Impact of EBV infection and immune function assay for lymphoproliferative disorder in pediatric patients after liver transplantation: A single-center experience
2020-03-03TinQinXingQinGuSeogSongJeongYnYnSongJinChunLiuJinXinZhengFengXueQingXi
Tin Qin , Xing-Qin Gu , Seog-Song Jeong , Yn-Yn Song , Jin-Chun Liu , Jin-Xin Zheng , Feng Xue ,, Qing Xi
a Department of Liver Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, China
b Department of Biostatistics, Institute of Medical Sciences, Shanghai Jiaotong University School of Medicine, Shanghai 20 0 025, China
Keywords: Post-transplant lymphoproliferative disorder Pediatric liver transplantation Epstein-Barr virus
ABSTRACT Background: Post-transplant lymphoproliferative disorder (PTLD) is a lethal complication after pediatric liver transplantation, but information regarding risk factors for the development of PTLD remains unclear. This study was to identify characteristics and risk factors of PTLD. Methods: A total of 705 pediatric patients who underwent liver transplantation between January 2017 and October 2018 were studied. Impact of clinical characteristics and Epstein-Barr virus (EBV) infection on the development of PTLD was evaluated. In addition, ImmuKnow assay was adopted in partial patients to analyze the immune status. Results: Twenty-fvie (3.5%) patients suffered from PLTD with a median time of 6 months (3-14 months) after transplantation. Extremely high tacrolimus (TAC) level was found in 2 fatal cases at PTLD onset. EBV infection was found in 468 (66.4%) patients. A higher peak EBV DNA loads ( > 9590 copies/mL) within 3 months was a significant indicator for the onset of PTLD. In addition, the ImmuKnow assay demonstrated that overall immune response was significantly lower in patients with EBV infection and PTLD ( P < 0.0 0 01). The cumulative incidence of PTLD was also higher in patients with lower ATP value ( ≤187 ng/mL, P < 0.05). Conclusions: A careful monitoring of EBV DNA loads and tacrolimus concentration might be support- ive in prevention of PTLD in pediatric patients after liver transplantation. In addition, application of the ImmuKnow assay may provide guidance in reducing immunosuppressive agents in treatment of PTLD.
Introduction
Post-transplant lymphoproliferative disorder (PTLD) is a lethal complication in liver recipients. Most cases of PTLD are associated with Epstein-Barr virus (EBV) infection and impaired immune surveillance [1-3] . Because of the immature immunity and po- tential chance of EBV transmission from EBV-positive donor, pediatric liver recipients are more susceptible for PTLD, which led to approximately 30% -60% of mortality [4 , 5] . The applica- tion of rituximab, an anti-B-cell monoclonal, has been shown to improve prognosis of PTLD. Previous studies have indicated that the administrations of tacrolimus (TAC), antithymocyte globulin and anti-CD3 antibody are the major risk factors for PTLD [5] . Therefore, the immunosuppression and EBV viral load monitoring were considered the main pre-emptive strategy for PTLD [6 , 7] .
High blood TAC concentration was found suppressive to the T-cell function, resulting in more elevated EBV DNA replication and increased resistance to immune cell killing by promoting the expression of anti-apoptotic genes [8] . Recently, the immune function (ImmuKnow) assay that allows measurement of the cell-mediated immunity based on the peripheral CD4 + T cell ATP activity, could be helpful for monitoring infectious events during the perioperative period after transplantation. ImmuKnow assay could provide a quantization parameter of patients’ dynamics of functional immunity and a direct basis for individualized immune status [9-12] . Hence, we conducted this study to describe char- acteristics and outcomes of PTLD based on a single center experience. In addition, impacts of EBV loads, TAC level, and Im- muKnow adenosine triphosphate (ATP) value to the development of PTLD were evaluated.
Methods
Patients
From January 2017 to October 2018, 745 pediatric patients re- ceived liver transplantation in Renji Hospital (Shanghai, China). Only Chinese patients with a minimum follow-up period of 3 months were included in the present study, thus a total of 705 patients were finally enrolled in the analyses. The institutional database was retrospectively reviewed for clinical characteristics, demographic data of donor, EBV infection status, and the develop- ment of PTLD. This study was conducted in accordance to the Dec- laration of Helsinki and approved by the Ethics Committee of Renji Hospital.
Immunosuppressive regimens and EBV viral load monitoring
All patients were administered to a routine immunosuppres- sive protocol consisting of TAC and steroids with or without my- cophenolate mofetil. TAC was started at day 2-3 after pediatric transplantation, and the initial dose was 0.15 mg/kg body weight per day orally. The target whole blood trough concentration of TAC was 8-12 ng/mL for the first month, 7-10 ng/mL between the second to sixth months, and 5-8 ng/mL thereafter. At each follow-up, TAC concentration was measured and recorded by a mi- croparticulate enzyme immunoassay (IMx or i10 0 0, Abbott Co. Ltd., Tokyo, Japan) according to the manufacturer’s instructions. Rejec- tion episode which has been pathologically proved would be re- viewed as well.
The pediatric liver recipients who have participated in the study were followed up weekly within 3 months after surgery, biweekly from the fourth to sixth months, monthly from the seventh to twelfth months, and once every three months thereafter. The re- cipients were routinely screened by peripheral blood polymerase chain reaction (PCR) for EBV DNA loads at follow-up visit every 2-3 months. The recipients who showed a positive result would be recorded for their viral copy numbers. If all the EBV DNA load tests were negative, the pediatric patients would be clarified to be in the EBV negative group, otherwise in the EBV positive group.
Diagnosis and treatment of PTLD
PTLD is diagnosed by a [18F]-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG)-PET-CT and biopsies of enlarged lymph nodes along with resected tissues. PTLD was histopatho- logically classified into early type, polymorphic type, monomor- phic type, and classical Hodgkin lymphoma according to the World Health Organization classification [13] . The clinical mani- festations, such as lymphadenopathy, fever, diarrhea, and weight loss, were closely monitored. The standard treatment for PTLD included immunosuppressant discontinuation, a monoclonal anti CD20 antibody (rituximab, Roche Pharma Schweiz, Basel Switzer- land), and anthracycline-containing chemotherapy, which was con- sidered when rituximab showed no effect.
ImmuKnow assay
The ImmuKnow assay have been applied in our center from April, 2017 to July, 2018. The collected peripheral whole blood samples (2 mL) were put into green-capped sodium heparin an- ticoagulant tubes within 12 h. Anticoagulated whole blood (250 μL) was diluted and added to a 96-well microtiter plate, which was then incubated for 16-18 h with phytohemagglutinin stim- ulation in a 5% CO 2 37 °C incubator. Magnetic particles coated with anti-human CD4 monoclonal antibodies were added to CD4 + cells in the next day. Subsequently, the selected cells were washed, and the lysing reagent was added to release the intracellular ATP, which was measured using the luciferase by a luminometer (Inzex Biotechnology Company, Shanghai, China).
We have categorized the patients into three status as: EBV neg- ative, EBV positive but not PTLD, and PTLD. Stable status was de- fined as normal clinical experimental and imaging examinations without any noticeable symptoms.
Statistical analysis
All statistical analyses were performed using the SPSS, ver- sion 23.0 (SPSS Inc., Chicago, IL, USA). Graphical plots were gen- erated using the GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Continuous variables were described as median with range. Categorical data were described as numbers with percent- ages. Receiver operating characteristic (ROC) curves were generated to evaluate the cut-off values of EBV viral load and ImmuKnow ATP predicting the development of PTLD. The Cox hazard regression was applied to identify risk factors of PTLD. Kaplan-Meier curves were drawn to compare time-dependent cumulative incidence of PTLD. A P value < 0.05 was considered statistically significant.
Results
Clinical features
A total of 705 pediatric liver transplantation (LT) patients with a median follow-up of 14 months (range 3-24 months) were in- cluded in the analyses. Demographic and clinical characteristics of the patients were shown in Table 1 . The major primary disease for transplantation was biliary atresia (BA; n = 582, 82.6%), followed by methylmalonic acidemia ( n = 27, 3.8%), cryptogenic cirrhosis ( n = 12, 1.7%), propionic acidemia ( n = 10, 1.4%), Alagille syndrome ( n = 8, 1.1%), Caroli’s disease ( n = 5, 0.7%), and hepatoblastoma ( n = 3, 0.4%). The median age of pediatric patients at LT was 7 months (range 4-181 months). The proportion of male patients were 47.2% ( n = 333). The follow-up data showed that 468 (66.4%) patients had EBV infection episodes during the study. In addition, 25 (3.5%) PTLDs were detected, which were diagnosed at a median of 6 months. All PTLDs occurred in patients with history of EBV infection. The serum peak EBV DNA loads within 3 months of PTLD patients were significantly higher compared to those without PTLD (median, 52900 vs 1770 copies/mL; P < 0.001). No significant difference was observed in terms of age, sex distribution, height, weight, pediatric of end-stage liver disease (PELD) score, donor-to- recipient ABO compatibility, and donor related parameters.
Characteristics of PTLD patients
The detailed data of each pediatric PTLD patient were listed in Table 2 . There were 14 male and 11 female patients. The primary diagnosis was mostly BA (23, 92%). Two (8.0%) patients died due to PTLD. The dose of TAC usage ranged from 0 to 3.0 mg/day. TAC levels varied among patients and the median TAC concentration at the onset of PTLD was 3.9 ng/mL. Two cases of death showed an extremely high TAC level (14.1 and 22.4 ng/mL) compared to others who survived from PTLD.
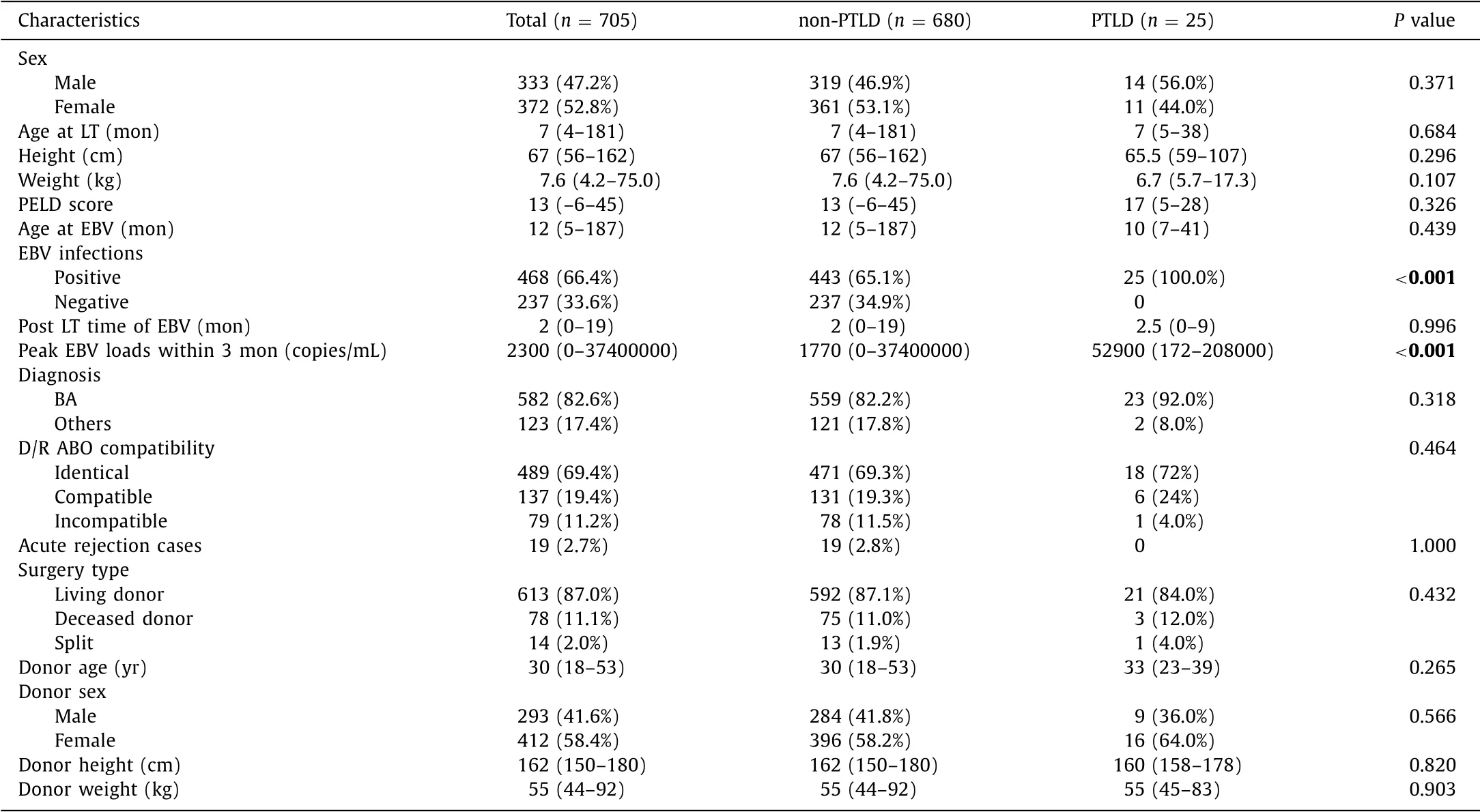
Table 1 Baseline characteristics of the pediatric patients who underwent liver transplantation according to PTLD.
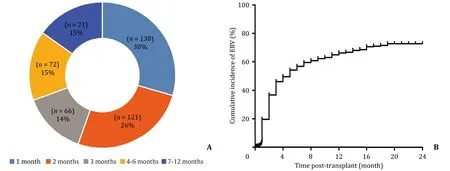
Fig. 1. A : The distribution of EBV positive time post-surgery. About 70% ( n = 325) of the follow-up patients got EBV infection within 3 months after liver transplantation; B : The cumulative incidence of EBV infections showed that the incidence went up sharply before 3 months and slowly paced down thereafter.
EBV infection
The distribution of the time from LT to EBV infection was shown in Fig. 1 A. Three hundred and twenty-five (70%) patients with EBV infection were infected within 3 months after trans- plantation, whereas the others 143 (30%) had EBV infection after 3 months. EBV infection mostly occurred between 1 and 3 months after transplantation with 1-year incidence of 64.8% and 2-year in- cidence > 70% ( Fig. 1 B).
The generated ROC curve showed an optimal cut-off threshold for PTLD with peak EBV DNA loads of 9590 copies/mL ( Fig. 2 A). The area under curve (AUC) was 0.81 with sensitivity of 88.0% and specificity of 67.2% ( P < 0.001). Subsequently, we adopted the de- fined cut-off along with sex, primary diseases, and age in the uni- variate and multivariate analyses for identification of risk factors for PTLD ( Table 3 ). The Cox regression revealed that the peak EBV DNA loads > 9590 copies/mL was a significant risk factor for PTLD (HR = 13.184, P < 0.001). As shown in Fig. 2 B, the generated Kaplan- Meier curves showed that the cumulative incidence of PTLD was higher in patients with peak EBV loads > 9590 copies/mL com- pared to those of peak EBV loads ≤9590 copies/mL (9.9% vs 0.66%, P < 0.05).
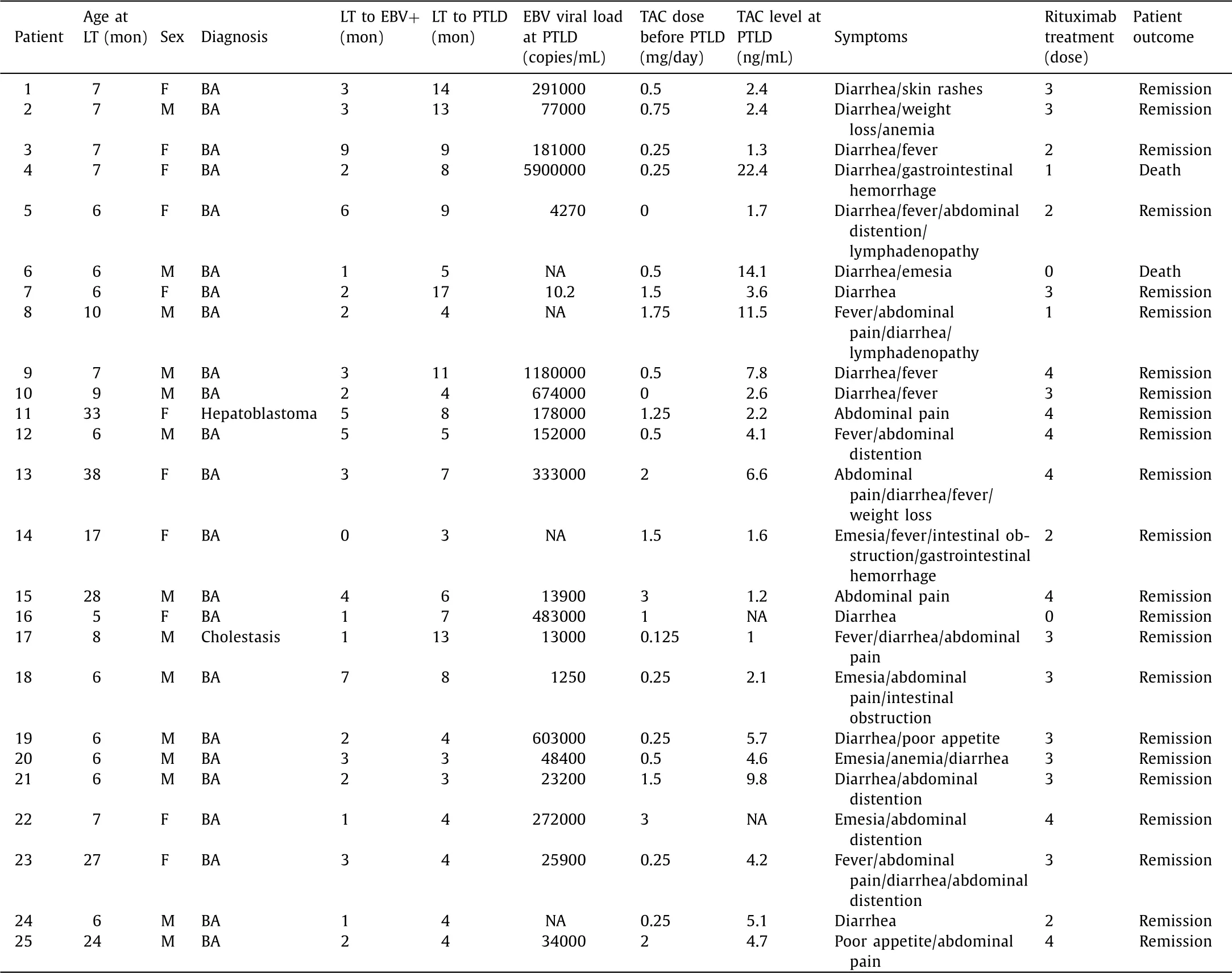
Table 2 Clinical characteristics, tacrolimus usage, and outcomes of 25 patients with PTLD.
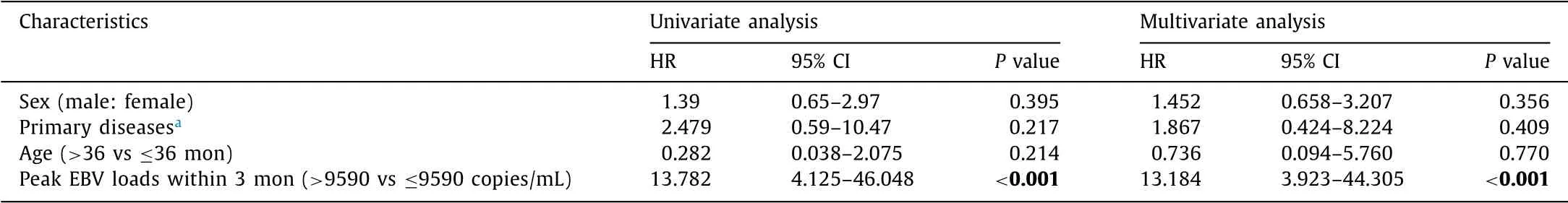
Table 3 Predictive value of EBV loads for PTLD by cox proportional hazard model.

Fig. 3. A: The ImmuKnow ATP values of EBV positive but not PTLD patients and PTLD patients were significantly lower than EBV negative patients ( P < 0.0 0 01); B: The ROC curve for ImmuKnow ATP value to diagnosis EBV infection; C: The cumulative incidence of PTLD was higher in those with ATP values within 3 months post-transplant ≤187 than > 187 ng/mL ( P < 0.05).
ImmuKnow assays
Furthermore, 134 patients received ImmuKnow assay within 3 months post-transplant. Another 15 patients got tested when diagnosed PTLD (more than 3 months post-transplant). The me- dian peripheral blood CD4 + T-cell ATP values at the onset of PTLD ( n = 15, median ATP 111 ng/mL) were significantly lower than those without EBV infection within 3 months post-transplant ( n = 83, median ATP 233 ng/mL; Fig. 3 A). The median ATP values of EBV infected patients without PTLD were 134 ng/mL ( n = 51), also lower than the EBV negative group.
Further Cox regression analysis was performed with the 134 pa- tients’ ImmuKnow assay results within 3 months post-transplant, to reveal the predictive value of ATP level for PTLD. Basic descrip- tion data was shown in Table 4 . Fig. 3 B showed that ImmuKnow ATP had significant diagnostic value for EBV infections. The AUC was 0.91 (95% CI: 0.85-0.96) with sensitivity of 75.9% and speci- ficity of 94.1% at the cut-off point of 187 ng/mL ( P < 0.01). Mul- tivariate analysis showed that ATP value ≤187 ng/mL was a sig- nificant risk factor for PTLD (HR = 14.111, P = 0.027). While EBV DNA loads > 9590 copies/mL still acted as a higher risk factor for PTLD (HR = 15.654, P = 0.013, Table 5 ). The Kaplan-Meier curves showed that the cumulative incidence of PTLD was higher in pa- tients with ATP value ≤187 ng/mL compared with those of ATP value > 187 ng/mL ( P < 0.05, Fig. 3 C).
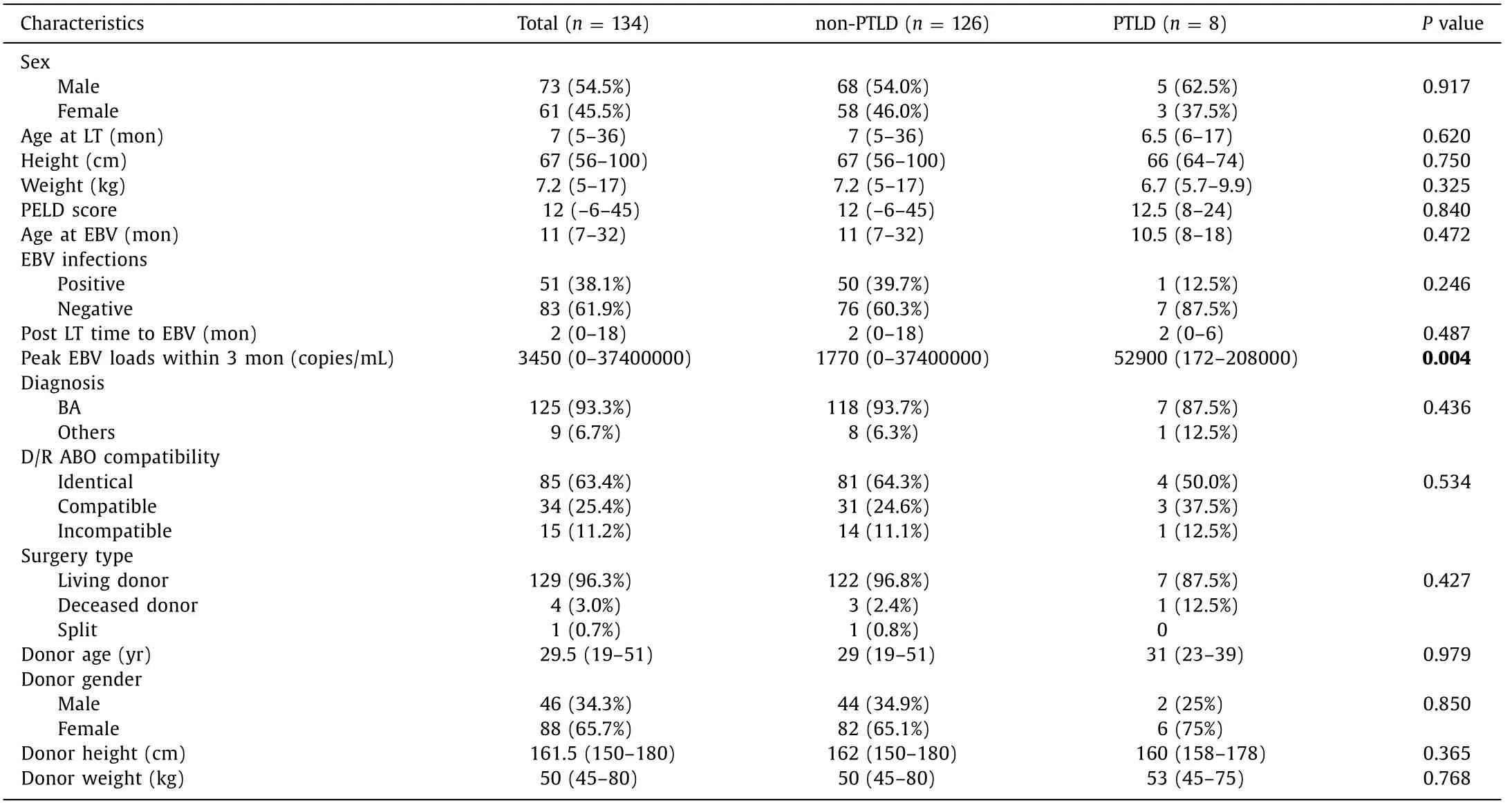
Table 4 Baseline characteristics of the pediatric patients who had ImmuKnow assays within 3 months post-surgery.
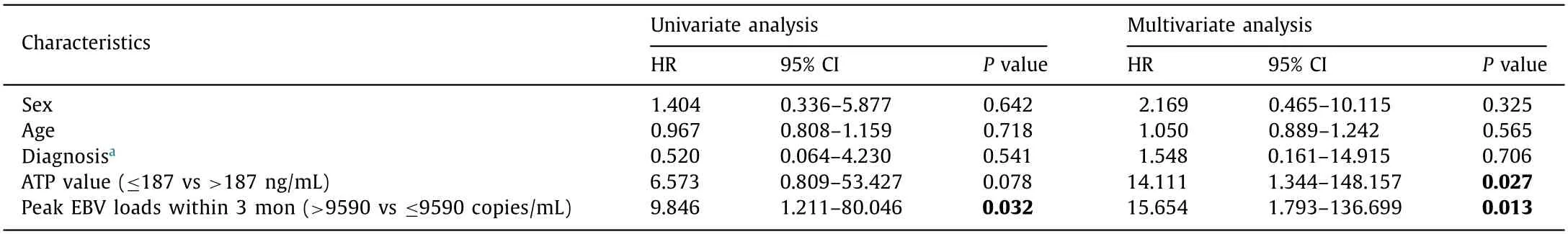
Table 5 Predictive value of EBV loads and ATP values for PTLD by cox proportional hazard model.
The relationship between ImmuKnow ATP levels and other clinical parameters
According to the Spearman’s Rank correlation coefficient test at each visiting-point, there was no correlation between the Immu- Know ATP levels and white blood cell counts ( r = 0.22, P = 0.01), TAC concentrations ( r = -0.02; P = 0.81), and EBV DNA loads ( r = -0.23, P = 0.11), respectively ( Fig. 4 ). The coefficient value ( r value) was quite low between ImmuKnow ATP levels and white blood cell counts despite of a significant P value, which demon- strated a weak relationship.
Discussion
PTLD is a life-threatening complication for pediatric recipients undergoing LT. The incidence of PTLD was 3.5% in our hospital, which is comparable to other centers [14 , 15] . Previous studies have found that TAC levels, EBV DNA loads, and observation of PTLD- associated signs and symptoms are related to the development of PTLD in children. Thus a careful monitoring of EBV DNA loads and a proactive intervention might be helpful to decrease the incidence of PTLD [7 , 15] . Moreover, we initially derived a specific threshold of EBV DNA copies within 3 months after transplantation for clini- cal drug regimen adjustment that may support prevention of PTLD in patients with EBV infection.
All PTLD patients were EBV-positive at the onset. According to our data, patients with more than 9590 copies/mL of peak EBV loads exhibited higher cumulative incidence of PTLD. In addition, patients with EBV loads higher than 9590 copies/mL were about 13 times more common to have PTLD compared to the others. There- fore, we suggest transplant doctors pay more attention to antivi- ral treatment, and to the recovery and restoration of damaged im- mune system. Although the value of decreasing EBV loads to avoid the incidence of PTLD remains uncertain, it is helpful to monitor the EBV infection and immunosuppression protocol. The frequency of surveillance for EBV DNA loads should also be increased when the loads are increasing.
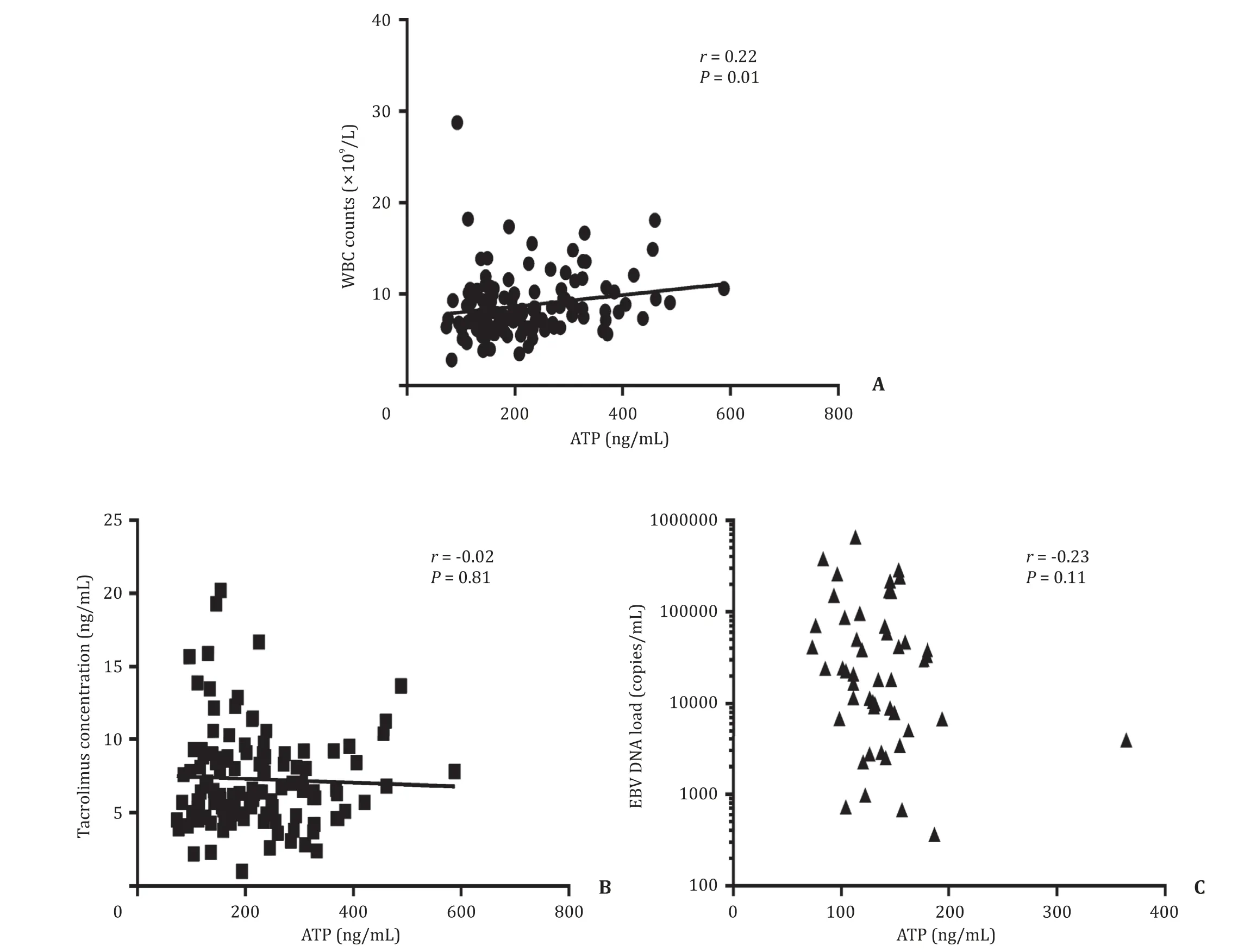
Fig. 4. The relationship between ImmuKnow ATP values and other clinical indexes according to Spearman’s Rank correlation analysis.
As we know, the diagnosis, evaluation and treatment of PTLD still remains a crucial challenge. Some biomarkers, such as EBV DNA in blood and tissues [7 , 16-18] , lymphocyte subsets [19] , and negative EBV serology [20] , might be considered as risk factors of PTLD [21 , 22] . Chen et al. [7] reported that the peak EBV viral load > 4100 copies/ μg peripheral blood mononuclear cell (PBMC) DNA within 3 months after liver transplantation helped to predict a high risk of PTLD, which was in correspondence to our result while we have a larger group for Chinese pediatric recipients. However, the EBV DNA load monitoring in each medical center might be quite different in samples, methods and diagnostic values. Wang et al. recommended that EBV-DNA load in both PBMC and plasma should be tested in PTLD disease [23] . Further studies are still needed to establish a universal standard for EBV DNA monitoring. In addition, researchers have found that EBV-related PTLD usually occurs early after transplantation, mostly in the first year [24] , while EBV-negative PTLD typically takes place later, more than 5 years after transplantation [25 , 26] . Due to the limitation of the follow-up period in our study, there was no EBV-negative PTLD cases in our study, and a research with a longer follow-up would be needed.
Approximately 70% patients suffered from EBV infection within 3 months after transplantation. It inspired us that the donor- related EBV infections may be responsible for its high incidence. A previous study of heart transplant recipients identified that even asymptomatic EBV infection may be a significant risk factor for the development of PTLD, emphasizing importance of routine antiviral therapy [27] . In the present study, all patients administered intra- venous acyclovir for 2 to 3 weeks, which turned into oral adminis- tration of acyclovir for 2-3 months after transplantation. However, some other centers have applied antiviral prophylaxis to prevent EBV infection, as well as EBV-associated PTLD, of which its impact remains to be confirmed [28-30] .
Two patients who did not get remission had a high TAC level at the onset of PTLD, while there were no differences in TAC dose before PTLD. This may remind us that an over regimen level of TAC concentration may be predictive to the prognosis of PTLD. TAC pharmacokinetics (PKs) is thought to be considerably unexplained variable, especially in the early period after transplantation [31 , 32] . Slight change of TAC dosage affects the prognosis of pediatric pa- tients in terms of acute organ rejection and transplant related in- fection [33] . Drug administration during the first 3 months after transplantation is even crucial since the under immunosuppression would cause the rejection so that dropping the dosage is impro- priate. However, PTLD usually occurs more than 3 months post- transplant. The continual over-immunosuppression status would make the recipients vulnerable to viral infections. It may be bet- ter to decrease the dosage in time to regimen level when TAC concentration is higher than usual [34] . Additional intensive mon- itoring is also urgently needed to protect against poor prognosis. Among PTLD cases found in this study, 24 of 25 patients were aged under 3 years at the time of transplantation. Thus, age at transplantation also needs to be considered as an important factor for PTLD.
Reduced immunosuppressive dosage remains a key component of therapy for EBV-positive PTLD and may lead to remission in early disease [35] . Part of our patients’ immune statuses were mea- sured by ImmuKnow from the adenosine 5-triphospate level pro- duced CD4 + T helper cells, which might help provide evidence for immunosuppressive dosage reduction [36] . After all, we furthered our study by finding another predictive value for PTLD incidence, which has never been reported before. As the possible mechanism for EBV DNA loads to predict PTLD, EBV transmission could hap- pen via EBV positive donor or by entry through oropharynx. The duplication of EBV would be harbored by B cells as an episome and is mainly targeted by T cells and NK cells. However, the func- tion of T cells is mostly inhibited by immunosuppressant in trans- plant patients which may lead to an intermittently viral reactiva- tion by active viral duplication and high loads of EBV and finally result in PTLD. Although many studies have demonstrated that the immune protection against EBV is mainly dominated by the expan- sion of CD8 + T cells in the circulation, CD4 + T cell was found to be acutely activated in EBV infection [37] . In our study, the Immu- Know assay mainly detects the peripheral CD4 + T cell ATP activity, and the underlying mechanism between ATP activity and EBV still needs further investigation.
Despite novel findings, some underlying limitations need to be considered when interpreting our results. First, we retrospectively reviewed the patients in our center over the last 2 years, but a multi-center prospective analysis might give a more accurate ob- servation for PTLD patients with a longer observation period. Sec- ond, not all PTLD patients in our center have biopsy data, thus we suggest future studies to include detailed examinations, such as PET-CT and biopsy for PTLD to support treatment strategies. More- over, a test of recipients and donors for serum EBV antibody prior to the transplantation may be helpful for PTLD prevention.
In conclusion, we demonstrated that EBV DNA > 9590 copies/mL has a predictive value of the development of PTLD within 3 months after LT. The ImmuKnow ATP levels ≤187 ng/mL is another param- eter for higher risk of EBV-associated PTLD. Therefore, lower TAC dosage should be recommended for those pediatric patients who have a high EBV DNA loads and/or low ImmuKnow ATP values af- ter transplantation.
CRediT authorship contribution statement
Tian Qin:Data curation, Writing - original draft, Writing - re- view & editing.Xiang-Qian Gu:Data curation.Seog-Song Jeong:Formal analysis.Yan-Yan Song:Formal analysis, Methodology.Jin-Chuan Liu:Data curation.Jian-Xin Zheng:Data curation.Feng Xue:Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing - review & editing.Qiang Xia:Funding acquisition, Supervision.
Funding
This study was supported by grants from Shanghai Munici- pal Hospital Three-year-Project for Clinical Skills’ Promotion and Innovation ( 16CR1003A ), Shanghai Jiaotong University School of Medicine ( DLY201606 ), and National Natural Science Foundation of China ( 81670602 ).
Ethical approval
This study was approved by the Ethics Committee of Renji Hos- pital, Shanghai Jiaotong University School of Medicine.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub- ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary&Pancreatic Diseases International
- Intraoperative management and early post-operative outcomes of patients with coronary artery disease who underwent orthotopic liver transplantation
- Acute onset of autoimmune hepatitis in children and adolescents
- Liver stiffness as a predictor of hepatocellular carcinoma behavior in patients with hepatitis C related liver cirrhosis ✩
- Treatment and prognosis of hepatic epithelioid hemangioendothelioma based on SEER data analysis from 1973 to 2014
- Cholecystoenteric fistula with and without gallstone ileus: A case series
