abdominal pain was registered in 2 patients (0.7%) in the control group ( P = 0.23 and P = 1.00, respectively; Table 2 ). Two cases of bleeding (0.7%), of which 1 serious, were ob- served in the control group versus no bleeding event in the statin users group with no significant difference.
Mild events were managed conservatively, whereas the patient who experienced severe bleeding underwent surgery. All of com- plications recovered successfully.
Discussion
Although EUS-guided sampling of PCLs is a safe procedure with limited complications [5 , 6] , AP still remains an issue in particular after EUS-FNA of cystic lesions in communication with the pancre- atic duct [7] .
A growing body of evidence supports the statement that the beneficial effects of statins are not only limited to lowering choles- terol serum levels but include also other pleiotropic effects [23] . While their clinical relevance is still controversial, statins clearly show anti-inflammatory properties [8 , 9] . In fact, statins are well known to improve endothelial function by inducing eNOS within endothelial cells and decreasing exocytosis of Weibel-Palade bod- ies, granules whose contents promote vascular thrombosis and in- flammation [24] . Furthermore, statin use impairs leukocytes re- cruitment in the sub-endothelial space through down-regulation of several chemokines such as MCP1, IL-8, and RANTES and inhibition of β2-integrins [9] .
According to prospective cohort studies and several meta- analyses [10-14] , statins seem to play a protective role against AP; moreover, patients undergoing chronic statin treatment seem to develop milder episodes of AP and even decreased mortality [14] . In a large retrospective cohort study based on data from an inte- grated health-care system, simvastatin consumption was indepen- dently associated with a lower probability of having an episode of AP and similar results were noted for atorvastatin, suggesting a class effect [11] .
These results paved the way to an extensive line of research involving observational and experimental studies aiming to ascer- tain whether statins are useful for preventing AP. A retrospective series showed decreased risk of AP after ERCP [17] and an interna- tional prospective protocol is currently ongoing aiming to find out whether statins may influence positively on the incidence and the clinical course of post-ERCP AP [18] .
To the best of our knowledge, the current study represents the first series evaluating the potential beneficial role of statins in pre- venting AP after EUS-guided FNA of PCLs.
Overall, incidence of AP after EUS-FNA was in keeping with the current literature (3.6%, of which only 0.8% severe), thus confirm- ing the low-risk profile of this procedure. Interestingly enough, all of AP events occurred in the control group, hence none of statin users experienced post-FNA AP. Therefore, as already shown in other settings (namely, in the prophylaxis of post-ERCP pancre- atitis), statins were proved to play a significant preventive role against post-procedural AP. Although no difference was registered with regard to severe AP (1% in the control group versus 0% in the statin users group, P = 1.00), this finding is probably due to the low incidence of post-FNA severe pancreatitis and we may postu- late that in a larger series (hence with higher absolute number of severe AP events) statins would provide significant results also in the prevention of serious adverse events.
The striking results observed in the statin users group were confirmed even in the multivariate analysis, where a significant protective effect for AP incidence was observed (OR = 0.15; 95% CI: 0.03-0.98; P = 0.03).
As expected, AP occurred more frequently in IPMN patients with direct communication between the aspirated cyst and the main pancreatic duct and in subjects with history of previ- ous pancreatitis. However, multivariate analysis confirmed the beneficial effects of statins in preventing AP independently of these two variables, already known to impact on post-FNA AP incidence [7 , 25-29] .
As none of statin users experienced AP, no difference accord- ing to the drug used was observed hence the prophylactic role of statins was a class effect, as suggested in previous reports [11] .
These findings, if confirmed in further series, are undoubt- edly of interest since statins may represent an inexpensive and safe pharmacological tool to prevent AP, a dreadful and po- tentially serious complication of interventional procedures in pancreatology.
No difference was observed in terms of other complications, namely infection, bleeding and non-specific abdominal pain, which were reported infrequently and homogeneously distributed be- tween the two study groups.
This study has a number of strengths: firstly, it is the only pub- lished series evaluating the beneficial role of statins in preventing post-FNA AP. Although EUS-FNA is considered a safe procedure (as confirmed in our study), previous reports properly posed a note of caution in the sampling of IPMNs with a reported incidence of AP up to six times greater as compared to other cyst types [7] . Therefore, a cheap, widely available, and easy to manage pharma- cological agent such as statins may represent a useful preventive tool at least in this high-risk subset of patients. Other elements of strength in our study are the accurate statistical design and the completeness of the collected data as well as the unicentricity of the current report which is a guarantee against eventual biases due to different treatment procedures or endoscopic training.
Nevertheless, the paper has some weaknesses. Its main limi- tation is the retrospective nature of the study which could have led to selection biases. However, the two study groups resulted well-balanced in terms of baseline variables as reported in Table 1 . Secondly, the small number of patients who developed AP after EUS-FNA may have the potential to introduce bias when identi- fying significant predictors of this complication. Furthermore, the difference in sample size between the two study groups might constitute an additional source of bias in the comparative anal- ysis. Nevertheless, a rigorous uni/multivariate logistic regression analysis was performed and statin use resulted as a significant protective factor against AP occurrence independently of other predictors of pancreatitis such as previous anamnesis of AP and cyst histology. Finally, cost considerations were beyond the scope of the present study and could not be addressed.
Despite such limitation, we think that our analysis provides ro- bust evidence supporting the beneficial role of statins in prevent- ing post-FNA AP.
In conclusion, basic and clinical studies suggest that statins may prevent and even ameliorate AP. Our study points out the effective- ness of statins as a prophylactic agent of AP in PCLs patients un- dergoing EUS-FNA. Although EUS-FNA is a relatively safe procedure burdened by a low rate of complications, our findings may be of particular interest in the subset of patients with IPMNs that, given the direct communication between aspirated cyst and pancreatic duct, are at higher risk of post-FNA pancreatitis. Moreover, our study represents a further step to support the anti-inflammatory effects of statins in pancreatology, thus pushing the extensive use of these drugs in the prevention of AP after other invasive proce- dures such as ERCP or therapeutic EUS.
Acknowledgments
We thank Miss Elektra Fike-Data for her help in reviewing and revising the manuscript for grammar and syntax.
CRediT authorship contribution statement
Antonio Facciorusso:Conceptualization, Formal analysis, Su- pervision, Writing - review & editing.Vincenzo Rosario Buc-cino:Data curation, Writing - original draft.Valentina Del Prete:Methodology, Writing - original draft.Matteo Antonino:Method- ology, Writing - original draft.Antonella Contaldo:Methodology, Writing - original draft.Nicola Muscatiello: Investigation, Supervi- sion, Writing - review & editing.
Funding
None.
Ethical approval
The study was approved by the local Institutional Review Board and patients provided written informed consent to perform the procedure.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Impact of EBV infection and immune function assay for lymphoproliferative disorder in pediatric patients after liver transplantation: A single-center experience
- Hepatobiliary&Pancreatic Diseases International
- Intraoperative management and early post-operative outcomes of patients with coronary artery disease who underwent orthotopic liver transplantation
- Acute onset of autoimmune hepatitis in children and adolescents
- Liver stiffness as a predictor of hepatocellular carcinoma behavior in patients with hepatitis C related liver cirrhosis ✩
- Treatment and prognosis of hepatic epithelioid hemangioendothelioma based on SEER data analysis from 1973 to 2014
