Treatment and prognosis of hepatic epithelioid hemangioendothelioma based on SEER data analysis from 1973 to 2014
2020-03-03KyuNohSoonSunKimMinJeYngSunGyoLimJeChulHwngHyoJungChoJeYounCheongSungWonCho
O Kyu Noh , b , c , Soon Sun Kim , , Min Je Yng , Sun Gyo Lim , Je Chul Hwng , Hyo Jung Cho , Je Youn Cheong , Sung Won Cho
a Department of Radiation Oncology, Ajou University School of Medicine, Suwon, Korea
b Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea
c Office of Biostatistics, Ajou Research Institute for Innovative Medicine, Ajou University Medical Center, Suwon, Korea
d Department of Gastroenterology, Ajou University School of Medicine, Suwon, Korea
Keywords: Hemangioendothelioma Epithelioid SEER program Liver transplantation Hepatectomy Liver neoplasms
ABSTRACT Background: Hepatic epithelioid hemangioendothelioma (HEH) is a rare tumor of vascular origin with an unknown etiology, a low incidence, and a variable natural course. We evaluated the management and prognosis of HEH from the Surveillance, Epidemiology and End Results (SEER) program and changes in treatment modalities of HEH over 30 years. Methods: From 1973 to 2014 in the SEER database, we selected patients diagnosed with HEH. We ana- lyzed the clinical characteristics, patterns of management, and clinical outcomes of patients with HEH. Results: We identified 79 patients with HEH (median age: 54.0 years; male to female ratio: 1:2.6). The initial extent of disease was local in 22 (27.8%) patients, regional metastasis in 22 (27.8%), distant metas- tasis in 31 (39.2%) and unknown in 4 (5.1%). The median size of primary tumor was 3.85 cm (interquartile range, 2.50-7.93 cm). Among 74 patients with available management data, the most common manage- ment was no treatment (29/74, 39.2%), followed by chemotherapy only (22/74, 29.7%), liver resection- based (13/74, 17.6%), and transplantation-based therapy (6/74, 8.1%). The 5-year cancer-specific survival rate was 57.8%. Patients who underwent surgical treatment had significantly higher survival than those who underwent non-surgical treatment (5-year survival; 88% vs. 49%, P = 0.019). Multivariate analysis revealed that surgical therapy was the only independent prognostic factor for survival (hazard ratio: 0.20, P = 0.040). Conclusions: Resection or liver transplantation is worth considering for treatment of patients with HEH.
Introduction
Epithelioid hemangioendothelioma is a rare tumor of vascular origin that can involve soft tissues, as well as visceral organs, in- cluding the liver, lung, spleen, stomach, and heart. Hepatic ep- ithelioid hemangioendothelioma (HEH) is a kind of clinical form of the disease [1] . Due to its low incidence and variable clinical course, the standard treatment has not been established. Liver re- section, liver transplantation, chemotherapy, radiotherapy, and/or immunotherapy have been used in the treatment of patients with HEH [2] . Several studies reported that liver transplantation can prolong survival by preventing liver failure even in patients with a known extrahepatic disease [3-6] .
The Surveillance, Epidemiology and End Results (SEER) registry has been used extensively over the last two decades in oncology research [7] . The SEER registry currently covers 17 geographical areas in the United States representing 28% of the United States population. SEER reports information on patient demographics, tu- mor data, primary tumor site, the extent of disease, use of cancer- directed surgery and radiation therapy, and follow-up for vital sta- tus [7] .
To the best of our knowledge, there was only one study on HEH using SEER registry data until now. The previous study re- ported data from 56 patients with HEH from SEER database until 2007 [8] . In this study, we updated the management and prognosis of patients with HEH using the SEER database from 1973 to 2014. Furthermore, we evaluated the changes in treatment modalities of HEH over 30 years.
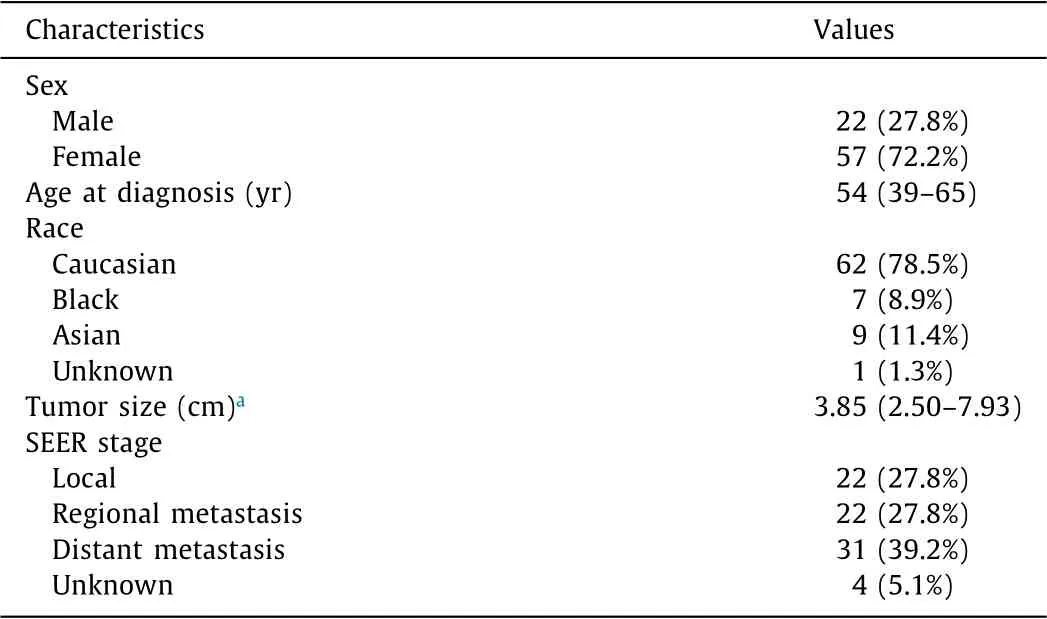
Table 1 Clinical summary of hepatic epithelioid hemangioendothelioma.
Methods
Patient selection
A retrospective cohort study was performed using data from the SEER registry between 1973 and 2014. International Classification of Diseases for Oncology, 3rd Edition histology codes 9130 (he- mangioendothelioma) was combined with site code C22.0 (liver) to identify patients with HEH. The extent of disease was classi- fied by the SEER stage: local (confined to the liver), regional (ei- ther direct tumor extension or limited to regional lymph nodes), and distant (metastatic). Surgical treatments were coded as trans- plant (70), resection (20-32), and local ablation (10) until 2003. From 2004, these were coded as transplant (61), resection (20-59), and ablative procedures (10-17).
Statistical analysis
Continuous variables were expressed as median (interquartile range, IQR). Categorical variables were expressed as numbers and percentage (%). Treatment pattern over time was analyzed using linear trend analysis. We calculated the cancer-specific survival us- ing the Kaplan-Meier method. The Cox proportional hazards mod- els were used for univariate and multivariate analyses. Factors with a P value of less than 0.20 in the univariate analysis were included in the multivariate analysis. Two-sided P values less than 0.05 were considered statistically significant. All statistical analyses were per- formed with IBM SPSS statistical software, version 23 (IBM SPSS, Armonk, NY, USA).
Results
Baseline characteristics of the study population
A total of 79 patients were identified with HEH in the SEER database from 1973 to 2014. The patient characteristics are sum- marized in Table 1 . Out of 79 patients, 57 (72.2%) were women, and the median age was 54 years (IQR, 39-65). Sixty-two (78.5%) patients were Caucasian. Local, regional, and distant diseases were in 22 (27.8%), 22 (27.8%), and 31 (39.2%), respectively. The me- dian tumor size was 3.85 cm (IQR, 2.50-7.93). Regarding the year of diagnosis, the first patient with HEH was registered in 1986, and most of the HEH cases were diagnosed after 1998. The largest number of patients was registered in 2009 ( Fig. 1 ).
Treatment strategy and its changes over time

Fig. 1. Distribution of registered patients with hepatic epithelioid hemangioendothelioma according to year.
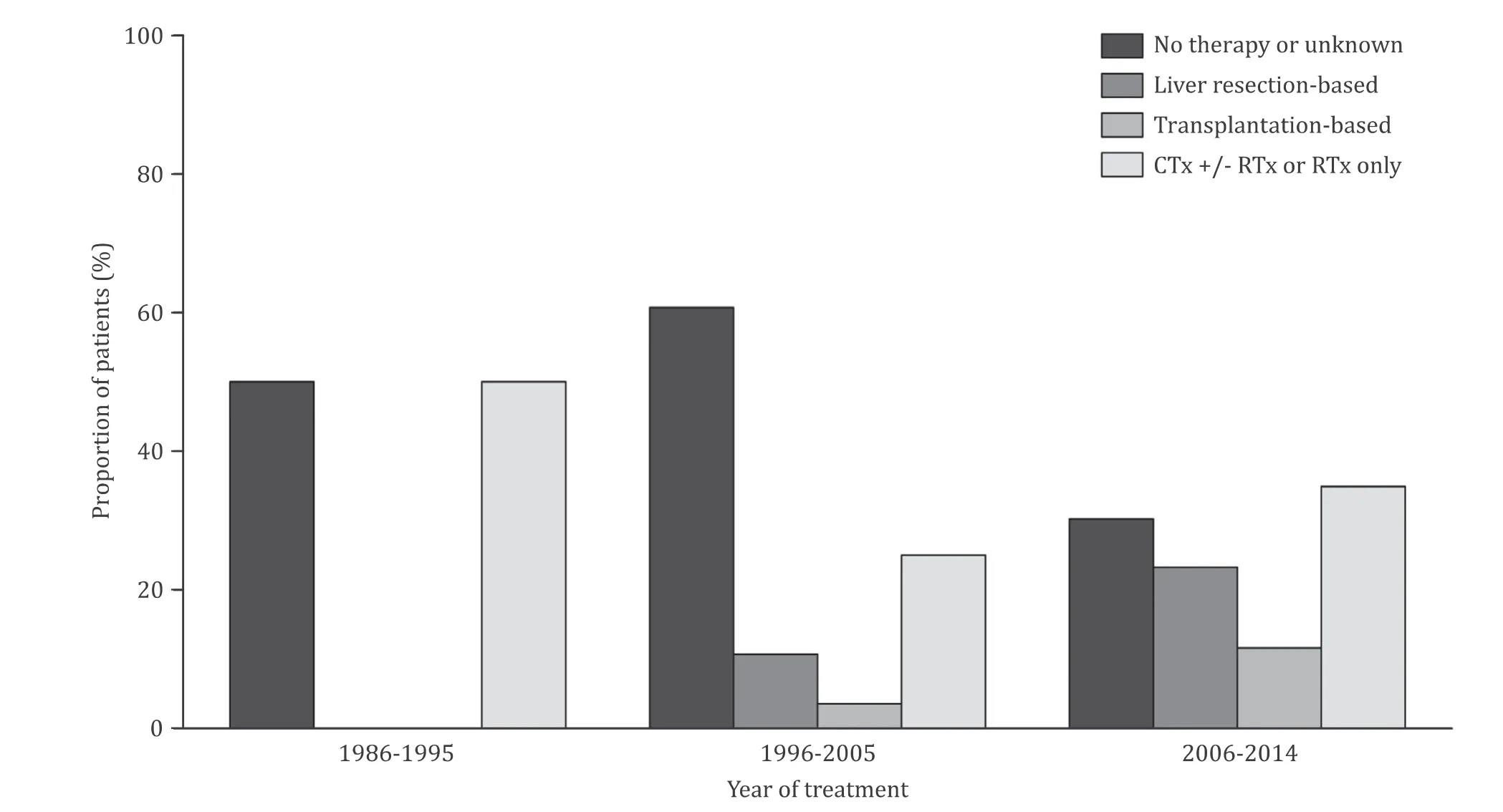
Fig. 2. Types of therapies according to the period of treatment. Linear trend P value < 0.001. CTx: chemotherapy; RTx: radiotherapy.

Table 2 Treatment summary of hepatic epithelioid hemangioendothelioma.
The types of management are listed in Table 2 . For 5 pa- tients, the data on therapy were not available. Among 74 pa- tients with available management data, the most common man- agement was no treatment (39.2%), followed by chemotherapy only (29.7%), liver resection-based (17.6%), and transplantation-based therapy (8.1%). Nineteen patients (25.7%) underwent surgical treat- ment (liver resection-based or transplantation-based). We divided the period into three stages (1986-1995, 1996-20 05, and 20 06-2014) based on decades, and compared the patterns of treatment over time. During the first decade, no patients underwent surgical therapy, and no therapy or unknown cases were predominant. Af- ter that, the number of no treatment or unknown cases decreased, and the number of patients who received surgical procedure in- creased ( P < 0.001, Fig. 2 ).
Cancer-specific survival and prognostic factors
In the whole cohort, the 1-year and 5-year cancer-specific sur- vivals were 74.1% [95% confidence interval (95% CI), 63.9%-84.3%] and 57.8% (95% CI, 45.3%-69.8%), respectively. The cancer-specific survival was different among the groups with various therapies ( P = 0.033, Fig. 3 A). Those who underwent surgical treatment had a significantly higher survival rate than those who underwent non- surgical treatment (5-year survival: 88% vs. 49%, P = 0.019; Fig. 3 B). Survival rates were not significantly different according to the SEER stage ( P = 0.150, Fig. 3 C) and the year of treatment ( P = 0.530, Fig. 3 D). Multivariate analysis showed that the independent prog- nostic factor affecting cancer-specific survival was the treatment type favoring surgical therapy (liver resection or transplantation- based) (hazard ratio = 0.20, P = 0.040; Model 1 in Table 3 ). Chemotherapy based treatment was associated with poor overall survival (hazard ratio = 2.34, P = 0.039; Model 2 of Table 3 ).
Discussion
The current study updated the management and prognosis of 79 patients with HEH using the most recent SEER database from 1973 to 2014. The most common treatment was conservative care. In patients who were treated with surgical therapy (liver resection or transplantation-based), the 5-year cancer-specific survival was 88%. Additionally, we evaluated the changes of treatment modal- ities of HEH over 30 years. Compared to that in the first decade, the use of surgical therapy slightly increased in the other decades. However, the survival rate of patients with HEH has not changed over time.
About two-thirds of patients with HEH were diagnosed with regionally or distantly metastatic disease. The advanced stages of HEH might be due to its unclear etiology and indolent nature. One- fourth of patients were asymptomatic. Even most tumor markers, such as α-fetoprotein, carcinoembryonic antigen, and cancer anti- gen 19-9, were negative [2] .
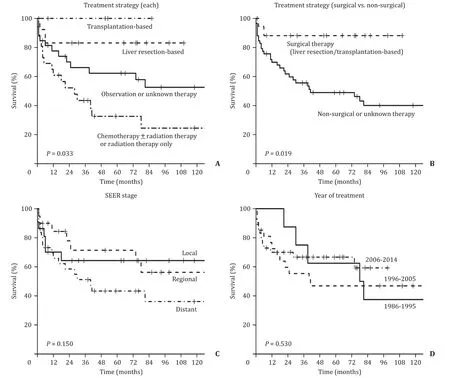
Fig. 3. Cancer-specific survival. A : Each treatment strategy; B : Surgical versus non-surgical treatment; C : SEER stage; D : Year of treatment. SEER: the Surveillance, Epidemi- ology and End Results program.
HEH is often diagnosed incidentally on imaging modality including ultrasonography, computed tomography (CT), magnetic resonance image (MRI), or positron emission tomography recom- mended for other indications. Typical CT findings of HEH include multiple hypervascularized nodules or large hypodence lesion with peripheral enhancement with contrast. When tumor size increases, it can be presented with a halo or target-type pattern of contrast enhancement. Fine calcifications may also appear in 20% of cases. MRI usually reveals hypo-intense lesions on T1- weighted images and a hyperintense heterogeneous pattern on T2-weighted images. Diffusion-weighted imaging usually identifies a rim of diffusion restriction in the periphery of the mass and variable signal in the central core because of T2 shine through effects [9 , 10] . Nonetheless, many findings are non-specific and are often diagnosed as liver metastases or primary liver tumors. Therefore, the definitive diagnosis is made based on histopatho- logical finding. The characteristic histopathological features of HEH are based on evidence of endothelial differentiation, as demonstrated by histochemical positivity of CD34, CD31 and factor VIII-related antigen. Nuclear calmodulin-binding transcription activator 1 expression, which is verified in 85% -90% of patients, is currently the main diagnosis of HEH. The other method using fluorescence in situ hybridization or reverse transcription poly- merase chain reaction to detect the WW domain- containing transcription regulator (WWTRI)-calmodulin-binding transcription activator 1 fusion gene was reported to show with high sensitivity and specificity [11] . The histopathological differential diagnosis includes other vascular tumors including angiosarcoma, metastatic adenocarcinoma, and different tumors with a fibrous stroma such as cholangiocarcinoma, scirrhous hepatocellular carcinoma, or sclerosed hemangioma, as well as nonneoplastic conditions such as veno-occlusive disease. Tumor cells containing the characteristic vascular vacuole may be mistaken for steatotic or mucin vacuoles of an adenocarcinoma, but mucin staining is negative. Angiosar- coma is much more aggressive and destructive, eliminating acinar landmarks and leading to the appearance of cavities. For chola- giocarcinoma, tumor cells are often placed in a tubular or linear pattern with mucin production, positive staining for cytokeratin and negative staining for endothelial markers [9] .
HEH showed a variable clinical course. There are some reports of long-term survival in the presence of stable HEH without any treatment [ 1 , 12 ]. In contrast, the other studies reported an unpre- dictable and aggressive prognosis in some patients [13-16] . Due to the rarity of HEH, previous studies have focused on case series and national registry data. The overall survival rates of the previously reported studies are briefly presented in Table 4 [ 2-4 , 8 , 17-21 ]. In 2006, Mehrabi et al. comprehensively analyzed a total of 434 pa- tients with HEH using a literature review of case reports [18] . This study investigated the most significant number of patients with HEH in a single report. According to this report, liver transplanta- tion was the most common treatment modality (44.8%), followed by no treatment (24.8%), chemotherapy with or without radiother- apy (21.0%), and liver resection (9.4%). Their findings are different from the present study that the most common management was no treatment, followed by chemotherapy with or without radio- therapy and liver resection-based and liver transplantation-based therapies. These discordant results may be related to publication bias and difference in data source (case series vs. national registry database). The results of the present study suggest that liver transplantation was not actively applied in patients with HEH despite of several studies which reported the survival benefits of liver transplantation [ 2 , 4 , 6 , 22 , 23 ]. Recently, a study suggested the HEH treatment algorithm based on data from the European Liver Transplant Registry [2] . After review of 149 HEH patients who underwent liver transplantation, the authors revealed that the risk factors for recurrence after liver transplantation are pathological macrovascular invasion, pathological hilar lymph node invasion, and shorter pre-liver transplantation waiting time ( ≤120 days). This study suggested that patients with aggressive tumor behavior could be excluded with 120 days of waiting time. Importantly, extrahepatic metastasis was not a risk factor for post-liver transplantation recurrence.
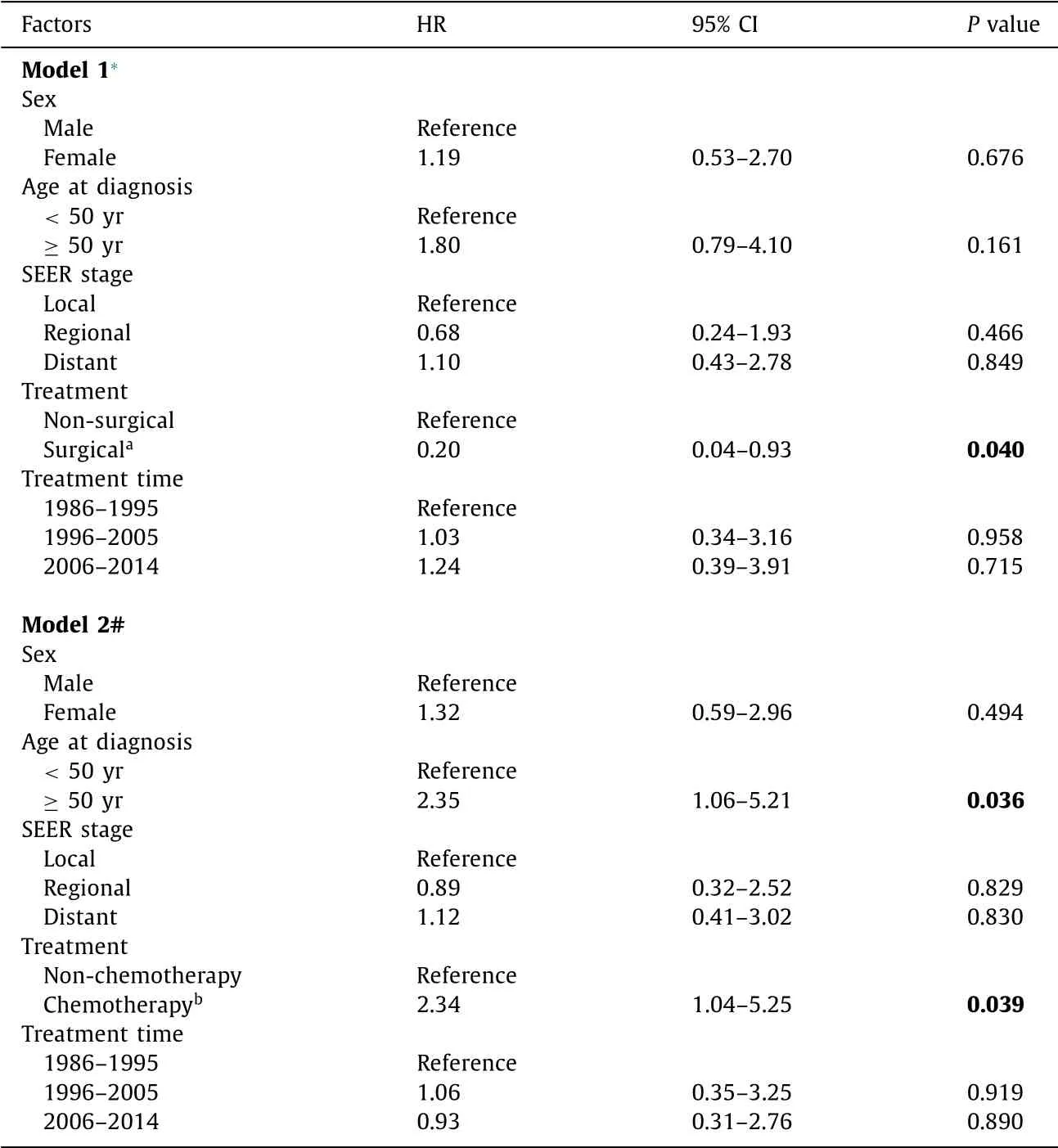
Table 3 Multivariate Cox regression analyses for factors predicting overall survival.
The previous study published in 2014 reviewed the malignant vascular tumor of the liver using the SEER database from 1973 to 2007 [8] . Groeschl’s report briefly examined the 56 patients with HEH and also showed that only 13 patients underwent surgery, including liver transplantation. The 5-year survival rate was ap- proximately 50%, which does not significantly differ from our data. However, they did not evaluate the survival benefit of surgical treatment compared to that of other therapy or conservative care.
Our study had limitations. First limitation is the retrospective nature. Therefore, not all patients had treatment information. Al- though 93.7% of our study patients had treatment data, these data did not include information about the use of systemic or catheter- based therapies. Furthermore, survival analyses of small popula- tions are less reliable, and these results should be interpreted with caution. We did not compare the survival rate of liver resection with those of liver transplantation due to small number of patients in each group. Since recent studies were limited to case series or transplantation registry [3-6] , the current research using SEER reg- istry data is worth considering in reflecting the actual treatment reality of HEH and is representative of patients who are not candi- dates for surgical treatment.
In conclusion, HEH showed different prognosis compared to other liver malignancies. Despite growing evidence of survival ben- efit with surgical treatment, especially liver transplantation, the majority of patients were still treated with conservative care or chemotherapy. These patterns of treatment may contribute to the similar survival rate in the recent 30 years. Nevertheless, surgical resection or liver transplantation is worth considering for treat- ment of patients with HEH.

Table 4 Reported overall survival rate of patients with HEH according to treatment modality.
CRediT authorship contribution statement
O Kyu Noh :Data curation, Formal analysis, Writing - review & editing.Soon Sun Kim :Conceptualization, Funding acquisition, Writing - original draft.Min Jae Yang :Investigation, Methodology.Sun Gyo Lim :Resources, Visualization.Jae Chul Hwang :Investi- gation, Validation.Hyo Jung Cho :Funding acquisition.Jae Youn Cheong :Conceptualization, Supervision.Sung Won Cho :Supervi- sion, Writing - review & editing.
Funding
This research was supported by the Bio & Medical Tech- nology Development Program of the National Research Foun- dation (NRF) funded by the Korean government (MSIT) (NRF- 2018M3A9E8023861) and by a grant from the Korean Health R & D Project. Ministry of Health Welfare, Korea (HI18C0531).
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub- ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Impact of EBV infection and immune function assay for lymphoproliferative disorder in pediatric patients after liver transplantation: A single-center experience
- Hepatobiliary&Pancreatic Diseases International
- Intraoperative management and early post-operative outcomes of patients with coronary artery disease who underwent orthotopic liver transplantation
- Acute onset of autoimmune hepatitis in children and adolescents
- Liver stiffness as a predictor of hepatocellular carcinoma behavior in patients with hepatitis C related liver cirrhosis ✩
- Cholecystoenteric fistula with and without gallstone ileus: A case series
