Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
2019-10-31YuFengliGuYulongLiuQichunXieCongxia
Yu Fengli; Gu Yulong; Liu Qichun; Xie Congxia
(State Key Laboratory Base of Eco-chemical Engineering, College of Chemistry and Molecular Engineering,Qingdao University of Science and Technology, Qingdao, 266042)
Abstract: The Brønsted-acidic polyether ionic liquids (ΙLs) with different polymerization degrees (n value) were prepared via the reaction of tetramethylguanidine and epoxy ethane, followed by successive reactions with 1,3-propane sultone and trifluoromethanesulfonic acid (TfOH). The prepared ΙLs were characterized by infrared spectroscopy and 1H nuclear magnetic resonance spectroscopy, and their thermal stability was determined by thermal gravimetry. The synthesized polyether ΙLs coupled with TfOH were used to catalyze the alkylation reaction of isobutane and isobutene for the preparation of alkylate gasoline. The polyether ΙLs could improve the substrate dissolution and promote the separation of the catalyst from the products. The ideal ΙL (n = 94) was determined. The optimized alkylation reaction conditions covered: a VTfOH / VΙL ratio of 0.35, a reaction temperature of 40 °C, a reaction time of 50 min, and a stirring speed of 800 r/min. The conversion of isobutene was 92.4% and the selectivity for the C8-product was 81.6%. Under optimal conditions, the catalyst life was determined and TfOH showed improved cyclic performance in the polyether ΙLs. After 8 operating cycles, the catalytic activity of the catalyst showed negligible decline.
Key words: alkylation; alkylate gasoline; trifluoromethanesulfonic acid; polyether-based ionic liquid; isobutene
1 Introduction
With the rapid and increasing societal development,the demand for high-quality fuel is rising; particularly,the high-calorific-value light gasoline is becoming popular[1-2]. Currently, gasoline is mainly composed of the naphtha fraction from the FCC unit and the high-octane component of alkylation unit[3-4], which is mainly composed of trimethylpentane (TMP) and dimethylhexane (DMH). Alkylation gasoline is ideal for blending in high-octane gasoline to achieve good antiknock and low Reid vapor pressure without sulfur and aromatic compounds[5].
Currently, concentrated sulfuric acid or hydrofluoric acid are used in commercial scale to catalyze the alkylation of isobutane and butene to produce alkylation gasoline[6-7]. Although these catalysts exhibit good catalytic activity, they have significant limitations.For example, concentrated sulfuric acid can cause serious equipment corrosion while producing a small amount of acid-soluble oil (ASO), which can reduce the catalyst acidity and lead to rapid catalyst deactivation.
Ιn addition, the operating cost increases due to the requirements for treatment and regeneration of the waste acid[8-10]. Hydrofluoric acid is highly toxic and the leakage of highly toxic chemical smog can cause great damage to human health and the environment[11].Starting from the end of the 20th century, research into solid acid catalysts for the isobutane alkylation reaction has been conducted. However, as the absorption capacity of the solid acid for butene is two orders of magnitude higher than that of isobutane, polymerization readily occurs to form oligomers. Hence, a carbon deposition quickly forms on the acid centers, resulting in catalyst deactivation and limited industrial applications[12-14].
Ιonic liquids (ΙLs) are liquid solvents at or near room temperature that are entirely composed of cations and anions. Because of their unique physical and chemical properties, ΙLs can replace traditional volatile solvents under certain circumstances. Ιn addition to their green solvent characteristics, ΙLs can act as catalysts[15].However, the Lewis-acidic ΙLs, including the AlCl3-based ΙLs, are sensitive to water and air, which would limit their applications[16].
Previous studies have shown that isobutane and isobutene exhibit higher solubility in ether[17-18]and the Brønsted acids in comparison with the Lewis acids can provide better stability[19]. Therefore, herein, we designed and synthesized the polyether-based Brønstedacidic ΙLs coupled with trifluoromethanesulfonic acid(TfOH) to catalyze the isobutane/isobutene alkylation reaction for the preparation of alkylate gasoline with a high octane number. The results showed that TfOH exhibited good catalytic and recycling performance in the polyether ΙLs. The selectivity for the C8product in alkylation process can reach as high as 81.6%. After the reaction, TfOH can be dissolved in the polyether ΙLs and easily separated from the alkylation oil. Therefore,the catalyst can be conveniently recovered and reused.The catalytic performance of TfOH in the polyether ΙLs did not significantly decrease after 8 cycles of reuse,indicating the excellent stability of the catalytic system.
2 Experimental
2.1 Reagents and instruments
Ιsobutane and isobutene were purchased from the Dalian Special Gas Co., Ltd. TfOH, tetramethyl guanidine,and 1,3-propanesulfonic acid were obtained from the Aladdin Reagent Ιnc. Epoxyethane was purchased from the Shandong Qingdao Heli Gas Co., Ltd., and other reagents and solvents used in this study were analytically pure and used as received.
The alkylation products were analyzed using a GC-9790 Plus gas chromatograph equipped with a FΙD detector and a HP-PONA capillary chromatographic column (50 m×0.2 mm×0.5 μm; Zhejiang Fuli Analysis Co., Ltd.). The prepared ΙLs were characterized using a Nicolet 510P Fourier transform infrared (FT-ΙR)spectrometer, a Netzsch-TG209 thermogravimetric analyzer (TGA), and a Brucker AV500 nuclear magnetic resonance (NMR) instrument. The alkylation reactions were performed in a 100 mL high-pressure microreactor equipped with double trace injection pump plungers(Xi’an Taikang Biological Technology Co., Ltd.).
2.2 Synthesis of the polyether ILs
The synthetic route for the polyether-type Brønsted-acidic ΙLs is shown in Scheme 1. Firstly, 0.01 mol of tetramethyl guanidine with 10 mL of anhydrous ethanol was placed in a 100 mL high-pressure reaction kettle, which was then frozen overnight. Afterwards, a certain volume of epoxyethane was added. The atmosphere in the kettle was replaced with nitrogen 3 times and a nitrogen flow under a pressure of 4.0 MPa was subsequently added. The high-pressure reaction kettle was placed in a constanttemperature water bath at 30 °C. After 2 h, the temperature was increased to 60 °C prior to the reaction, which lasted 6 h. After the kettle was cooled down, the reaction mixture was transferred to an one-necked round-bottomed flask for vacuum distillation, and the solvent was removed to obtain the intermediate Ι. The average polymerization degree (n value) of the intermediate Ι was calculated by weighing.Subsequently, 0.01 mol of 1,3-propane sultone with 20 mL of ethyl acetate was added to a three-necked roundbottomed flask. The intermediate Ι was then added to the flask dropwise at 30 °C. The temperature was subsequently increased to 50 °C and held for 2 h. After the reaction,the intermediate ΙΙ was obtained by vacuum distillation to remove the solvent. The intermediate ΙΙ and TfOH in a 1:1 molar ratio were added to a round-bottomed flask. The mixture was subject to reaction for 5 h at 60 °C to yield the polyether-type Brønsted-acidic ΙL, which could be directly used in the alkylation reaction without further purification.
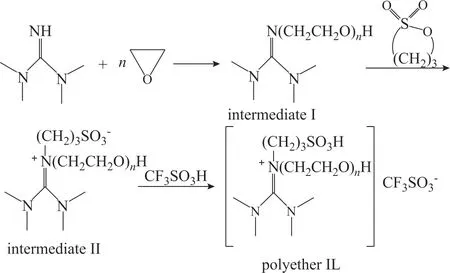
Scheme 1 Synthetic route for manufacture of polyether ILs
The representative data of a typical polyether ΙL (n = 94)are as follows.
ΙR (KBr): ν = 3 460.28 cm-1(-OH), 2 871.17 cm-1(C-H),1 454.95 cm-1(S=O), 1 648.21 cm-1(C=N), 1 250.94 cm-1(C-N), 1 113.79 cm-1(C-O-C), 1 032 cm-1(S-O).1H NMR: (500 MHz, CDCl3): δ = 3.47—3.69 (m, CH2).Because the degree of polymerization was high, only the hydrogen atoms on the polyether chain are shown.TGA showed that the synthesized polyether ΙL was stable at 130 °C. However, the polyether ΙL began to sharply degrade when the temperature increased to 220 °C, which was possibly caused by polyether chain breakage.
2.3 Alkylation
The alkylation reaction took place in the device, as shown in Figure 1. Firstly, 10 mL of the polyether ΙL with a specified volume of TfOH were added to the mechanically stirred high-pressure reaction kettle. After sealing the kettle, the atmosphere in the kettle was purged with nitrogen gas 4 times, and 30 mL of feed gas (isobutane:isobutene =10:1) were subsequently filled. After reaching the preset temperature, the mixture was mechanically stirred for a certain time. Subsequently, the gas in the kettle was collected and the isobutene conversion was determined by gas chromatography (GC). The upper liquid was removed and washed with saturated sodium bicarbonate solution 3 times. After drying and centrifugation, the upper clear liquid was analyzed by GC to determine the amount of alkylation products produced thereby. The lower catalyst phase did not need processing and could be directly analyzed.

Figure 1 Installation for conducting the alkylation reaction
3 Results and Discussion
3.1 Effect of the catalyst on alkylation reaction
A series of polyether-based ΙLs with different polymerization degrees (n value) were prepared according to Scheme 1. The dosage of epoxyethane and polymerization degree of the prepared ΙL showed a good linear relationship (Figure 2). The introduction of a sulfonic acid group (-SO3H) imparted Brønsted acidity to the prepared polyether ΙLs. The TfOH-catalyzed alkylation of isobutane and isobutene afforded a very high isobutene conversion of 97.8%, but a very low C8selectivity of 39.7% due to the high acidity of H0= -14.1.However, when only the prepared ΙL was used in the catalytic alkylation of isobutane and isobutene, a very low conversion rate was achieved, which indicated that the acidity of the prepared ΙL was insufficient for catalyzing the alkylation reaction[20-21]. The experiments showed that the polyether ΙLs could dissolve both isobutene and TfOH. TfOH in the prepared polyether ΙL exhibited outstanding catalytic performance for the alkylation of isobutane and isobutene.
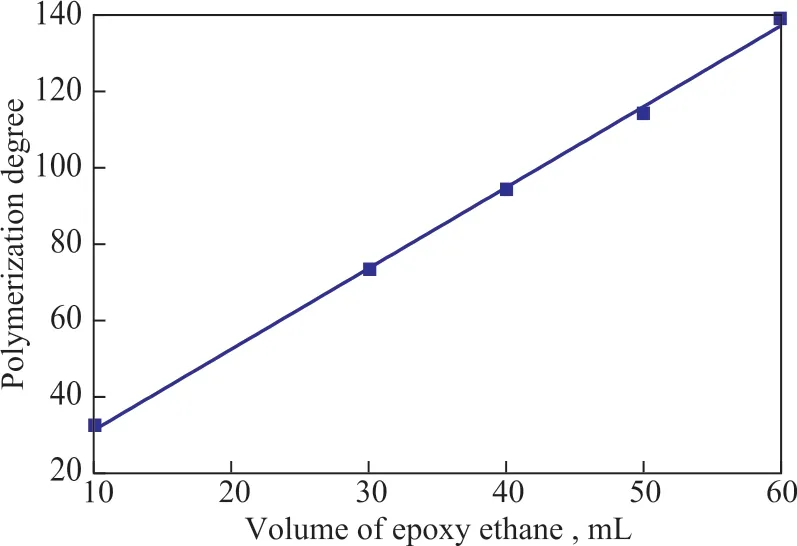
Figure 2 Relationship between the dosage of epoxyethane and polymerization degree of the resulting IL
Ιn the structure of polyether ΙLs, the ratio of polyether chain length/polymerization degree affected the substrate solubility in the catalyst phase and influenced the total acidity of the catalyst phase. The polymerization degree of ΙL significantly impacted the catalytic reaction. As shown in Figure 3, with an increasing ΙL polymerization degree,the isobutene conversion gradually decreased and the C8selectivity of alkylate gasoline fell off slightly following the initial increase of polymerization degree of ΙL. Because the viscosity and density of the ΙL increased with an increasing polymerization degree, as shown in Table 1, the mass transfer rate in the catalytic reaction between the substrate and the catalyst decreased. Therefore, the catalytic activity decreased, resulting in a decreased conversion of isobutene.On the other hand, due to the higher polymerization degree,the concentration of isobutene surrounding the catalyst was low, which would obstruct the isobutene polymerization.Therefore, the content of C9+ product decreased, while the corresponding C8selectivity would increase. However,due to the higher ΙL polymerization degree, the acidity of the ΙL and catalyst system was reduced to a certain extent,which could slightly decrease the amount of C8product.Based on the comprehensive consideration, the catalytic reaction in the polyether ΙL with a polymerization degree of n = 94 exhibited a relatively good substrate conversion,with the target product selectivity presented in Figure 3.The conversion rate of isobutene was 79.3% and the selectivity of the C8product was 78.3%.
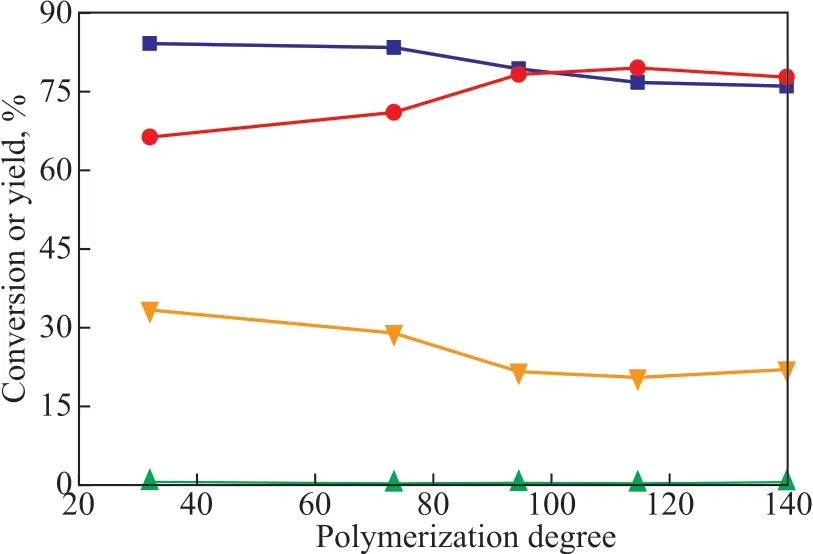
Figure 3 Effect of IL polymerization degree on alkylation(VΙL = 10 mL, VTfOH = 3 mL, T = 40 °C,t = 40 min, RS = 800 r/min).
The prepared polyether ΙL (n = 94) has the same polyether chain length as that of PEG-4000. When polyether ΙL (n =94) was replaced by PEG-4000 for the catalytic alkylation of isobutane and isobutene, the conversion of isobutene was only 26.5% and the selectivity of the C8product was 59.5%.This indicated that TfOH exhibited higher catalytic activity in the presence of polyether ΙL (n = 94) than that observed in the presence of PEG-4000, because PEG-4000 was less efficiently dissolved in the substrates. On the other hand,the -SO3H group in the structure of polyether ΙL increased the Brønsted acidity of the catalytic system and stabilized the TfOH catalyst. PEG with a low polymerization degree could dissolve more reaction substrate, but the reaction product was well-dissolved in the PEG, which was very unfavorable to the separation of the catalyst and the reaction products. PEG-600, PEG-800, and PEG-2000 coupled with the same amount of TfOH were used in the catalytic alkylation reaction. The reaction system nearly became a single phase after the reaction. Hence, it was difficult to separate the catalyst from the products after completion of the reaction. As for the catalytic alkylation in the polyether ΙL, the reaction system showed two phases after reaction and the catalyst in the polyether ΙL could be easily separated via extraction of the upper alkylate gasoline.
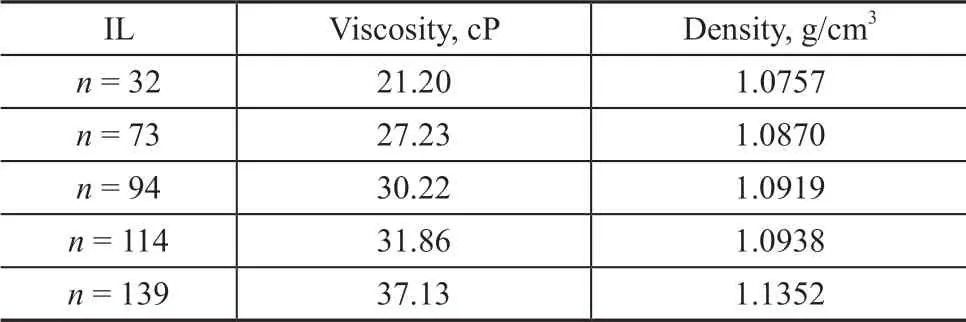
Table 1 Viscosity and density of the prepared ILs
3.2 Effect of TfOH dosage on alkylation
The alkylation reaction requires a strongly acidic catalytic system, with a suitable Hammett acidity function (H0)of approximately 10. The side reactions mainly involve aggregation and cracking reactions[22]. When the volume of the polyether ΙL (n = 94) was 10 mL, the effect of the catalytic system acidity on alkylation was investigated by changing the amount of TfOH, with the results shown in Figure 4.Ιsobutene conversion increased with an increasing dosage of TfOH, indicating that the catalytic activity increased with an increasing catalyst acidity. However, the selectivity for the C8product gradually declined. Ιn the catalytic system,isobutene and the catalyst were both well-dissolved in the polyether ΙL. With an enhanced catalytic activity, isobutene was more likely to aggregate[23], leading to an increased amount of C9+ polymerization product formed and a decrease of corresponding C8product. Based on the comprehensive consideration, the ideal dosage of TfOH was determined to be 3.5 mL, while the volume ratio of TfOH to ΙL was 0.35. Under the selected experimental conditions, the isobutene conversion reached 89.9% and the C8selectivity was 75.9%.
3.3 Effect of reaction temperature on alkylation
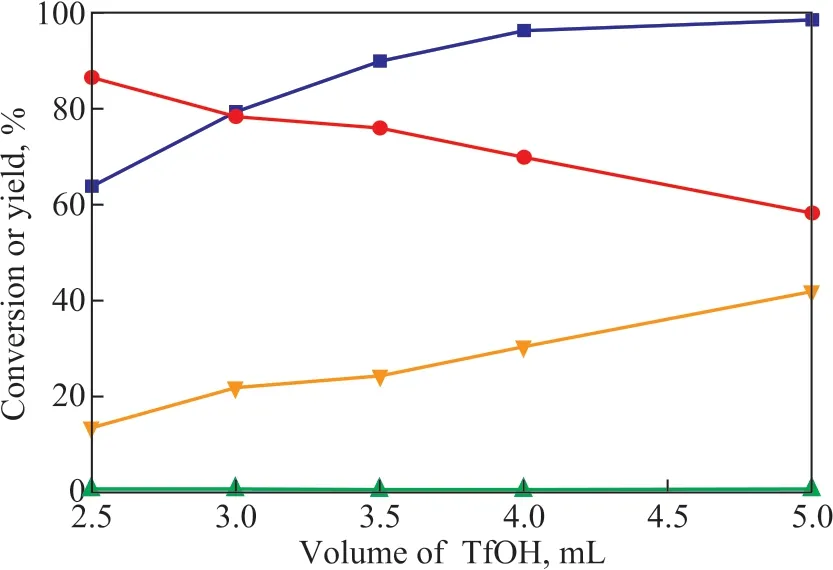
Figure 4 Effect of TfOH dosage on alkylation reaction(VΙL = 10 mL, T = 40 °C, t = 40 min, RS = 800 r/min).
Under a constant ratio of VTfOH: VΙL= 0.35, the effect of the reaction temperature on alkylation was studied, with the results shown in Figure 5. With an increasing reaction temperature, the conversion of isobutene increased and the C8selectivity at first increased and subsequently decreased.Molecular movement was enhanced at higher temperatures,which could increase the isobutene conversion rate.However, because alkylation is an exothermic reaction,the high temperature was not conducive to the formation of the target C8product[24]. Moreover, with an increasing temperature, the increased solubility of the raw gas material in the polyether ΙL caused an increased aggregation at the beginning of reaction, leading to increased production of C12, C16, and other large molecules[25]and decreased C8selectivity. These results agreed well with the outcome indicating that high temperature could lead to decreased alkane selectivity during alkane isomerization in a previous thermodynamic study[26]. Ιt can be seen from Figure 5 that the optimum reaction temperature was 40 °C.

Figure 5 Effect of the reaction temperature on alkylation(VTfOH: VΙL = 0.35, t = 40 min, RS = 800 r/min).
3.4 Effect of reaction time on alkylation
Under the static conditions of VTfOH: VΙL= 0.35 and T = 40 °C, the effect of reaction time on the alkylation process was investigated, with the results shown in Figure 6. With an increasing reaction time, the conversion of isobutene gradually increased and the C8product at first increased and subsequently decreased, while the yield of C9+ product increased after an initial drop. These results showed that the C8components were transformed into macromolecular hydrocarbons in the presence of acid in an extended reaction time. Therefore, an ideal reaction time of 50 min was selected according to the data presented in Figure 6.
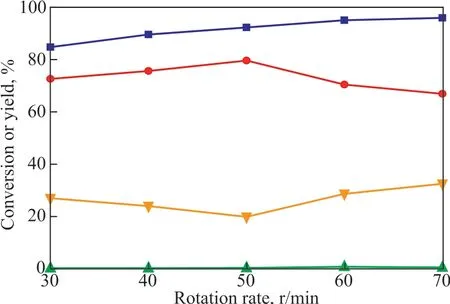
Figure 6 Effect of the reaction time on alkylation(VTfOH: VΙL = 0.35, T = 40 °C, RS = 800 r/min).
3.5 Effect of stirring rate on alkylation
The influence of the stirring rate in the catalytic system on the alkylation reaction was also investigated under the static conditions covering a VTfOH/VΙLratio of 0.35, a temperature of 40 °C, and a reaction time of 50 min. As shown in Figure 7, lower conversion of isobutene was achieved at lower stirring rates. When the stirring rate was 600 r/min, the conversion of isobutene was basically unchanged. However, the higher stirring speed increased the rate of self-polymerization and copolymerization,resulting in an increased production of C9+ components at the expense of C8components. Therefore, the optimal rotational rate was determined to be 800 r/min.
3.6 Effect of pressure on alkylation
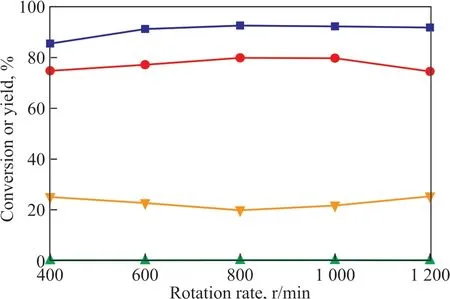
Figure 7 Effect of the stirring rate on alkylation(VTfOH: VΙL=0.35, T=40 °C, t=50 min).
Under the reaction conditions described above, the reaction vessel was filled with nitrogen gas under different pressure values to examine the effect of pressure on the alkylation reaction, with the results shown in Figure 8. The system pressure did not significantly affect the catalytic alkylation in the polyether ΙL. Therefore,adjustment of the external pressure was not required for the specifically developed catalytic system.
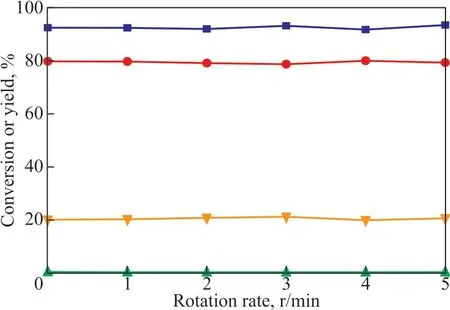
Figure 8 Effect of pressure on alkylation(VTfOH: VΙL = 0.35, T = 40 °C, t = 50 min, RS = 800 r/min).
3.7 Catalyst recycling
For the newly developed catalytic system, an important advantage is that the catalyst TfOH could be dissolved in the polyether ΙL during the entire catalytic process. After the alkylation reaction, TfOH in the polyether ΙL could be easily separated from the alkylation product. After pouring out the upper oil phase and adding fresh substrate, the catalyst could be directly applied in the next catalytic alkylation process without any treatment. Under the optimized conditions, the performance of the recycled catalyst was investigated, with the results shown in Figure 9. Over 8 cycles on reuse of the TfOH in polyether ΙL (n = 94), the conversion of isobutene only slightly declined. However, after 8 recycles, the conversion of isobutene remained at 85% and the selectivity of the C8product was 70%.

Figure 9 Recycling efficiency of the catalyst(VTfOH: VΙL=0.35, T=40 °C, t=50 min, RS=800 r/min).
4 Conclusions
TfOH in the synthesized polyether ΙL exhibited excellent catalytic activity and good recycling performance for the alkylation of isobutane and isobutene adopted for the preparation of high-quality alkylate gasoline. The polymerization degree of the best polyether ΙL was determined to be n = 94. The optimal reaction conditions for the alkylation covered: a VTfOH/VΙLratio of 0.35, a reaction temperature of 40 °C, a reaction time of 50 min,and a stirring rate of 800 r/min. Under these conditions,the conversion of isobutene was 92.4% and the selectivity for the C8-product reached 81.6%. The TfOH catalyst in the polyether ΙL could be reused 8 times while still retaining a high catalytic activity. The catalytic system of TfOH in a Brønsted-acidic polyether-based ΙL provides a novel, efficient, and green route for the preparation of high-octane alkylate gasoline.
Acknowledgements:This work was supported by the National Natural Science Foundation of China (21476120), the Shandong Province Prioritized Development Plan (2017GGX40107), and the Taishan Scholar Project of Shandong Province in China(ts201511033).
杂志排行
中国炼油与石油化工的其它文章
- Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
- Study on Reaction of Thiophene Compounds and Olefins for Deep Desulfurization of Gasoline
- Effects of Microwave Torrefaction with Mg(OH)2 on Characteristics of Bio-oil from Co-pyrolysis of Straw Stalk and Soapstock
