急性冠脉综合征患者CYP2C19基因多态性与氯吡格雷疗效的关系
2019-12-16王光亮林爽吴雪梅
王光亮 林爽 吴雪梅


【摘要】 目的 探討急性冠状动脉(冠脉)综合征患者CYP2C19基因多态性与氯吡格雷疗效的关系。方法 42例急性冠脉综合征患者, 根据CYP2C19基因多态性分为A组(正常代谢+快代谢型, 23例)和B组(中间代谢+慢代谢型, 19例)。。两组患者均行CYP2C19基因型检测、血栓弹力图检测。观察比较两组患者二磷酸腺苷(ADP)抑制情况、ADP抑制率, 并统计基因分型。结果 A组中野生型纯合子组包括正常代谢型患者23例, 均为CYP2C19*1/*1基因型;快代谢型0例。B组中野生型与突变基因杂合子组包括中间代谢型患者15例, 其中CYP2C19*1/*2基因型13例, CYP2C19*1/*3基因型2例;突变基因纯合子或杂合子组包括慢代谢型患者4例, 其中CYP2C19*2/*2基因型3例, CYP2C19*2/*3基因型1例。B组中ADP抑制率<30%的发生率为58%, 高于A组的57%, 但差异无统计学意义(P>0.05)。42例患者中服用氯吡格雷后ADP抑制率<30%患者24例(57%), 其中停用氯吡格雷更换为替格瑞洛继续服用患者14例(33%), 14例患者中替格瑞洛顿服12 h后复查血小板ADP抑制率均>30%患者13例(31%), 但仍有ADP抑制率<30%患者1例(2%)。A组平均ADP抑制率为(39.5±28.4)%, B组平均ADP抑制率为(31.5±21.6)%, B组平均ADP抑制率低于A组, 但差异无统计学意义(P>0.05)。结论 本研究结果支持CYP2C19多态性仅为众多影响氯吡格雷疗效的因素之一, 并不能最终决定氯吡格雷疗效。氯吡格雷服用后的抗血小板聚集疗效, 还受众多其他因素的影响, 仍需进一步研究。
【关键词】 CYP2C19基因;血栓弹力图;血小板;二磷酸腺苷抑制率
DOI:10.14163/j.cnki.11-5547/r.2019.31.003
Correlation between CYP2C19 gene polymorphism and clopidogrel efficacy in patients with acute coronary syndrome WANG Guang-liang, LIN Shuang, WU Xue-mei. Northeastern International Hospital Geriatrics Center, Shenyang 110000, China
【Abstract】 Objective To discuss the correlation between CYP2C19 gene polymorphism and clopidogrel efficacy in patients with acute coronary syndrome. Methods A total of 42 patients with acute coronary syndrome were divided into two groups: group A (normal metabolism type + fast metabolism type,23 cases) and group B (intermediate metabolism type + slow metabolism type, 19 cases) according to CYP2C19 gene polymorphism. All patients were tested for CYP2C19 genotype and thromboelastography. The general information, adenosine diphosphate (ADP) inhibition, CYP2C19 gene polymorphism and ADP inhibition rate of the two groups were observed and compared, and the genotypes were analyzed. Results In group A, the wild-type homozygous group included 23 patients with normal metabolic type, all of whom were CYP2C19 * 1 / * 1 genotype, and 0 patient with fast metabolic type. In group B, the heterozygotes of wild type and mutant gene group included 15 patients with intermediate metabolism, of which 13 patients with CYP2C19 * 1 / * 2 genotype and 2 patients with CYP2C19 * 1 / * 3 genotype. The homozygous or heterozygous group of the mutant gene included 4 patients with slow metabolism, of which 3 patients with CYP2C19 * 2 / * 2 genotype and 1 patient with CYP2C19 * 2 / * 3 genotype. The incidence of ADP inhibition rate <30% was 58% in group B, which was higher than 57% in group A, but the difference was not statistically significant (P>0.05). Of the 42 patients,24 cases (57%) had ADP inhibition rate <30% after taking clopidogrel, of which 14 cases (33%) who discontinued clopidogrel and changed to telgrelor. Among the 14 patients, 13 cases (31%) had platelet ADP inhibition rate >30% after 12 h of tegriloton administration, but 1 case (2%) had ADP inhibition rate of<30%. The mean ADP inhibition rate was (39.5±28.4)% in group A, which was (31.5±21.6)% in group B, the mean ADP inhibition rate in group B was lower than that in group A, but the difference was not statistically significant (P>0.05). Conclusion The results of this study support that CYP2C19 polymorphism is only one of the factors that affect the efficacy of clopidogrel, and cannot ultimately determine the efficacy of clopidogrel. The anti-platelet aggregation effect of clopidogrel is also influenced by many other factors, which need further study.
【Key words】 CYP2C19 gene; Thromboelastogram; Platelet; adenosine diphosphate inhibition rate
氯吡格雷是一种需要细胞色素P450(CYP450)进行生物转化的前药[1, 2], 需要肝细胞色素P450 2C19(CYP2C19)的干预才能激活[3], 所以肝细胞色素P450 2C19(CYP2C19)基因多态性简称CYP2C19基因多态性, 有可能会影响到氯吡格雷的抗血小板聚集作用[4-6]。氯吡格雷的反应存在个体间差异, 不同患者服用氯吡格雷后会出现不同的抗血小板聚集疗效[3]。服用氯吡格雷后的抗血小板聚集疗效, 可以通过血栓弹力图ADP抑制率表达出来[7, 8]。本研究主要为评价CYP2C19 基因多态性与血栓弹力图ADP抑制率之间是否存在联系展开病例统计分析, 进而探讨CYP2C19 基因多态性是否会决定患者服用氯吡格雷后的抗血小板聚集疗效。现报告如下。
1 资料与方法
1. 1 一般资料 选取2018年4~11月本院住院的42名急性冠脉综合征患者作为研究对象, 患者中男24例, 女18例;年龄39~82岁。根据CYP2C19 基因多态性分为A组(正常代谢+快代谢型, 23例)和B组(中间代谢+慢代谢型, 19例)。
A组中野生型纯合子组包括正常代谢型(CYP2C19*1/*1)、快代谢型(CYP2C19*1/*17、CYP2C19*17/*17)。B组中野生型与突变基因杂合子组包括中间代谢型(CYP2C19*1/*2、CYP2C19*1/*3、CYP2C19*2/*17、CYP2C19*3/*17);突变基
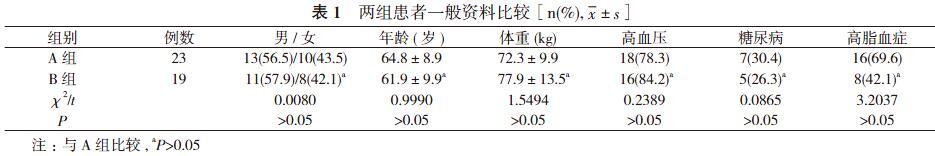
因纯合子或杂合子组包括慢代谢型(CYP2C19*2/*2、CYP2C19*2/*3、CYP2C19*3/*3)。两组患者性别、年龄、体重等一般资料比较差异无统计学意义(P>0.05), 具有可比性。见表1。入选患者均同期行CYP2C19基因多态性检测及血栓弹力图ADP抑制率检测, 所有患者行血栓弹力图ADP抑制率检测前确定已顿服300 mg氯吡格雷[商品名:波立维, 赛诺菲(杭州)制药有限公司]或75 mg氯吡格雷1次/d至少4 d, 保证行血栓弹力图ADP抑制率检测采血前氯吡格雷累积量>300 mg。排除标准:①血小板计数>400×109/L或<100×109/L;②阿司匹林或氯吡格雷使用禁忌证;③严重肝脏疾病或凝血功能存在异常者;④严重贫血、感染或甲状腺功能亢进等疾病者。
1. 2 方法
1. 2. 1 CYP2C19基因型检测方法 采集患者静脉血2 ml
(EDTA-K2抗凝), 使用天根血液基因组DNA提取试剂盒 (TIANamp Blood DNA Kit DP 318) 提取血液基因组DNA。应用美国Light Cycler cobas z480仪器检测, 应用武汉友芝友医疗科技股份有限公司生产的人类CYP2C19基因检测试剂盒, 根据基因检测结果及试剂盒说明书分组。
1. 2. 2 血栓弹力图检测方法 血小板ADP抑制率检测使用 TEG5000型凝血分析仪, 试剂包括高岭土(含15% Kadin液)、激活剂、花生四烯酸(arachidonicacid, AA)和二磷酸腺苷(adenosine diphosphate, ADP), 均为美国Haemonetics公司产品。所有患者抽取静脉血, 置于含3.13%枸橼酸钠进行检查。
1. 2. 3 用药方法 服用氯吡格雷后ADP抑制率<30%患者, 依据患者病情停用氯吡格雷更换为替格瑞洛继续服用, 替格瑞洛方法为首剂180 mg顿服后改为90 mg/次, 2次/d 口服。
1. 3 观察指标及判定标准 观察比较两组患者ADP抑制情况、ADP抑制率, 并统计基因分型。判定标准:以ADP诱导的血小板抑制率<30%为氯吡格雷低反应(LCR), ≥30%定义为氯吡格雷正常反应。ADP诱导聚集后的血小板抑制率(ADP抑制率)用于监测氯吡格雷疗效。ADP抑制率<30%为无效, 30%≤ADP抑制率≤75%为起效, 75% 1. 4 统计学方法 采用SPSS19.0统计学软件处理数据。计量资料以均数±标准差( x-±s)表示, 采用t检验;计数资料以率(%)表示, 采用χ2检验。P<0.05表示差异有统计学意义。 2 结果 2. 1 两组患者基因分型统计 A组中野生型纯合子组包括正常代谢型患者23例, 均为CYP2C19*1/*1基因型;快代谢型0例。B组中野生型与突变基因杂合子组包括中间代谢型患者15例, 其中CYP2C19*1/*2基因型13例, CYP2C19*1/*3基因型2例;突变基因纯合子或杂合子组包括慢代谢型患者4例, 其中CYP2C19*2/*2基因型3例, CYP2C19*2/*3基因型1例。见表2。 2. 2 两组患者ADP抑制情况比较 A组中服用氯吡格雷后ADP抑制率<30%患者13例(57%)。见图1。其中停用氯吡格雷更换为替格瑞洛继续服用患者10例(43%)。替格瑞洛顿服12 h后复查血小板ADP抑制率, ADP抑制率>30%患者9例(39%), ADP抑制率<30%患者1例(4%), 具体原因不详。见图1。B组中服用氯吡格雷后ADP抑制率<30%患者11例(58%), 其中停用氯吡格雷更换为替格瑞洛继续服用患者4例(21%)。替格瑞洛顿服12 h后复查血小板ADP抑制率, ADP抑制率均>30%患者4例(21%)。见图2。B组中ADP抑制率<30%的发生率高于A组, 但差异无统计学意义(P>0.05)。 A组+B组42例患者中服用氯吡格雷后ADP抑制率<30%患者24例(57%), 其中停用氯吡格雷更换为替格瑞洛继续服用患者14例(33%), 14例患者中替格瑞洛顿服12 h后复查血小板ADP抑制率均>30%患者13例(31%), 但仍有ADP抑制率<30%患者1例(2%), 具体原因不详。见图3。 2. 3 两组患者ADP抑制率比较 A组平均ADP抑制率为(39.5±28.4)%, B组平均ADP抑制率為(31.5±21.6)%, B组平均ADP抑制率低于A组, 但差异无统计学意义(t=1.0094, P>0.05)。 3 讨论 急性冠脉综合征是常见的心血管系统疾病, 影响患者的生存时间及生存质量[9-11]。我国人群CYP2C19基因多态性多以基因型(*1/*1)为主。氯吡格雷是急性冠脉综合征患者广泛应用的药物。 针对CYP2C19基因多态性与氯吡格雷疗效的研究, 目前主要有以下两种观点:一种是支持CYP2C19基因多态性与氯吡格雷疗效有关的研究:①Hou等[12]的荟萃分析表明, CYP2C19基因多态性中的CYP2C19*2基因型可能与氯吡格雷抵抗有关。②Idrissi等[13]的研究结果支持CYP2C19基因多态性与氯吡格雷抵抗具有明显相关性。③Li等[14]研究发现:CYP2C19基因多态性与PCI术后1年心血管事件的高风险具有明显相关性。④Zhong等[15]的文章提出CYP2C19基因突变(CYP2C19*2)和(CYP2C19*3)影响中国较多的人口, 并且这种突变与氯吡格雷抵抗和主要心血管不良事件的风险增加密切相关。⑤研究表明[16], 支架血栓形成与CYP2C19*2 突变导致的氯吡格雷抵抗有关, 而CYP2C19*17可能在这一过程中起到保护作用。⑥Chen等[17]研究表明, 性别、年龄、糖尿病、高血压和高脂血症可能导致患者对氯吡格雷低反应性。 另一种是支持CYP2C19多态性与氯吡格雷疗效无关的研究:①Charfi等[18]研究了接受氯吡格雷治疗1个月的71例冠心病患者, 没有观察到CYP2C19*2等位基因与心血管事件的发生有显著相关性。②Notarangelo等[19]的文章指出CYP2C19基因型仅解释了12%的氯吡格雷反应变异性, 这表明除CYP2C19以外的因素可能是更重要的。③Bouman等[20]的研究显示CYP2C19基因型无论是慢代谢还是快代谢, 均未对氯吡格雷疗效产生明显的影响。④Calderon-Cruz等[21]提出氯吡格雷药物低反应性与基因多态性无关。 正是由于目前以上两种观点争论较为激烈, 本研究主要围绕基因型分组, 分别检测血小板聚集率, 探讨急性冠脉综合征患者CYP2C19基因多态性与氯吡格雷疗效的关系。 本研究结果显示, 急性冠脉综合征患者中, B组中ADP抑制率<30%的发生率高于A组, 但差异无统计学意义(P>0.05)。说明CYP2C19基因多态性并不能完全决定服用氯吡格雷后的血栓弹力图ADP抑制率, 支持CYP2C19多态性仅为众多影响氯吡格雷疗效的因素之一, 并不能最终决定氯吡格雷疗效。同时本研究结果提示, 服用氯吡格雷后血小板ADP抑制率<30%的发生率高, 但更换成替格瑞洛后ADP抑制率多数均>30%, 但仍有ADP抑制率<30%患者1例, 具体原因不详。 本研究入组人群数量有限, 推断氯吡格雷疗效还受众多其他因素的影响, 但是为何种因素具体如何影响, 仍需进一步研究。 参考文献 [1] Hankey GJ. Prasugrel or clopidogrel for long-term secondary stroke prevention? Lancet Neurol, 2019, 18(3):222-223. [2] Prasad K, Siemieniuk R, Hao Q, et al. Dual antiplatelet therapy with aspirin and clopidogrel for acute high risk transient ischaemic attack and minor ischaemic stroke: a clinical practice guideline. BMJ, 2018(363):k5130. [3] Jovani M, Chan AT. Do Aspirin and Clopidogrel Follow the Same Road Toward Prevention of Colorectal Cancer?Clinical Gastroenterology and Hepatology, 2019, 17(10):1945-1947. [4] Tangamornsuksan W, Thiansupornpong P, Morasuk T, et al. A pharmacokinetic model of drug-drug interaction between clopidogrel and omeprazole at CYP2C19 in humans. Conf Proc IEEE Eng Med Biol Soc, 2017(2017):2704-2707. [5] 杨玲, 刁珊珊, 丁意平,等. 负荷剂量氯吡格雷治疗轻型缺血性脑卒中/短暂性脑缺血发作的作用及机制. 中华医学杂志, 2019, 99(5):349-353. [6] Larson EA, Miller NJ. Point-Counterpoint: CYP2C19 Genotyping for Clopidogrel. SD Med, 2017, 70(1):13-15. [7] Li RH, Stern JA, HoV, et al. Platelet Activation and Clopidogrel Effects on ADP-Induced Platelet Activation in Cats with or without the A31P Mutation in MYBPC3. Journal of Veterinary Internal Medicine, 2016, 30(5):1619-1629. [8] Jin L, Yu H, Dong T, et al. The Prognostic Value of ADP-Induced Platelet Aggregation for Bleeding Complications in Low- Intermediate Risk Patients With Acute Coronary Syndrome Taking Clopidogrel After Percutaneous Coronary Intervention. Heart Lung & Circulation, 2017, 26(1):49-57. [9] Bonello L, Laine M, Lenesle G, et al. Meta-Analysis of Potent P2Y12-ADP Receptor Antagonist Therapy Compared to Clopidogrel Therapy in Acute Coronary Syndrome Patients with Chronic Kidney Disease. Thromb Haemost, 2018, 118(10):1839-1846. [10] Siasos G, Kioufis S, Oikonomou E, et al. Impact of C34T P2Y12 ADP receptor polymorphism and smoking status on cardiovascular outcome in coronary artery disease patients receiving clopidogrel. International Journal of Cardiology, 2016, 210(12):161-163. [11] Martischnig AM, Mehilli J, Pollak J, et al. Impact of Dabigatran versus Phenprocoumon on ADP Induced Platelet Aggregation in Patients with Atrial Fibrillation with or without Concomitant Clopidogrel Therapy (the Dabi-ADP-1 and Dabi-ADP-2 Trials). Biomed Research International, 2015(2015):1-10. [12] Hou X, Shi J, Sun H. Gene polymorphism of cytochrome P450 2C19*2 and clopidogrel resistance reflected by platelet function assays: a meta-analysis. European Journal of Clinical Pharmacology, 2014, 70(9):1041-1047. [13] Idrissi HH, Hmimech W, Khorb NE, et al. A synergic effect between CYP2C19*2, CYP2C19*3 loss-of-function and CYP2C19*17 gain-of-function alleles is associated with Clopidogrel resistance among Moroccan Acute Coronary Syndromes patients. Bmc Research Notes, 2018, 11(1):46. [14] Li X, Wang Z, Wang Q, et al. Clopidogrel-associated genetic variants on inhibition of platelet activity and clinical outcome for acute coronary syndrome patients. Basic Clin Pharmacol Toxicol. 2019, 124(1):84-93. [15] Zhong Z, Hou J, Li B, et al. Analysis of CYP2C19 Genetic Polymorphism in a Large Ethnic Hakka Population in Southern China. Medical Science Monitor International Medical Journal of Experimental & Clinical Research, 2017(23):6186-6192. [16] Kirac D, Erdem A, Avcilar T, et al. Effects of genetic factors to stent thrombosis due to clopidogrel resistance after coronary stent placement. Cell Mol Biol (Noisy-le-grand), 2016, 62(1):51-55. [17] Chen K, Zhang R, Liu H, et al. Impact of the CYP2C19 Gene Polymorphism on Clopidogrel Personalized Drug Regimen and the Clinical Outcomes. Clinical Laboratory, 2016, 62(9):1773-1780. [18] Charfi R, Mzoughi K, Boughalleb M, et al. Response to clopidogrel and of the cytochrome CYP2C19 genepolymorphism. La Tunisie medicale, 2018, 96(3):209-218. [19] Notarangelo MF, Bontardelli F, Merlini PA. Genetic and nongenetic factors influencing the response to clopidogrel. Journal of Cardiovascular Medicine, 2013, 14(14 Suppl 1):S1-S7. [20] Bouman HJ, Sch?mig E, Werkum JWV, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nature Medicine, 2011, 17(1):110-116. [21] Calderón-Cruz B, Rodríguez-Galván K, Manzo-Francisco LA, et al. C3435T polymorphism of the ABCB1 gene is associated with poor clopidogrel responsiveness in a Mexican population undergoing percutaneous coronary intervention. Thrombosis Research, 2015, 136(5):894-898. [收稿日期:2019-06-21]
