Liver stiffness as a predictor of hepatocellular carcinoma behavior in patients with hepatitis C related liver cirrhosis ✩
2020-03-03SmehLshenMohmedElshfeiFhmyHlssEmnAlsyedAsmHssn
Smeh A Lshen , , Mohmed M Elshfei , Fhmy H Hlss , Emn A Alsyed , Asm A Hssn
a Department of Internal Medicine, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
b Department of Radiology, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
c Department of Biomedical Informatics and Medical Statistics, Medical Research Institute, University of Alexandria, Alexandria, Egypt
Keywords: Fibrosis Hepatitis C Hepatocellular carcinoma Point shear wave Liver cirrhosis
ABSTRACT Background: Risk stratification and prognostication of hepatocellular carcinoma (HCC) help to improve patient outcome. Herein we investigated the role of liver stiffness measurement (LSM) in the prediction of HCC behavior. Methods: Totally 121 naïve patients with HCC were included. HCC radiological evaluation and staging were done. LSM was measured using virtual touch quantification. Patients were divided into early to intermediate HCC (BCLC-0, A and B) and late HCC (BCLCC and D). HCC was treated according to the BCLC stage. HCC recurrence-free interval was estimated. Results: The mean LSM inside the tumor was significantly lower than the peri-tumoral area and the cirrhotic non-cancerous liver parts ( P < 0.001). In late HCCs stage, the mean LSM inside the tumor and in the peri-tumoral tissue was lower than the corresponding values in the early to intermediate HCCs stage ( P < 0.001). LSM inside the tumor and in the peri-tumoral tissue negatively correlated with serum AFP, tumor vascular invasion, and stage ( P < 0.05). The recurrence-free interval was directly correlated to LSM inside the tumor and inversely to LSM in cirrhotic non tumorous liver part. Kaplan-Meier analysis showed that the recurrence-free interval was significantly longer in patients with LSM inside the tumor of ≥1.25 m/s compared to those with LSM inside the tumor of < 1.25 m/s. Conclusions: LSM can serve as a potential non-invasive predictor for HCC clinical behavior and the recurrence-free interval following loco-regional treatments.
Introduction
The invasive nature of liver biopsy for diagnosing and grading of liver fibrosis and the possibility of sampling error necessitate the availability of non-invasive methods for fibrosis assessment [1] . In spite of the multiplicity of hepatocellular carcinoma (HCC) risk factors, liver fibrosis is the most important irrespective of its eti- ology [2-4] . For improvement of patients outcome, early detection and treatment of HCC are mandatory. Extensive investigations for screening, risk stratification and prognostication of HCC are essen- tial [2] .
In the last decade, image-based quantification of liver fibrosis by transient elastography (TE) has been introduced into practice. Several studies have addressed a high accuracy of TE in defining significant fibrosis ( F > 2) and cirrhosis (F4) in chronic hepatitis C patients [5 , 6] . In addition, it is more representative of the hepatic parenchyma, where it can evaluate a larger area compared to a sin- gle liver biopsy [5] . The risk for development of HCC has been as- sessed via liver stiffness measurement (LSM) in patients with hep- atitis B (HBV) and hepatitis C virus (HCV) infections [7 , 8] . Never- theless, in most reports, the risk for HCC was only indirectly eval- uated based on the value for liver cirrhosis as measured by TE. LSM related to HCC has not been directly evaluated [7 , 9] .
Shear wave elastography techniques have been applied in the real-time ultrasound systems, and their accuracy in the assessment of liver fibrosis has been addressed in several studies [10-14] . These techniques have the advantage of perform- ing an examination in real-time. In the point shear wave elastogra- phy (pSWE), the shear wave elastometry is done at a certain tissue point by measurement of the speed of shear waves that are gener- ated using acoustic radiation force impulse (ARFI) [15] .
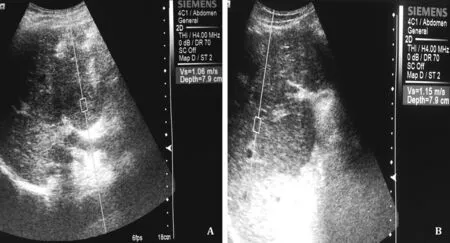
Fig. 1. Image showing how to measure the liver stiffness using acoustic radiation force impulse ultrasound probe. A: Inside the hepatocellular carcinoma mass; B : At the zone within 1 cm around the tumor mass. The mean of twelve readings is recorded.
To our knowledge, no studies were directed to the evaluation of the impact of liver stiffness or tumor stiffness on HCC clinico- radiological characteristics or response to treatment.
Methods
Patients
Prospectively, our research included 128 naïve patients who were diagnosed as HCC on top of HCV related liver cirrhosis. Pa- tients were recruited from the Hepatology Clinic at Alexandria Main University Hospital, Alexandria, Egypt. Patients with mor- bid obesity, marked ascites, seropositivity to HBV, other causes of chronic liver diseases, were excluded. We also excluded 7 pa- tients with failed LSM. The study was approved by the local ethics committee at Faculty of Medicine, University of Alexandria ( http://www.med.alexu.edu.eg/ethics-committee/ ), Institutional Re- view Board number (IRB): 0 0 0 07555; Federal Wide Assurance (FWA) number: 0 0 015712; review serial number: 0302801. Also, the study was conducted in accordance with the 1975 Helsinki Dec- laration and its later amendments as revised in 2008, and Good Clinical Practice guidelines. Informed consent was obtained from all patients included in the study.
Patient evaluation
All the patients were evaluated clinically. Liver tests profile and serum alfa-fetoprotein (AFP) were measured. The AST/platelet ra- tio index (APRI) was calculated (with a cut-off value of 1.5 to rule in cirrhosis) [16] , and the severity of liver disease was assessed by Child-Turcotte-Pugh classification (CTP). The diagnosis of HCC was based on the wash in/wash out pattern in triphasic CT. HCC eval- uation included the number of lesions, maximum tumor diame- ter, vascular invasion, extra-hepatic nodal spread. The BCLC staging was used to evaluate the applicability of different treatment strate- gies [17] . Patients were classified as having early to intermediate stage HCC including BCLC stage 0, A and B, or as having late-stage HCC including BCLC stage C and D.
Liver stiffness measurement
Point shear wave elastography was performed by ACUSONTMS20 0 0 ultrasound system (Siemens Mountain View, CA, USA), equipped with a 4C1 MHz frequency convex transducer, using the virtual touch quantification (VTQ) method. While pa- tients were fasting for 6 h, lying in decubitus (or 30 ° left lateral) position, and the right arm in maximal abduction, the transducer of VTQR○apparatus in the B-mode was placed between ribs, per- pendicular to the liver capsule, and the liver was scanned to reach the most accessible positions. LSM was measured for the tumor mass, within 1 cm around the tumor, and at the non-malignant cirrhotic part of the liver ( Fig. 1 A and B). Measures were taken while the patient was holding the breath at the end of expira- tion. Verifying that the examined area was free of vascular and biliary structures and rib shadows were ascertained. LSM values were calculated as the average of 12 successful measurements and expressed as a meter per second (m/s). For every patient, the examination was annotated failed if the ratio of the number of valid measurements to the total number of measurements is < 60% [18] . After LSM, patients who were eligible for treatment were treated by appropriate modality according to the BCLC stag- ing system, and after tumor ablation, the recurrence-free interval was calculated starting from last CT evidence of tumor ablation to 1st CT evidence of tumor recurrence or de novo lesions.
Statistical analysis
The analysis was done for 121 patients. The data were ex- pressed as mean ± standard deviation (SD). Comparison between proportions was determined by the Chi-square test or Fisher’s Exact test. Comparisons between groups were made by the Stu- dent’s t- test or Mann-Whitney test as appropriate. Correlations between variables were done using the Pearson’s or Spearman Rho coefficient, when appropriate. The sensitivity and specificity of LSM by VQT in differentiating early-intermediate from late stages HCC was assessed by receiver-operating characteristic (ROC) curve. The area under the curve (AUC) and its 95% confi- dence interval (CI) were calculated. A binary logistic regression model using the “enter” method was applied for prediction of early-intermediate stage HCC, significant predictors by univari- ate analysis were entered in the model and predictors causing multi-collinearity problem were excluded. Kaplan-Meier survival analysis with log-rank test and Cox regression hazard models were applied for recurrence-free survival. Statistical signifi- cance was assessed at P < 0.05. All calculated P values were two-tailed.
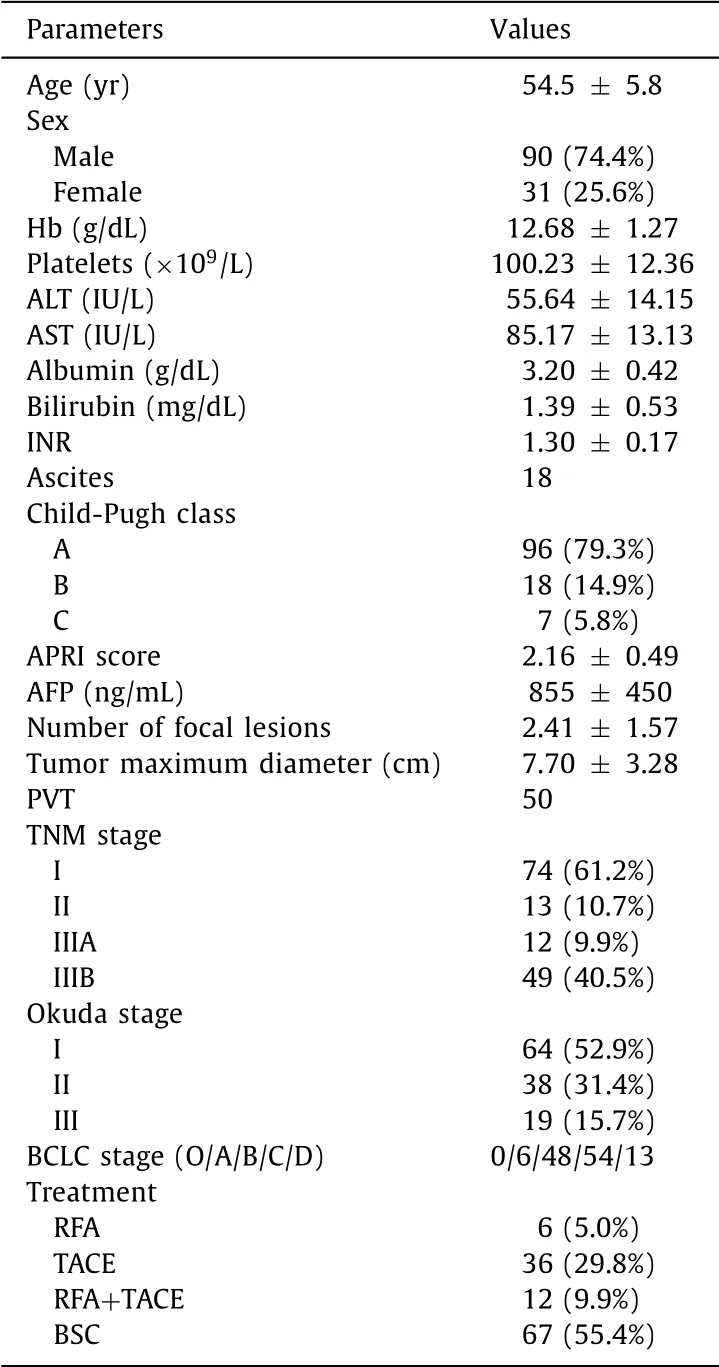
Table 1 Clinico-laboratory characteristics of the patients included in the study ( n = 121).
Results
Clinical laboratory parameters
Our study included 121 patients with their baseline data shown in Table 1 . Among our patients, 54 (44.6%) have early-intermediate stage HCC (BCLC 0, A or B), while 67 (55.4%) have late-stage HCC (BCLC C and D). In patients with early-intermediate stage HCC who were eligible for treatment, RFA was used in 6 (11.1%) patients, TACE in 36 (66.7%), and combined RFA + TACE in 12 (22.2%). We did not have cases of liver transplantation or hepatic resection.
During the follow-up period after HCC complete ablation, the recurrence-free interval in this group ranged between 3-17 months. Patients in the late HCC group according to the BCLC al- gorithm were managed by the best supportive treatment or so- rafenib according to the availability of resources ( n = 67). Patients with late HCC have a significantly lower platelet count and serum albumen compared to early-intermediate stage HCC ( P < 0.001), while they have significantly higher serum bilirubin, INR, Child- Pugh score, and AFP levels ( P < 0.001, Table 2 ).
LSM
In all patients ( n = 121), the LSM value inside the tumor, in the peri-tumoral area, and in the cirrhotic non-malignant parts were compared. The median value of the LSM inside the tumor was sig- nificantly lower than that in the peri-tumoral area [1.26 (0.51-3.71) m/s vs. 2.03 (1.10-4.39) m/s, P < 0.001, Z = -7.47] and in the cir- rhotic non-malignant parts of the liver [1.26 (0.51-3.71) m/s vs. 2.73 (1.22-4.04) m/s, P < 0.001, Z = -10.1], while there was no statistically significant difference between the peri-tumoral area and the cirrhotic non-malignant parts [2.03 (1.10-4.39) m/s vs. 2.73 (1.22-4.04) m/s, P = 0.14, Z = -1.46] ( Fig. 2 A).
We compared the early-intermediate vs. late HCC subgroups as regards the LSM value. The LSM value inside the tumor in the late HCC group were significantly lower than their corresponding val- ues in the early-intermediate HCC group [1.04 (0.51-2.38) m/s vs. 1.75 (0.63-3.71) m/s, P < 0.001, Z = 4.88].
Similarly, the median value of LSM in the peri-tumoral area in the late HCC group were significantly lower than their correspond- ing values in the early-intermediate HCC group [1.57 (1.10-3.92) m/s vs. 3.35 (1.27-4.39) m/s, P < 0.001, Z = -5.69], while there was no significant statistical difference between the two groups as regards LSM in the cirrhotic non-malignant part of the liver [2.70 (1.22-4.04) m/s vs. 2.78 (1.98-3.4) m/s, P = 0.36, Z = -0.91] ( Fig. 2 B).
We subdivided all patients ( n = 121) into two subgroups based on presence ( n = 50) or absence ( n = 71) of tumoral portal vein invasion. The median (range) of LSM inside the tumor and in the peri-tumoral area were significantly lower in patients with portal vein invasion compared to patients without portal vein invasion [0.97 (0.51 - 2.38) m/s vs. 1.74 (0.63-3.71) m/s, P < 0.001; and 1.42 (1.10-3.29) m/s vs. 3.29 (1.27-4.39) m/s, P < 0.001, respec- tively]. However, there was no significant difference between the two subgroups as regards the median value of LSM in the cirrhotic non-malignant liver parts [2.65 (1.22-3.43) m/s vs. 2.79 (1.98-4.04) m/s, P = 0.08].
Correlation of LSM to different study parameters
The value of LSM inside the tumor showed negative correlation with the following parameters: serum AFP ( r = -0.57, P < 0.001), the number of HCC lesions ( r s = -0.36, P < 0.001), HCC maximum diameter ( r = -0.401, P < 0.001), CLIP ( r s = -0.45, P < 0.001), OKUDA ( r s = -0.27, P = 0.002), TNM ( r s = -0.43, P < 0.001), and BCLC stages ( r s = -0.35, P < 0.001).
The value of LSM in the peri-tumoral area showed negative cor- relation with serum AFP ( r = -0.49, P < 0.001), the number of HCC focal lesions ( r s = -0.55, P < 0.001), HCC maximum diame- ter ( r = -0.51, P < 0.001), CLIP ( r s = -0.57, P < 0.001), OKUDA ( r s = -0.46, P < 0.001), TNM ( r s = -0.55, P < 0.001), and BCLC stages ( rs= -0.53, P < 0.001), while the value of LSM in the cir- rhotic non-malignant part showed no significant correlation with all tumor-related parameters.
ROC curve
By plotting the ROC curve, at cut-off value of 1.25 m/s, LSM inside the tumor was found to have a sensitivity of 77.8% and a specificity of 71.6% in differentiating early-intermediate stages of HCC from late-stage HCC (AUC = 0.76; 95% CI: 0.67-0.85). At cut- off values of 2.22 m/s, LSM in the peri-tumoral area was found to have a sensitivity of 77.8% and a specificity of 73.1% in dif- ferentiating early-intermediate stages of HCC from late stage HCC (AUC = 0.80; 95% CI: 0.72-0.88), and at cut-off values of 2.72 m/s, LSM in the cirrhotic non-malignant part of the liver was found to have a sensitivity of 60% and a specificity of 53.1% in differentiating early-intermediate stages of HCC from late-stage HCC (AUC = 0.55; 95% CI: 0.45-0.65; Fig. 3 ).

Table 2 Comparison between early and late hepatocellular carcinoma patients as regards clinico- laboratory parameters.

Fig. 2. Liver stiffness measurements. A : Inside the hepatocellular carcinoma (HCC) mass, in the peri-tumoral area, and in the non-malignant cirrhotic liver part; B : Early- intermediate versus late HCC.
Predictors for tumor recurrence free interval
Univariate analysis showed that the following parameters were significant predictors for the early recurrence of HCC following tu- mor ablation by loco-regional modalities: APRI score ( P = 0.004), Child-Pugh class ( P = 0.012), serum AFP ( P < 0.001), LSM inside the tumor ( P < 0.001), number and maximum diameter of the tu- mor ( P < 0.05), and TNM ( P < 0.001), CLIP ( P = 0.004), and BCLC stages ( P = 0.001).
Cox-regression analysis models were run for predictors of recurrence free interval. We found that LSM inside the tu- mor (HR = 0.21; 95% CI: 0.10-0.43; P < 0.0 0 01), TNM staging (HR = 11.7; 95% CI: 1.81-76.3; P = 0.01), BCLC staging (HR = 0.26; 95% CI: 0.06-0.98; P = 0.04), number of tumor foci (HR = 4.2; 95% CI: 1.66-41.1; P = 0.01), and maximum size of the tumor (HR = 2.03; 95% CI: 1.21-3.41; P = 0.007) were predictors for re- currence free interval ( Table 3 ).
Kaplan-Meier with log-rank test was run for LSM inside the tu- mor at a cut-off value 1.25 m/s (as obtained from the ROC curve analysis). The recurrence-free interval was significantly longer in patients with LSM inside the tumor of ≥1.25 m/s [median (in- terquartile range, IQR) 14 months (7-15)] compared to patients with LSM inside the tumor of < 1.25 m/s [6 months (IQR: 5-6.9), P < 0.0 0 01, Fig. 4 A]. However, for the LSM at the peri-tumoral area, there was no statistically significant difference as regards the median duration of the recurrence-free interval at the cut-off value of 2.22 m/s ( P = 0.78, Fig. 4 B). For the LSM at the cirrhotic non-malignant part, the recurrence-free interval was significantly shorter in patients with LSM ≥2.72 m/s [7.5 months (IQR: 6-12)] compared to patients with LSM < 2.72 m/s [15 months (IQR: 6.38-16), P = 0.001, Fig. 4 C].
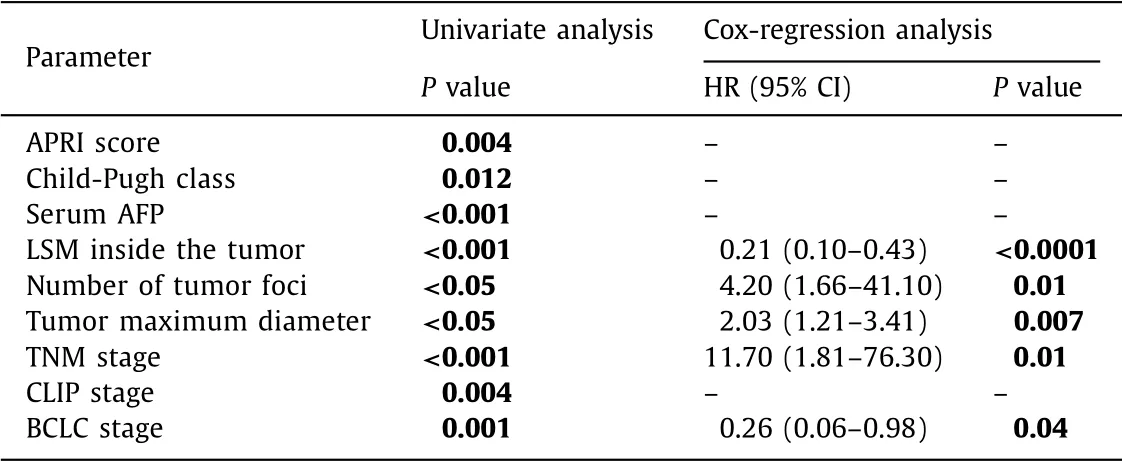
Table 3 Univariate and multivariate analysis for predictors of recurrence-free interval.
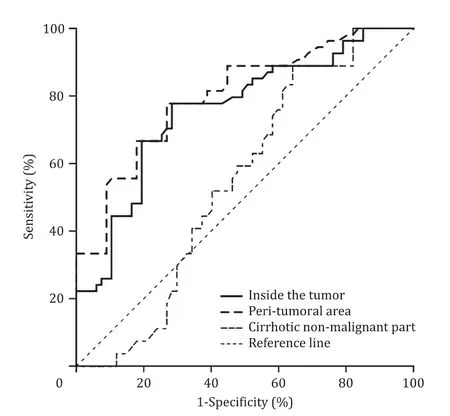
Fig. 3. Receiver operating characteristic curve (ROC): the sensitivity and specificity of liver stiffness measurement (LSM) at a cut-off values of 1.25 m/s, 2.22 m/s, and 2.72 m/s inside the tumor, in the peri-tumoral tissue, and in cirrhotic non-malignant liver parts respectively have sensitivities and specificities of 77.8% and 71.6% (AUC = 0.76), 77.8% and 73.1% (AUC = 0.80), and 60.0% and 53.1% (AUC = 0.55) respectively in differentiating early from late HCC.
Kaplan-Meier with log-rank test was run for the type of loco- regional treatment effect on the recurrence-free interval. There was no significant difference in the median duration of the recurrence- free interval among patients who were managed by RFA, TACE, or combined RFA + TACE (median, 15 months, 8 months, and 6 months, respectively) ( P = 0.06, Fig. 4 D).
Discussion
Liver stiffness measurement - a non-invasive alternative for the assessment of the degree of liver fibrosis - has been explored in many cohort studies [10 , 11 , 19] . In addition, LSM was also evalu- ated as a predictor of HCC development in patients with liver cir- rhosis, and liver stiffness based risk scores have been developed to predict HCC occurrence in patients with hepatitis B. Moreover; LSM > 12.0 kPa using TE was an independent risk factor for new HCC development in patients with HCV [9 , 20] . In spite of this, data about the role of LSM in prognostication of HCC itself is scarce, and to our knowledge, this is the first study to address the role of LSM using VTQ as a predictor for HCC clinico-biological behavior.
In our study, the LSM inside the tumor and peri-tumoral area were significantly lower in patients with late stages of HCC than that in those with early to intermediate stage HCC, and the low LSM values were associated with worse characteristics of HCC in- cluding high serum AFP, larger tumor sizes and number, portal vein invasion, and late cancer stages with subsequent non-eligibility for treatment.
Point shear wave elastography generates a shear-wave inside the liver using a focused acoustic beam from an ultrasound trans- ducer. These waves travel through the liver, and LSM is expressed as the velocity of transmission of the shear waves inside the liver (the stiffer the liver, the higher the velocity) [21] . The lower stiff- ness reading inside the tumor could be explained by the higher vascularity and the non-organized cellular structure due to the rapid growth of the tumor cells. These two characteristics would help the cancer cells to grow rapidly and to spread earlier com- pared to the less vascular, more fibrotic (and so, stiffer) tumor tis- sues. In addition, a rapidly growing tumor may have an internal breakdown due to an imbalance between the blood supply and cel- lular growth.
Similarly, we proposed that the association between lower LSM at the peri-tumoral area and the worse HCC characteristics may be due to the loose peri-tumoral zone resulted from (a) tumor cell invasion of the nearby cirrhotic liver or microsatellite tumors, (b) the ramifying capillary network surrounding the highly vascular tumors [22] , or (c) leaky peri-tumoral zone formed by substances released by aggressive HCCs e.g. metalloproteinases, which would help cancer cells to spread and get access to multi-centricity. We suggest that a lower peri-tumoral stiffness represents either a port for microsatellites extension (this may be missed and represent a source for early recurrence) or an indicator for more biologically aggressive cancer with a worse prognosis. It has been found that tumor blood supply may not be enough for rapidly growing HCCs; as a result, the enhanced neoangiogenesis around the tumor leads to the formation of tumors with softer tissue structure, a finding linked to worse tumor characteristics [23 , 24] .
However, opposing data about the influence of microenviron- ment stiffness on HCC growth and chemotherapy responsiveness were obtained from in vitro studies which showed that higher stiffness in the tumor supporting microenvironment was associ- ated with a worse prognosis [25] . This influence may be attributed to the difference in the growth factors milieu and the cell cross- linkage pattern in the in vitro , rather than in vivo , studies.
In our study, lower LSM at the cirrhotic non-malignant liver parts were associated with a higher incidence of vascular inva- sion by the tumor. A soft liver matrix/stiffness will facilitate cancer spread inside the liver including vascular channels. On the other hand, the higher LSM in the cirrhotic non-malignant part in our study was associated with a shorter recurrence-free interval. It has been shown previously that higher liver stiffness is associated with a higher incidence of HCC, but the ideal cut-off value of liver stiff- ness in the implementation of HCC surveillance program is still un- known [9] . Again, data available about liver cirrhosis stiffness and prognostication of HCC are scarce. An in-vitro study showed that increasing matrix stiffness promotes proliferation and chemother- apy non-responsiveness of cancer cells [25] . LSM using TE has been also investigated for prediction of HCC recurrence after liver re- section where LSM was an independent predictor of recurrence, whereas histological fibrosis status was not [26] . These findings are in accordance with data obtained in the current study and rein- force the suggested possible role of LSM as a predictor for early occurrence/recurrence of HCC.
After HCC ablation in our eligible patients, we found that the lower values of pre-ablation LSM inside the tumor were associ- ated with shorter recurrence-free interval (irrespective to the type of the loco-regional treatment). Because a lower LSM was linked to poor tumor characteristics, this makes sense to be linked to a shorter recurrence-free interval. Highly vascular and aggressive tumors may be associated with the early micro-vascular spread and synchronous/metachronous micro-satellites. This may draw attention to the possible use of LSM before planning to treat HCC. On the other hand, we found that higher LSM in the cir- rhotic non-malignant liver parts was linked to shorter recurrence- free survival. This is explained by the relation between degree of liver fibrosis (hence, the stiffness) and the risk of HCC occur- rence/recurrence. Higher LSM means stiffer and more fibrotic liver, and the degree of fibrosis is a risk of earlier de novo tumor recur- rence [27] .
In HCC, it is clear that the large tumor size, multi-centricity, and vascular invasion already mean an advanced stage. Our study was to address the clinical significance of LSM in patients with early- intermediate HCC stages. LSM help us to predict the future be- havior and recurrence of the tumor. LSM adds more certainty and predictability to the BCLC algorithm. LSM, not like biological be- haviors, is a clinical approach which may be helpful for tumor treatment, especially with rising controversies about the BCLC treatment algorithm [28,29] . Our data may have clinical implica- tion in HCC treatment. Moreover; we highlighted the impact of liver stiffness on the clinical behavior of HCC.
The limitation of our study is that we did not correlate LSM with either histological grading of the tumors or with the grade of hepatic fibrosis due to difficulty in obtaining liver biopsies.
In conclusion, low LSM inside the HCC masses and at the peri-tumoral zone is a predictor of poor prognosis and shorter recurrence-free interval after HCC ablation. Also, the stiffness of cirrhotic liver tissues could predict the HCC recurrence-free inter- val. LSM inside HCC, at the peri-tumoral area, and at the cirrhotic non-malignant liver is a potential non-invasive marker for HCC prognostication and risk stratification especially in patients with early-intermediate HCC.
CRediT authorship contribution statement
Sameh A Lashen:Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writ- ing - original draft, Writing - review & editing.Mohamed M. Elshafei:Methodology, Validation, Visualization, Writing - original draft.Fahmy H. Hablass:Data curation, Writing - original draft, Writing - review & editing.Eman A. Alsayed:Data curation, Writ- ing - original draft, Writing - review & editing.Asmaa A. Hassan:Formal analysis, Validation.
Funding
None.
Ethical approval
The study was approved by the local ethics committee at Faculty of Medicine, University of Alexandria ( http://www.med. alexu.edu.eg/ethics-committee/ ), Institutional Review Board num- ber (IRB): 0 0 0 07555; Federal Wide Assurance (FWA) number: 0 0 015712; review serial number: 0302801.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub- ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Impact of EBV infection and immune function assay for lymphoproliferative disorder in pediatric patients after liver transplantation: A single-center experience
- Hepatobiliary&Pancreatic Diseases International
- Intraoperative management and early post-operative outcomes of patients with coronary artery disease who underwent orthotopic liver transplantation
- Acute onset of autoimmune hepatitis in children and adolescents
- Treatment and prognosis of hepatic epithelioid hemangioendothelioma based on SEER data analysis from 1973 to 2014
- Cholecystoenteric fistula with and without gallstone ileus: A case series
