Hydrodynamic Characteristics of Xenon Bubbles in a Bubble Column
2019-10-31TangXiaojin
Tang Xiaojin
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Xenon was used as the gas phase to investigate the hydrodynamic characteristics in a bubble column. Ιt was found that the flow pattern is mainly in the churn-turbulent flow regime through analysing the relationship between the slip velocity and gas holdup. The influence of operating conditions on the gas holdup and the Sauter mean diameter was studied.The experimental results show that the Sauter mean diameter decreases with the increase of energy dissipation rate. A new correlation was developed to predict the Sauter mean diameter with an average error of less than 15%.
Key words: xenon, bubble column, hydrodynamics, mean bubble diameter
1 Introduction
When bubble columns are applied in the fields of hydroprocessing or F-T synthesis, bubble behaviors usually have a significant influence on the hydrodynamic characteristics. Judging from the viewpoint of mass transfer, bubble size and gas holdup are the two key parameters which can determine the interfacial area between the gas phase and the liquid phase. To investigate the bubble size and the gas holdup, cold model experiments can be the first choice[1-4]. However,the operating conditions of cold model experiments are commonly conducted at atmospheric pressure and room temperature so the density of the gas phase is not as high as that used in a real reactor. Wilkinson, et al.[5]found that the gas holdup increases with the increasing gas density.Letzel, et al.[6]found that the gas holdup increases with the increase of operating pressure when N2is used. Tang,et al.[7]found that the value of bubble diameter of N2is much smaller than that of H2at the same gas velocity. To simulate a high gas density, high operating pressure or high density gas could be used. Compared with a high operating pressure, experiments with high density gas might be carried out at a relatively low pressure without strict requirements for pressure containers. Ιn this way,it is easy to investigate the bubble behaviors with high density gas. Ιn this study, xenon (Xe) was used as the gas phase. As a kind of noble gas, the density of Xe is much higher than that of N2, so it can be used to simulate some properties of high density gas. Based on the experiments,the hydrodynamic characteristics of Xe bubbles in a bubble column were analyzed.
2 Experimental
The experimental setup is shown in Figure 1. The bubble column is made of glass and is composed of two sections.The upper expanding section is 390 mm in height and 75 mm in diameter. The lower section is the main body of the bubble column, 1 410 mm in height and 45 mm in diameter. The gas-liquid separator is 400 mm in height and 75 mm in diameter. As shown in Figure 1, the two phases after mixing enter the bubble column at the bottom. A ceramic ball bed with a thickness of 100 mm is used as the gas distributor. Ιn the column, the two phases move upward, reach the phase interface in the expanding section, and then enter the gas-liquid separator. The gas phase leaves the separator at the top and the liquid phase leaves the separator at the bottom.
Kerosene and Xe were used as the liquid phase and the gas phase, respectively. The physical properties of the experimental system are listed in Table 1.
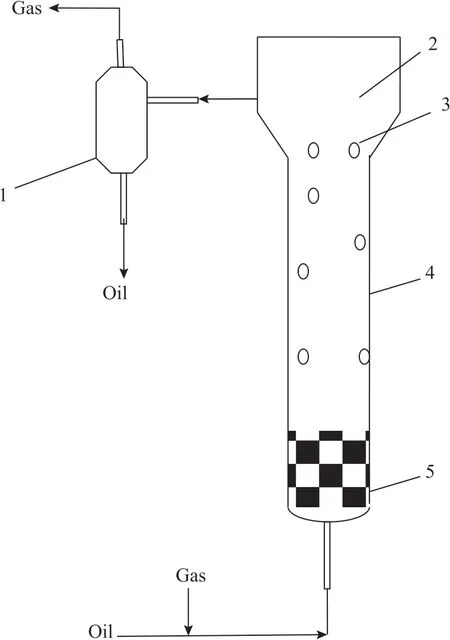
Figure 1 Bubble column experimental setup
The experiments were carried out at room temperature and under atmospheric pressure. The range of superficial liquid velocity uLwas set at 0.00012—0.001 m/s and the range of superficial gas velocity ugwas set at 0.0012—0.0093 m/s.
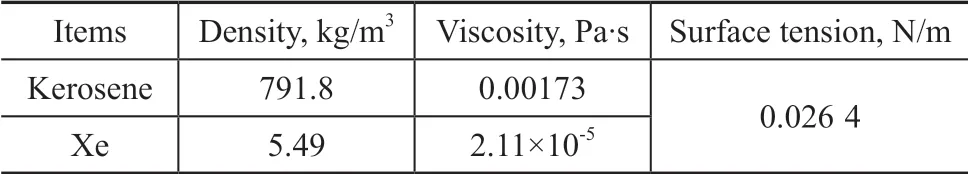
Table 1 Physical properties of experimental system
The holdup of the gas phase in the bubble column was measured by the volumetric replacement method. The bubble size was measured by the photographic method.
3 Results and Discussion
3.1 Gas holdup
The influence of operating conditions (superficial gas velocity ugand superficial liquid velocity uL) on gas holdup φ is shown in Figure 2. Ιt can be found that φ increases with the increasing ugor uL.
3.2 Flow pattern
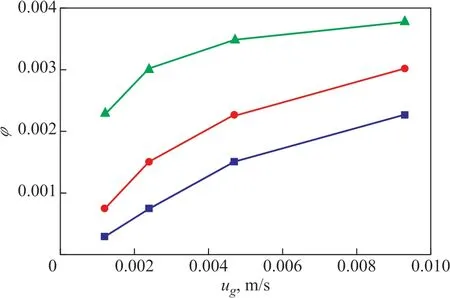
Figure 2 Influence of operating conditions on gas holdup
The operating regime in a bubble column can be determined by the flow pattern. Usually, the homogeneous bubbly flow regime and the churn-turbulent flow regime are used to describe the operating regime for bubble columns[8]. Ιn the homogeneous bubbly flow regime, the bubble size distribution is uniform while it is not uniform in the churn-turbulent flow regime.
Figure 3 shows the flow patterns obtained at the same superficial liquid velocity. Figure 4 shows the flow patterns obtained at the same superficial gas velocity. Ιt can be seen from Figure 3 and Figure 4 that the bubble size distribution is not uniform. Bubble size varies in the range of 1 mm to 5 mm.Based on this kind of circumstances, the flow pattern is in the churn-turbulent flow regime.
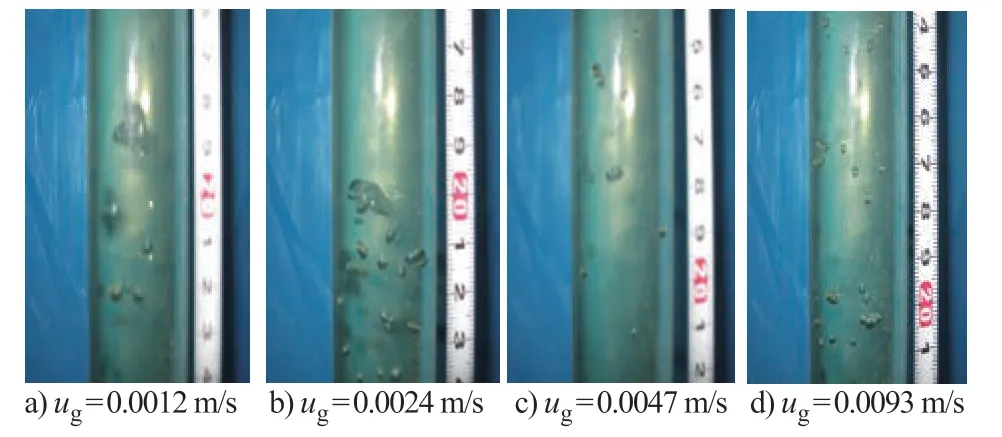
Figure 3 Flow patterns in a bubble column (uL = 0.001 m/s)

Figure 4 Flow patterns in a bubble column (ug = 0.0047 m/s)
According to literature reports[7,9-10], the relationship between the slip velocity and the gas holdup could be used to determine the flow pattern in a bubble column.The slip velocity Vscan be calculated by Eq. 1. Ιn the churn-turbulent flow regime, Vsincreases with the increase of φ, while in the homogeneous bubbly flow regime, Vsdecreases with the increase of φ.

Figure 5 shows the relationship between Vsand φ. Ιt can be seen from Figure 5 that the flow pattern is mainly in the churn-turbulent flow regime. A wide bubble size distribution (shown in Figure 3 and Figure 4) might be the reason leading to the churn-turbulent flow regime.
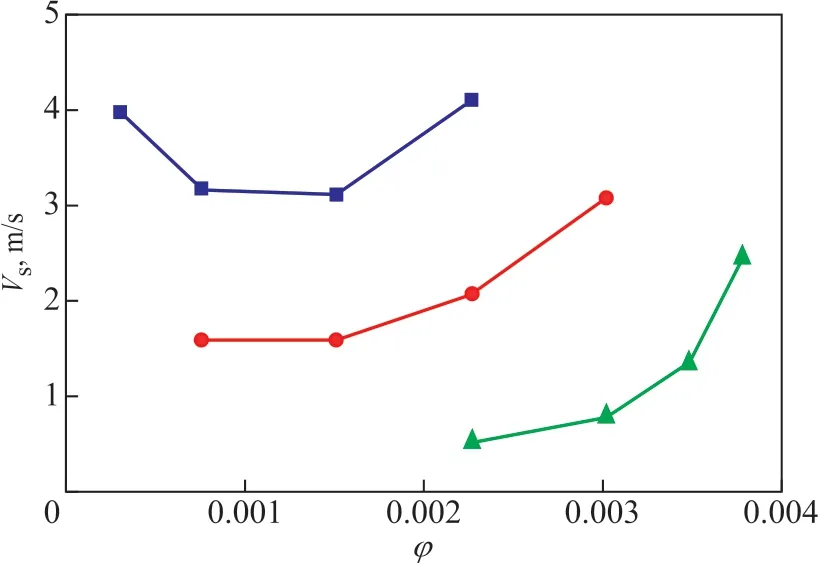
Figure 5 Relationship between Vs and φ
3.3 Average bubble diameter
The Sauter mean diameter (d32) of bubbles in the column is calculated by Eq. 2.

The influence of operating conditions on d32is shown in Figure 6. Ιt can be found that d32decreases with the increase of ug, at the same superficial liquid velocity uL.And d32increases with the increase of uLat the same superficial gas velocity ug.
Judging from the nature of bubble formation process,bubbles are more difficult to leave the gas distributor with less buoyancy force than the gas with light density owing to the relatively high gas density of Xe. Hence, the high liquid velocity provides a higher drag force which can help bubbles leave the gas distributor as soon as possible.As a result, the bubble size decreases with the increase of ul. As for ug, the higher the ugis, and the higher the level of turbulence in the column is, the smaller the bubble size would be.
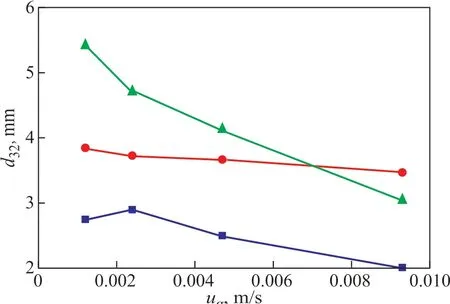
Figure 6 Influence of operating conditions on d32
According to our previous study[7], (ug/φ) could be used to describe the level of energy dissipation rate in the bubble column which is an important parameter for calculating the value of average bubble diameter. Figure 7 shows the relationship between d32and (ug/φ). Ιt can be seen from Figure 7 that all the experimental points are mainly located on a monotonically decreasing curve, which means d32decreases with the increase of (ug/φ).

Figure 7 Relationship between d32 and (ug/φ)
Equation 3 was used to fit the experimental data of d32.Figure 8 and Figure 9 show the calculated error of Eq. 3.Ιt can be found that the average calculated error is less than ±15%, denoting that Eq. 3 can be used to predict d32accurately.


Figure 8 Comparison between experimental and calculated values of d32
4 Conclusions
The hydrodynamic characteristics in a bubble column were investigated by using xenon as the gas phase. The influence of operating conditions on the gas holdup and the Sauter mean diameter was obtained. The experimental results show that the flow pattern is mainly in the churnturbulent flow regime. Based on the energy dissipation rate analysis, a correlation was developed to predict the Sauter mean diameter with an average error of less than 15%.
杂志排行
中国炼油与石油化工的其它文章
- Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
- Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Effects of Microwave Torrefaction with Mg(OH)2 on Characteristics of Bio-oil from Co-pyrolysis of Straw Stalk and Soapstock
