Effects of Microwave Torrefaction with Mg(OH)2 on Characteristics of Bio-oil from Co-pyrolysis of Straw Stalk and Soapstock
2019-10-31WenPingweiZhouYueDaiLeileiWangYunpuLiuYuhuanRuanRoger
Wen Pingwei; Zhou Yue; Dai Leilei; Wang Yunpu,; Liu Yuhuan; Ruan Roger
(1. College of Chemistry and Chemical Engineering, College of Life Science, Jiangxi Normal University,Nanchang 330022; 2. Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang Uniνersity, Nanchang 330047; 3. Center for Biorefining and Department of Bioproducts and Biosystems Engineering, University of Minnesota, MN 55108, USA)
Abstract: This study investigated the effects of torrefaction with Mg(OH)2 on the properties of bio-oil formed from the microwave-assisted catalytic fast co-pyrolysis of straw stalk and soapstock. The effects of torrefaction temperature and residence time on the yield and composition of bio-oil were discussed. Results showed that the torrefaction temperature and residence time remarkably influenced the yield and composition of bio-oil. With the increase in temperature and time, the bio-oil yield and the proportion of oxygen-containing compounds decreased, while the proportion of aromatic compounds increased. When the feedstocks were subject to torrefaction reaction for 20 min at 260 °C, the proportion of oxygencontaining compounds decreased from 29.89% to 16.49%. Meanwhile, Mg(OH)2 could render the deoxidization function of torrefaction process increasingly noticeable. The proportion of the oxygen-containing compounds reached a minimum(14.41%), when the biomass-to-Mg(OH)2 ratio was 1:1.
Key words: microwave torrefaction; Mg(OH)2; straw stalk; soapstock; bio-oil
1 Introduction
Fossil energy has become scarce with the increasing demand for energy resources. Moreover, the use of fossil energy has been causing a series of serious environmental problems. Thus, a new compatible substitute energy source should be determined. Biomass energy is considered to be the future main energy source[1]. Several techniques, such as biological, physical, and chemical methods, have been applied in converting biomass into valuable products[2]. Fast pyrolysis is most efficient among these approaches[3]. Compared with other methods,the fast pyrolysis can obtain a wide range of products,such as olefins, aromatics, alcohols, and acids, in a short period of time[4-6].
Recently, a new pyrolysis method called microwaveassisted catalytic fast pyrolysis (MACFP) has been attracting considerable attention[7-8]. This new technology can reduce the energy consumption and enhance the overall products yield compared with traditional pyrolysis methods[9-10]. Ιn the traditional method, which is known as conventional thermal heating, energy is transferred from the surface to the interior of the material[11], thereby resulting in low efficiency. By contrast, microwave can penetrate the material, and all parts of the material, rather than only the surface, can be heated simultaneously.Wu, et al.[12]evaluated the differences between the conventional and microwave-assisted methods for heating the biomass and found that the latter is faster. Recently,MACFP has emerged as a widely accepted method of bioenergy production. Borges, et al.[13]determined that the maximum bio-oil yields from wood sawdust and corn stover, which were treated with MACFP, reached 65%and 64%, respectively.
However, the bio-oil produced by direct biomass pyrolysis contains many oxygenic compounds, such as alcohols, acids, and ketones, which can result in several adverse properties, such as poor stability. This phenomenon is attributed to the low H:Ceffratio (<1) of biomass materials[14]. Materials with high H:Ceffratios can generate more aromatic components, compared with biomass with low H:Ceffratios[15]. Thus, several researchers have heated the mixtures of biomass and other materials with high H:Ceffratios to obtain highquality bio-oil. Xue, et al.[16]indicated that a substantial increase in the yield of aromatic hydrocarbons could be obtained during the co-pyrolysis of lignin and PE.Meanwhile, catalytic pyrolysis is an effective method used to reduce the content of oxygenated compounds.Bio-oil quality can be improved with the use of catalysts, such as ZSM-5, ZnO, and MCM-41[17-19]. Dai,et al.[20]investigated the effects of catalytic co-pyrolysis on bio-oil yield and found that the in-situ co-catalysis and the ex-situ catalysis could reduce the amount of oxygen-containing compounds in bio-oil and completely eliminate the N-containing compounds. Currently,biomass pretreatment is attracting considerable attention from researchers.
The biomass pretreatment techniques include sulfur acid hydrolysis, drying, torrefaction, and biological methods, among which torrefaction is the most frequently adopted. Torrefaction is a mild pyrolysis taking place at temperatures ranging from 200 °C to 300 °C[21]. This method can destroy the fiber structure and remove the excess oxygen in biomass[22]. Although the weight of biomass decreases, the energy density of biomass increases by approximately 30% after torrefaction[23]. This method can also reduce the water,acid, and oxygen compounds in bio-oil[24]. Meng,et al.[25]observed a decrease in the content of most oxygen compounds of bio-oil after torrefaction and an increase in the amount of phenolic compounds with the increasing torrefaction temperature and residence time. Meanwhile, the microwave technology has been used in torrefaction. Wang, et al.[26]indicated that microwave torrefaction can enhance the caloric value and decrease the oxygen content of materials, and the optimum microwave power level is between 250 W and 300 W. Ren, et al.[27]investigated the influence of microwave torrefaction on bio-oil and confirmed that it can reduce the amount of organic acids. Thus,microwave torrefaction can be used as an effective method for improving the bio-oil quality.
However, higher temperature can result in higher weight loss during torrefaction. Thus, a method that can enhance the deoxidation reaction at a low temperature should be developed to reduce the energy and mass losses.Recently, several CO2absorbents, such as zeolites and lithium zirconate, have been successfully used to capture CO2in gas stream[28-29]. Fisher, et al.[30]concluded that Mg(OH)2could capture CO2at 200—315 °C and could be regenerated at 400 °C.
Upon considering that integrating torrefaction with pyrolysis is an effective technique for upgrading the biooil quality[31], the combination of torrefaction and copyrolysis can further improve the bio-oil quality. This study aims at investigating the microwave-assisted pyrolysis of torrefied straw stalk and soapstock. The HZSM-5 zeolite was utilized as a catalyst to improve the bio-oil quality. The effects of torrefaction temperature and residence time on the yields and chemical compositions of bio-oil, char, and gas were investigated. Then, the influence of Mg(OH)2on bio-oil composition, when Mg(OH)2powder was mixed with straw stalk during torrefaction, was evaluated.
2 Experimental
2.1 Materials
Straw stalk was obtained from a farm in Nanchang,China. Prior to use, the straw stalk was mechanically pulverized with a grinder prior to being sieved through an 100-mesh sieve. Then, the sample was stored at room temperature for approximately one year. Soapstock was obtained from the Yihai Kerry Oils and Foodstuffs Co.,Ltd. (Jiangxi, China). Prior to use, the soapstock was saponified with excessive NaOH and absolute alcohol and then dried at 95 °C. Then, the soapstock was pulverized with a grinder and then passed through an 100-mesh sieve. Silicon carbide (SiC) powder was purchased from the Xinli Wear-Resistant Materials Co., Ltd. During the experiments, the SiC powder was used as the microwave adsorbent bed to rapidly increase the temperature. All the reagents used in the experiment were analytically pure.
The elemental compositions of dried straw stalk and soapstock are shown in Table 1. The carbon, hydrogen,and nitrogen contents of straw stalk and soapstock were determined by using an elemental analyzer (Elementar Vario EL Ⅲ, Germany), and the oxygen content was determined by difference.
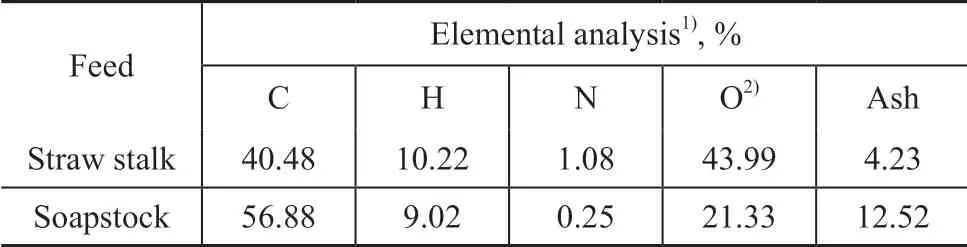
Table 1 Elemental analysis of dried straw stalk and soapstock
2.2 Catalyst
HZSM-5 (Si: Al = 50), a molecular sieve, was purchased from the Catalyst Plant of Nankai University (Tianjin,China) and was used as the catalyst for MACFP. Prior to use, the catalyst was calcined at 500 °C in air for 5 h.
2.3 Microwave-assisted torrefaction procedure
The torrefaction of straw stalk was performed in a microwave oven (MAX, CEM Corp.) operating at a power of 800 W and a frequency of 2 450 MHz. A quartz reactor was placed in a quartz beaker before the torrefaction experiment. Then, 900 g of SiC were added in the quartz beaker to bury the quartz reactor. To maintain an inert atmosphere and vacuum conditions during the reaction,the system was evacuated by using a vacuum pump at a pressure of 100 mm of Hg for at least 10 min prior to microwave heating. Then, the microwave oven was turned on to heat the system. At the specified temperature, 10 g of straw stalk were added in the quartz reactor through the feeder. Meanwhile, the microwave oven was turned on and off properly to maintain a stable temperature during torrefaction. The microwave oven was turned off when the designated processing time was reached. After the torrefied biomass was cooled down to approximately 100 °C, it was removed and placed in a desiccator for a few hours.
2.4 Microwave-assisted co-pyrolysis procedure
Co-pyrolysis of the torrefied straw stalk and soapstock was performed in the same microwave oven operating at a power of 800 W and a frequency of 2 450 MHz. A schematic of the experimental apparatus and process can be found in our previous research[11]. Co-pyrolysis was conducted at 550 °C under conditions covering a straw stalk/soapstock ratio of 1:1 and a catalyst/feed ratio of 1:2. The catalyst was placed in the tube, through which pyrolysis vapors passed, and was heated by the SiC bed.The yields of bio-oil and bio-char were calculated on the basis of their actual weights. The gas yield can be calculated as follows:

in which Yg, Yo, and Ycare the yields of gas, bio-oil, and bio-char, respectively.
2.5 Product analysis
Bio-oil was analyzed using a gas chromatographic column (HP-5ms; 30 m×0.25 mm×0.25 µm). The injector temperature was 250 °C, and a split ratio of 20:1 was used. The GC column was heated to 60 °C for 2 min and the temperature continued to increase at a rate of 10 °C/min until a temperature of 280 °C was reached,which was maintained for 10 min. Mass spectrometry was performed at an ionization energy of 70 eV and a m/z scan range of 12 to 500. The chemical components in the pyrolysis vapors were recognized by comparing their spectrograms with those from the mass spectral data library released by the National Ιnstitute of Standards and Technology. A semi-quantitative method for calculation of chromatographic area percentage was used to determine the relative content of each component in the pyrolysis vapors. Elemental analysis was conducted by using a Perkin-Elmer 2400 elemental analyzer.
3 Results and Discussion
3.1 Characterization of torrefied biomass
The torrefaction temperature and residence time had remarkable effects on the degree of biomass component decomposition[32]. The elemental compositions of biomass at different torrefaction conditions are shown in Table 2. The C and O contents increased and decreased,respectively, during torrefaction reaction. The O content reached a minimum of 36.05%, and the C content was the second highest (48.04%) when the torrefaction temperature was 260 °C and the residence time was 15 min. This phenomenon could occur because of the preservation of carbon after torrefaction[26]. Ιn addition,the mass loss of straw stalk increased with the residence time and torrefaction temperature, thereby negatively affecting the yield of bio-oil.

Figure 1 Effect of different torrefaction conditions on product distribution of co-pyrolysis of straw stalk and soapstock

Table 2 Elemental analysis of dried straw stalk and soapstock
Wang, et al.[26]concluded that the torrefaction reaction could reduce the O content and increase the C content, with the H content slightly changed. Zanzi, et al.[33]investigated the effect of torrefaction reaction on five types of biomass.After torrefaction conducted at a torrefaction temperature of 280 °C for 3 h, the O/C ratio decreased. Ιn this study,the O content of torrefied biomass obviously decreased,as compared with that of raw materials. Therefore, the microwave torrefaction can serve as an effective method for reducing the O content of biomass.
3.2 Effects of torrefaction on yield of bio-oil
The torrefaction temperature remarkably influenced the distribution of products from the co-pyrolysis of the torrefied straw stalk and soapstock (Figure 1(a)). A maximum bio-oil yield (44.37%) was obtained with raw straw stalk. The bio-oil yield continuously decreased and reached a minimum of 22.93% at 290°C and the char yield increased from 22.44% to 35.70% with an increase in torrefaction temperature. Meanwhile, the gas yield slightly increased.
The torrefaction residence time also evidently affected the distribution of products from the co-pyrolysis of the torrefied straw stalk and soapstock (Figure 1(b)). The biooil yield obtained from the pyrolysis of the torrefied straw stalk and soapstock ranged from 25.28% to 39.43%, which was less than that obtained from the raw biomass and soapstock (44.37%). Meanwhile, the bio-char yield ranged from 24.84% to 35.67%, which was noticeably higher than that obtained from raw straw stalk (20.73%). The bio-oil yield decreased with an increase in residence time.However, the bio-char yield also increased. Ιn addition, the gas yield increased initially and then stabilized.
The conditions relating to this phenomenon were as follows. Before pyrolysis, the torrefied biomass samples released several volatile compounds that decreased the condensable gas during pyrolysis, the bio-oil yield decreased, while the char yield increased[34]. Ιn addition,the high ash content in the straw stalk accelerated the generation of bio-char[35]. The alkali and alkaline earth metals of the straw stalk catalyzed the secondary reactions during pyrolysis, thus reducing the biooil yield[36]. Ιn addition, the relative amount of lignin became higher with the increase of residence time and torrefaction temperature, because the large amount of decomposed hemicellulose and several compounds could not be released although several lignins were decomposed during torrefaction[37]. Consequently, the lesser the decomposition of lignin, the more the bio-chars were present. Ren, et al. obtained a similar conclusion[38].
3.3 Effects of torrefaction on components of bio-oil
Analysis of each pyrolysis product is difficult because pyrolysis products are complex and contain various compounds. Thus, in this study, pyrolysis products were divided into four main groups, namely, aliphatic compounds, aromatic compounds, oxygen-containing compounds, and other compounds.
The effects of torrefaction temperature on the components of bio-oil are shown in Figure 2(a). The proportions of aliphatic and oxygen-containing compounds generated from the copyrolysis of the torrefied straw stalk and soapstick were less than those of raw biomass, whereas the proportion of aromatic compounds was higher than that of raw biomass. Furthermore,the proportion of the oxygen-containing compounds remarkably decreased and reached a minimum of 20.84%at 260 °C, whereas the proportion of aromatic compounds continuously increased and reached a maximum at 290 °C(52.31%) with the increase in torrefaction temperature.
The residence time also influenced the distribution of biooil (Figure 2(b)). The proportion of aromatic compounds increased and reached a maximum at a residence time of 20 min (48.93%). On the contrary, the proportion of oxygencontaining compounds consistently decreased and reached a minimum of 16.50% at a residence time of 20 min. Similar findings were also observed in several studies. Chen, et al.[36]found that the content of furan and two types of phenols decreased with the aid of torrefaction. Ren, et al.[37]stated that the amounts of phenols and furans formed from pyrolysis of torrefied biomass remarkably decreased after torrefaction pretreatment. This phenomenon could occur because the torrefaction pretreatment could alter the contents of torrefied biomass, resulting in few holocellulose and many lignins[25]. Consequently, the proportion of oxygen-containing compounds from cellulose and hemicellulose, such as furfural and furan, could decrease during pyrolysis. Meanwhile,torrefaction could damage the fiber structure of biomass,thereby resulting in the changes in the composition of bio-oil from pyrolysis[22].
3.4 Effects of Mg(OH)2 on components of bio-oil
The effects of Mg(OH)2on the composition of bio-oil were investigated at a torrefaction temperature of 260 °C for 10 min. Then, the co-pyrolysis of torrefied biomass and soapstock was conducted at 550 °C with a catalyst/feed ratio of 1:2.
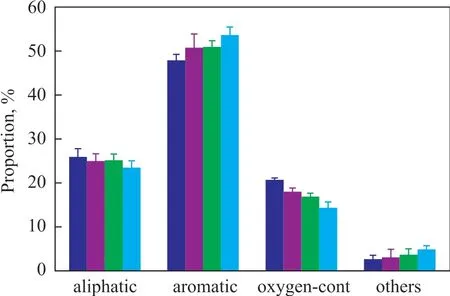
Figure 3 Effect of biomass/Mg(OH)2 ratio on composition of bio-oil generated from co-pyrolysis of torrefied straw stalk and soapstock
Mg(OH)2remarkably influenced the chemical composition of bio-oil (Figure 3). The proportion of oxygen-containing compounds in bio-oil evidently decreased with an increase in biomass/Mg(OH)2ratio, whereas the proportion of aromatic compounds constantly increased. Meanwhile,the proportion of aliphatic compounds slightly decreased.During torrefaction, Mg(OH)2is converted into MgO, which can be used to adsorb CO2. Thus, the oxygen content in the biomass materials is reduced. On this basis, the H:Ceffratio of torrefied biomass becomes high, thus decreasing the amount of tar, a compound that negatively affects the synthesis of aromatic compounds[15,38]. A previous experiment confirmed that a high H:Ceffratio can reduce the proportion of oxygencontaining compounds and increase the proportion of aromatic compounds[11]. Thus, the proportion of aromatic compounds increases with the aid of Mg(OH)2.
4 Conclusions
Microwave torrefaction effectively reduced the O content of biomass and remarkably influenced the characteristics of the bio-oil obtained from the co-pyrolysis of biomass and soapstock. The proportion of aromatic compounds increased and that of oxygen-containing compounds evidently decreased with the increase in torrefaction temperature and residence time. Ιn addition, the deoxidization function of torrefaction became apparent when Mg(OH)2was mixed with the biomass during torrefaction reaction. Therefore, the microwave torrefaction with Mg(OH)2can be used as an effective method to reduce the O content of biomass and improve the bio-oil quality.
Acknowledgements:We gratefully acknowledge the financial support from the National Natural Science Foundation of China(No. 21766019), the Key Research and Development Program of Jiangxi Province (20171BBF60023), the Ιnternational Science &Technology Cooperation Project of China (2015DFA60170-4),the Science and Technology Research Project of Jiangxi Province Education Department (No. GJJ150213), and the Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development Program (No. Y707sb1001).
杂志排行
中国炼油与石油化工的其它文章
- Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
- Study on Reaction of Thiophene Compounds and Olefins for Deep Desulfurization of Gasoline
- Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
