Effects of Recrystallization Parameters on the Formation of Hollow Silicalite-1 Zeolite
2020-01-13TanChengDongZhuoyaSunTuZhangYapingZhangGuanqunXiaChangjiuMaYanhang
Tan Cheng; Dong Zhuoya; Sun Tu; Zhang Yaping; Zhang Guanqun; Xia Changjiu; Ma Yanhang
(1. Shanghai Adνanced Research Institute, Chinese Academy of Sciences, Shanghai 201210; 2. School of Physical Science and Technology, ShanghaiTech Uniνersity, Shanghai 201210; 3. Uniνersity of Chinese Academy of Sciences, Beijing, 100049; 4. State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Abstract: Hollow silicalite-1 zeolite can be readily fabricated by hydrothermally treating the parent silicalite-1 with tetrapropylammonium hydroxide. A dissolution-recrystallization mechanism has been previously proposed to explain the formation of such hollow structures, but detailed information of this formation process still remains unclear. Herein, by tracking the evolvement of the hollow voids and morphology of the silicalite-1 under various treatments using XRD, SEM, TEM and N2 adsorption/desorption techniques, we systematically studied the formation process of the hollow structure of silicalite-1 zeolite and discovered that the organic template, water, treating temperature and time can significantly influence the morphology and size of hollow structure inside silicalite-1 zeolite crystals. Generally, a diluted synthesis medium with high template content under suitable temperature (for instance 170 °C) and extended treatment time favors the formation of single hexagonal hollow structure within silicalite-1 zeolites; while other conditions favor the formation of rounded hollow voids or even multiple-voids within silicalite-1 zeolites.
Key words: hollow silicalite-1; hydrothermal synthesis; dissolution, recrystallization
1 Introduction
Zeolites are the aluminosilicate members of the microporous solids family known as “molecular sieves”[1-3]. The ordered channel structure, high thermal/hydrothermal stability and adjustable acidity endow the zeolites with extensive application in catalysis, separation, and ionic exchange[4-7]. Recently, zeolites containing specifically designed structures, such as mesopores or hollow cavities, have gained increasing attention in both academia and industry[8-10]. One of the representatives is the hollow silicalite-1 zeolite (belonging to the MFI type framework) which can serve as a capsule to load target active centers, such as metal nanoparticles or organic molecules, for catalysis and drug delivery applications. The very recent example is the utilization of hollow silicalite-1 as the platform to encapsulate nanometric metal (Au, Co, Ni, etc.) clusters for a variety of catalytic reactions, in which these catalysts exhibit superior catalytic activity than the conventional metal-supported catalysts[11-16]. The main reason is ascribed to the hollow structure of silicalite-1, which traps the metal clusters but allows the reactant molecules to diffuse and interact with the metal clusters; moreover, the hollow structure prevents these nanoparticles from agglomerating even at high temperature, thus maintaining their catalytic activity. Besides the metal@silicalite-1, the hollow Tisubstituted silicalite-1 (commercially known as TS-1) zeolite can also achieve a remarkable improvement of reactant diffusion, resulting in an enhanced catalytic activity as compared with the solid TS-1 zeolites[17].
The employment of tetrapropylammonium hydroxide (TPAOH) is essential to the fabrication of hollow structure since it is necessary to the crystallization of solid silicalite-1[17-19]. The role of TPAOH in crystallization process was already well understood as the template with which the synthesis precursors can assemble in specific order to construct the MFI-type zeolite framework[20]; meanwhile, the role of TPAOH in the hollow voids fabrication process is somewhat elusive. A dissolution-recrystallization mechanism was proposed, denoting that at the initial stage the less-crystalline parts of the zeolite (i.e., the core and the inner parts) would be partially dissolved in the presence of OH-ions, and the dissolved silica species would diffuse into the solution, forming the void space; then the dissolved silica species would, upon being directed by TPA+, recrystallize as the MFI-type layers onto the outer surface of silicalite-1[21-23]. Although many characterization results would support this hypothesis, some key questions were still left to be answered. For instance, the shape of the void structure is highly sensitive to treatment procedures. Under specific conditions, regular hollow structures can be formed with its inner fringes parallel to the zeolite lattice[24], which is likely a result from a template-directed crystallization. However, due to the large size, the TPA+cation is unlikely to diffuse through the zeolite shell inside the hollow space to direct the recrystallization process. Another question is that by adjusting these procedures, a single hollow rectangular box, a hollow plate or even multiple hollow irregular cavities can be formed within silicalite-1 zeolites, but the key factor that can influence the shape of the inner voids is unknown.
In this research, we conducted a systematic study on the evolvement of hollow structures of silicalite-1 by recording the change of the crystallization, porosity and morphology of silicalite-1 during the hollow structure fabrication process under different conditions. We confirmed that a dissolution-recrystallization process could exist during this process. Interestingly, the dissolution and recrystallization do not happen on random zeolite surface, which is specifically parallel to the outside surface of the crystal.
2 Experimental
2.1 Synthesis of parent silicalite-1
Silicalite-1 was prepared using tetraethyl orthosilicate (TEOS) as the silicon source and TPAOH aqueous solution (40%) as the template. Firstly, TEOS and TPAOH were mixed with deionized water. The mixture was stirred in water bath until the hydrolyzed ethanol from TEOS completely evaporated. The final clear solution, with the components having a ratio of 1.0SiO2: 0.4TPAOH: 20H2O were transferred into a Teflon-lined autoclave and heated at 170 °C under static conditions for a specified time period. The resulting solid was recovered by filtration, and dried at 100 °C overnight. The solid was then calcined at 550 °C for 6 h in air to remove the organic compounds.
2.2 Fabrication of hollow silicalite-1
The hollow structures were obtained by hydrothermally treating the calcined silicalite-1 with TPAOH solution: 4.15 ml of 1 mol/L TPAOH solution were firstly added to 3.35 ml of deionized water, and then 1 g of silicalite-1 powder was suspended in this solution. The mixture was heated at 170 °C under static conditions for different duration periods (0 min, 5 min, 15 min, 30 min, 40 min, and 60 min,).
2.3 Characterization techniques
Powder X-ray powder diffraction (XRD) was performed on a Bruker D8 Advanced X-ray diffractometer equipped with a Pixel detector using Cu Kαradiation operated at 40 mA and 40 kV with a scanning speed of 5(°)/min. Scanning electron microscopy (SEM) was carried out on a JEOL JSM-7800F Prime scanning electron microscope operating at 1 kV. N2isotherm was measured with a Quantachrome Autosorb-iQ-MP-AG at -196 °C. Transmission electron microscopy (TEM) images were performed on a JEOL JEM-1400plus transmission electron microscope operating at an accelerating voltage of 120 kV.
3 Results and Discussion
Powder XRD patterns of all the samples before and after the treatment show only the typical diffraction peaks of MFI type zeolite, confirming that all samples are pure silicalite-1 zeolites without impurity (see Figure 1). No significant changes in the peak width or intensity can be observed among all treated samples and untreated ones, revealing that no remarkable structural changes happen during the treatment.

Figure 1 XRD patterns of silicalite-1 treated by TPAOH at 170 °C for different time
3.1 Influence of precursor compositions on the hollow structure
First, the morphologies of silicalite-1 treated by varying TPAOH contents are shown in Figure 2(a)—(c). The silicalite-1 zeolites treated by hollow structure fabrication (HSF) method with low TPAOH concentration have similar particle sizes and morphologies as those of the untreated silicalite-1; under higher TPAOH contents some larger silicalite-1 crystals can be seen (denoting that the large crystals have the similar hexagonal-like shape, and corresponding powder XRD patterns are typical of MFI type), while the small silicalite-1 crystals still retain the prevailing phase. As the TPAOH content increases, larger silicalite-1 crystals can be observed. This is probably due to the dissolution of the original small silicalite-1 crystals at high alkalinity caused by high TPAOH contents, and the subsequent recrystallization of silicalite-1 is directed by TPA+cations.
The corresponding TEM images (Figure 2(d)—(f)) show that all HSF derived silicalite-1 contain hollow structures of nonuniform morphologies including a majority of silicalite-1 containing single, hexagonal-like voids with fringes parallel to the silicalite-1 shells (Figure 2(g)) and a minority containing either single rounded voids or multiple-voids. Upon using higher TPAOH content, the silicalite-1 crystals containing hexagonal-like voids become predominant. The reason of this structural heterogeneity is unclear. More interestingly, this silicalite-1 containing multiple-voids crystals becomes prevailing under stirring conditions (Figure 2(h)—(i)).

Figure 2 SEM and TEM images of silicalite-1 treated by components at a ratio of: xTPAOH: 1silicalite-1: 22H2O with x equating to: 0.25 (a, d), 0.4 (b, e, g), and 0.6 (c, f); TEM images (h, i) of silicalite-1 samples treated by components at a ratio of: xTPAOH: 1silicalite-1: 22H2O with x equating to 0.25 and 0.4 at a stirring rate of 40 r/min
The impact of water content during HSF process was also investigated in this research, as water content not only impacts the TPA+cation concentration as well as the overall alkalinity, but also influences the mass transfer of synthesis precursors. The SEM images show that water contents do not impact remarkably on hollow silicalite-1, as all samples at different water contents have similar particle size and morphology (Figure 3(a)—(c)). The TEM images (Figure 3(d)—(e)) show that almost all samples have a single hollow structure within silicalite-1. When the water content is low, the shells of silicalite-1 are thick, and the hollow structures are roughly rounded; as the water content becomes higher, the hollow structure becomes larger (with the silicalite-1 shell becoming thinner) and can better evolve towards a hexagonal shape. Upon considering the positive effect of TPAOH content on the formation of single, hexagonal voids within silicalite-1, the positive effect of high water content (with a relatively low TPAOH content) on forming single, hexagonal voids of silicalite-1 is more likely a result from the improved mass transfer during the HSF procedure. Thus, the role of water in transferring the dissolved silica is crucial to the formation of single, hexagonal voids within silicalite-1 crystals.
3.2 Influence of HSF temperature on the hollow structure
The influence of HSF temperature on silicalite-1 varies in the range from 100 °C to 200 °C. Since the temperature does not pose a significant impact on the particle size or the morphology of silicalite-1 (Figure 4(a)—(c)), it can remarkably affect the formation of hollow structure within the zeolite crystals. At 140 °C, most HSF-treated silicalite-1 crystals contain multiple voids (Figure 4(d)); as the HSF temperature rises to 170 °C, most silicalite-1 crystals contain large, single hexagonal hollow voids (Figure 4(e)); however, when the HSF temperature further rises to 200 °C, most silicalite-1 crystals only contain single, small rounded voids (Figure 4(f)). The formation of multiple voids implies that the formation of hollow structure may be multi-originated, and at higher temperature these tiny multiple voids may merge into single large voids and can recrystallize into a hexagonal shape. Generally, the influence of HSF temperature on silicalite-1 reveals that the morphology of hollow structure is more likely a thermodynamically-driven than a kinetically-driven result, because the shape and size of hollow structure are highly dependent on the HSF temperature.
3.3 Influence of HSF time on the hollow structure
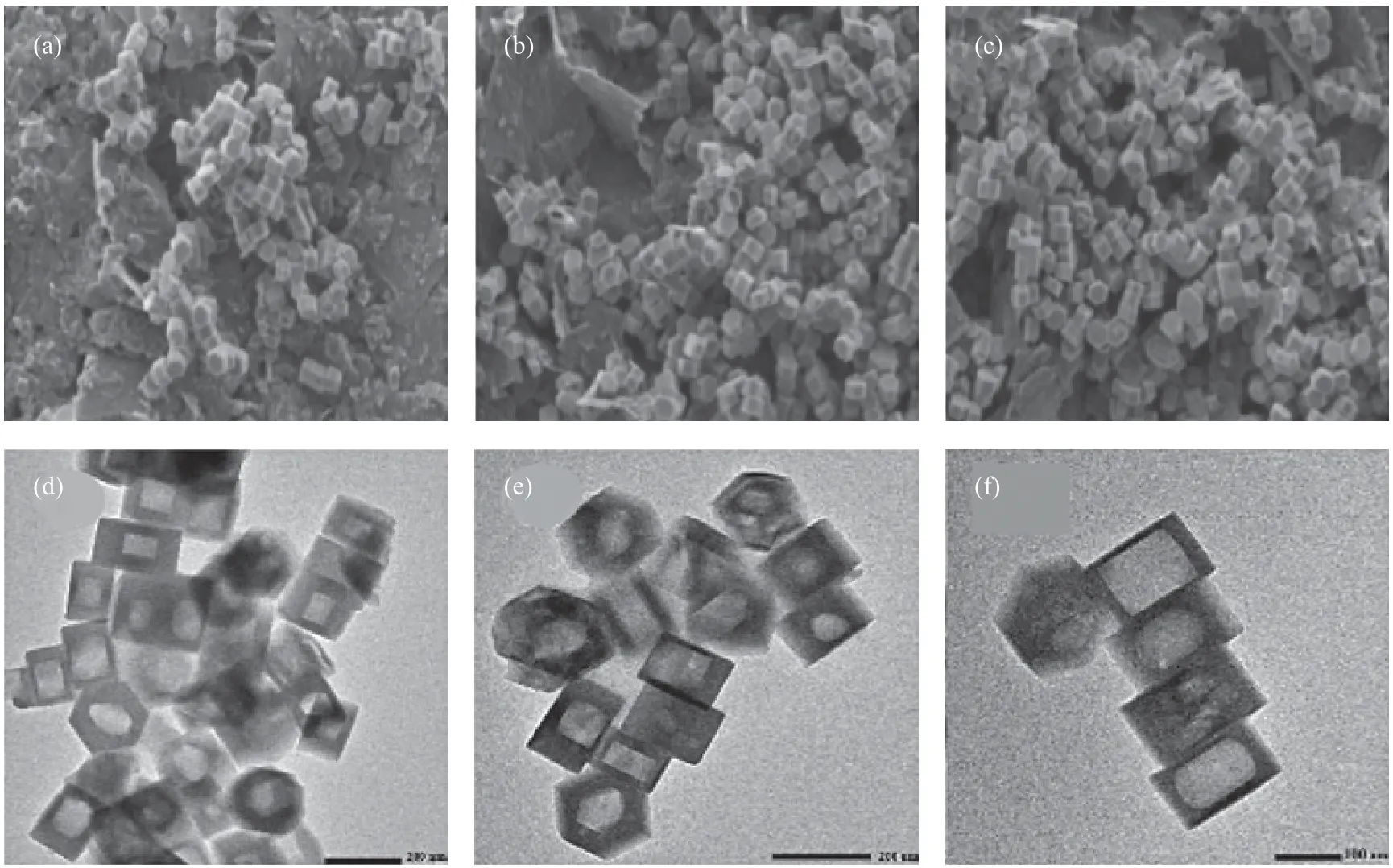
Figure 3 SEM and TEM images of silicalite-1 treated by components at a ratio of: 0.4TPAOH: 1silicalite-1: yH2O at 170 °C, in a day with y equating to: 0 (a, d), 2 (b, e), and 10 (d, f)
In this experiment, the HSF period was tracked from the initial state (0 min) continuously to the state where no further change occurs (60 min). With an increasing HSF time, the original solid crystals become thinner with the sharp edges and corners becoming curved, and after 60 min some crystals even evolve into cylindrical shapes (see Figure 5). On the other hand, a gradual evolvement of hollow structure is also observed after 15 min. As the HSF process continues, small rounded voids emerge from the cores of the crystals, and then expand outwards, with their fringes gradually becoming parallel to the silicalite-1 shell. Interestingly, when the silicalite-1 crystals are further treated by TPAOH for 7 days, the silicalite-1 crystals with a well-preserved flat surface and a well-developed hexagonal-like hollow structure become quite common. From the [010] direction, it can be easily observed that the dissolution and recrystallization reactions do not happen on random zeolite surface, which is specifically parallel to the outside surface of the crystal (Figure 6).
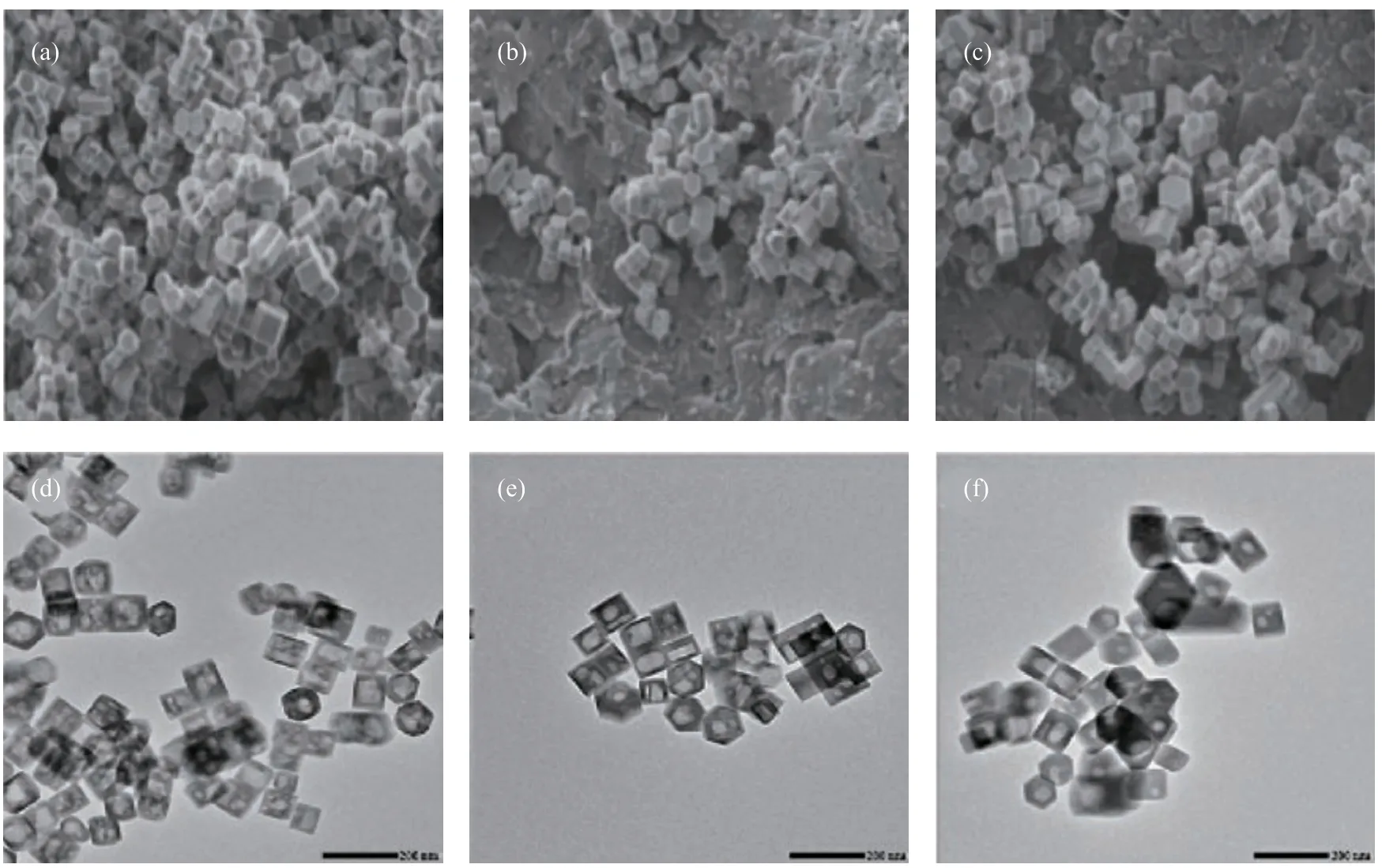
Figure 4 SEM and TEM images of silicalite-1 by HSF treatment with the solution of components at a ratio of: 0.4TPAOH: 1silicalite-1: 22H2O, in a day at: 140 °C (a, d), 170 °C (b, e), and 200 °C (c, f)
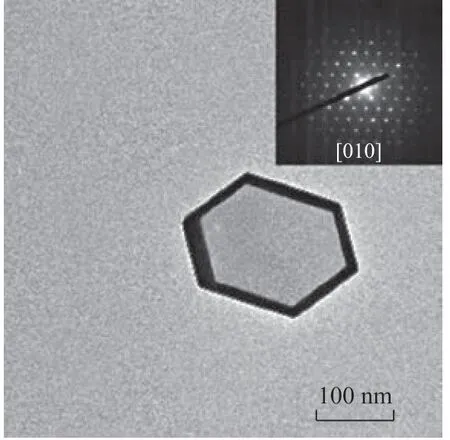
Figure 6 TEM image of silicalite-1 crystal treated by TPAOH at 170 °C for 7 days
A possible reason for this is that the structural defects are lattice-oriented, and are more concentrated along the direction parallel to the outside surface, so that the dissolution would preferentially take place along this direction[25]. An extended time provides suitable conditions for the re-crystallization to happen both in the inner part and in the shell. It should be mentioned that, even at high TPAOH content and long HSF time, the shell of silicalite-1 crystal was still completely intact without cleavages or perforated holes. This can be seen from Figure 7, where the high-voltage SEM images show that the shell of silicalite-1 was well-preserved while coexisting with the hollow structures (the shadow parts) inside the samples.N2adsorption data in Figure 8 show that with an increasing HSF time, the hysteresis loop becomes more and more pronounced, evidencing the existence of large hollow structure inside the silicalite-1 crystals[26-27].
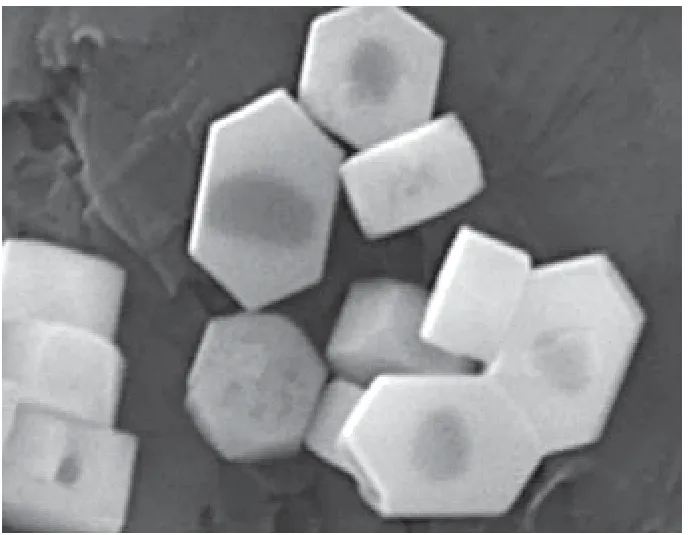
Figure 7 SEM images of HSF-treated silicalite-1 taken at an electron beam voltage of 5 kV

Figure 8 N2 adsorption and desorption isotherms of silicalite-1 treated by TPAOH for different period
Also, judging from Table 1, the surface area, the microporous volume, and the mesoporous volume do not change much during the treatment, while an increase of mesoporous volume is clearly observed. This observation is consistent with the hypothesis that mesopores are formed during the dissolution and re-crystallization process.

Table 1 Texture properties of HSF-treated silicalite-1 at different time
4 Conclusions
In this research, we conducted a systematic study on the evolvement of hollow structures of silicalite-1 by recording the change of the crystallization, porosity and morphology of silicalite-1 during the HSF process under different conditions. In the HSF process of silicalite-1 crystals, the TPAOH content, the water content, and the HSF temperature and time all have a remarkable influence on the shape and size of hollow structure inside the silicalite-1 zeolite crystals. Additionally, these factors also have their unique impact on the size and morphology of the shell of silicalite-1. Generally speaking, high TPAOH content and high water content, together with suitable HSF temperature (for instance 170 °C) and extended HSF time, silicalite-1 with a single hexagonal hollow structure becomes the predominant phase; while other conditions can favor the formation of silicalite-1 containing rounded hollow voids or even multiple-voids.
Acknowledgements:This work was supported by the Scientific Research Fund Project of National Natural Science Foundation of China (21835002) and the Young Elite Scientist Sponsorship Program by CAST (2017QNRC001). This work is partially supported by CħEM, SPST, ShanghaiTech (Grant 02161943).
杂志排行
中国炼油与石油化工的其它文章
- CoMnMgAl Hydrotalcite-like Compounds and their Complex Oxides: Facile Synthesis and FCC SOx Removal
- Effects of Blending Long-Chain Alcohols with Jet Fuel on the Macroscopic Spray Characteristics
- Effect of Zeolite 5A Particle Size on Its Performance for Adsorptive Separation of Ethylene/ Ethane
- Comparison on Adsorptive Separation of n-Paraffins Based on Binderless and Binder-containing Zeolite 5A Pellets
- Discussion on the Mechanism of Boric Acid and Phosphoric Acid to Improve the Hardening of Complex Calcium Lubricating Grease
- Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction
