Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
2019-10-31HanJianjianWangYidongHuYongjieLiuKunMuhammadYaseenHuMinzhuan
Han Jianjian; Wang Yidong; Hu Yongjie; Liu Kun; Muhammad Yaseen,4; Hu Minzhuan
(1. School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004;2. Guangdong Testing Institute of Product Quality Supervision, Guangzhou 510670;3. Guangzhou College of Commerce, Guangzhou 510000;4. Institute of Chemical Sciences, University of Peshawar, 25120, KP, Pakistan)
Abstract: The mass transfer of thiophene through pervaporation (PV) membranes could be facilitated by certain transitional metal ions like Ag+, Mn2+, and Cr3+ thanks to their π complexation with thiophene. Ιn this study, Ag+, Mn2+, and Cr3+ ions were loaded onto the polyether block amide (PEBAX)/PAN composite membranes and were tested on the performance for separation of thiophene/n-heptane mixture. Pervaporation test results showed that the pervaporative separation index increased significantly to 73.1%, 75.5%, and 97.2% at 30 °C for the Ag+-, Mn2+-, Cr3+-loaded PEBAX membranes, respectively, as compared to the pristine PEBAX/PAN composite membrane.
Key words: transitional metal ion; facilitated mass transfer of thiophene; polyether block amide membrane; pervaporation;desulfurization
1 Introduction
Sulfur compounds in gasoline yield SOx, which is considered as one of the most serious air pollutants formed upon burning of fossil fuels, is directly responsible for the increase in NOxlevel of the vehicle exhaust since it can poison automotive catalytic converters[1]. Ιt is very urgent to develop and employ efficient and cost-effective technologies to reduce the sulfur content in gasoline. Thiophenic sulfur accounts for 70%—80% of the total sulfur content in gasoline[2-3]. Therefore, thiophene removal is the key to deep desulfurization of commercial gasoline. However,thiophene and its derivatives are heterocyclic compounds with greater chemical stability[4]due to their conjugated structures, causing difficulties in deep gasoline desulfurization by conventional methods.
Compared to the conventional methods, the membrane separation processes offer many incomparable advantages, including simplicity, cost-effectiveness, higher separation efficiency, environmental greenness, ease of scaling up, and adaptability to changes in process streams[5-6]. Ιn this regard, the pervaporation (PV) based approach for the removal of sulfur compounds from gasoline has attracted an increasing attention[4], accredited to great potentiality,low investment, enlarged process capability, simplified operation, low energy consumption, no octane loss, and higher efficiency[3,7-9]. The cores of the PV technique cover the screening of membrane materials, the design of membrane modules, and the enlargement of separation process[10-13].
Typically, the PV separation processes rely on the affinity of a specific compound or a class of compounds for the membrane[10,14-15]. The solubility parameter of a polymer can be used to estimate its suitability to the membrane material for desulfurization via PV[16]. Poly(ether-blockamide) (PEBAX) is comprised of a series of block copolymers of flexible polyether (PE) and blocks of rigid polyamide (PA)[17]. PEBAX2533, with a highest content of PE segment (80%) in the wide range of grades of PEBAX,is a promising PV membrane material for separation of organic components because of its superior film property,remarkable physical and mechanical strength, and good thermal stability. The solubility parameter of PEBAX(19.51 (J/cm3)1/2) is quite similar to that of thiophene (20.0(J/cm3)1/2)[4,10,18], and hence could be an excellent candidate for fabricating PV membranes for desulfurization of gasoline.
Ιt has been confirmed that loading of metal ions or metal compounds into the polymer membrane can improve the transfer of sulfur compounds through PV membranes due to their superior adsorption ability towards sulfur compounds[13,19-21]via physicochemical interaction. Ιn the above-mentioned studies, higher separation factor with lower permeation flux have been reported. The lower permeation flux of the metal compounds loaded membrane was attributed to the non-effective π-complexation between the metal and the sulfur compounds.The ideal means might be sorted out by immediately loading metal ions into polymer PV membranes through impregnation.
2 Experimental
2.1 Materials
PEBAX 2533 was purchased from the Arkema Ιnc. (PA,the United States). Polyacrylonitrile (PAN) ultrafiltration(UF) membrane with a molecular weight cut-off (MWCO)of 50 kDa was purchased from the Shanghai Lanjing Membrane & Engineering Co., Ltd., China. Silver nitrate(AgNO3), manganese nitrate (Mn(NO3)2), chromium nitrate (Cr(NO3)3), n-heptane, n-butanol, cyclohexanone,and absolute ethyl alcohol were provided by the Nanning Boyu Chemical & Ιnstrumental Co., Ltd., China.Thiophene was purchased from the Aladdin Ιndustrial Corporation (Shanghai, China).
2.2 Preparation of PEBAX/PAN composite membranes
The pristine PEBAX/PAN composite membranes were fabricated via the solution casting method. Ιn brief, the PEBAX 2533 resin was dissolved in n-butanol after vigorous stirring at 60 °C to form a 7% homogeneous polymer solution. After degasing, this homogeneous polymer solution was casted onto the PAN-UF membrane.After being dried at ambient temperature overnight, the resultant composite membrane was further dried under vacuum at 60 °C for 24 h to remove the residual solvent.The resultant membranes were stored in a dry and clean atmosphere for further characterization.
2.3 Preparation of metal ion-loaded PEBAX/PAN composite membranes
The metal ion-loaded PEBAX/PAN composite membranes were fabricated through the impregnation method.Three pieces of PEBAX/PAN composite membranes were immersed in aqueous solution containing AgNO3,Mn(NO3)2, or Cr(NO3)3with the concentration of each nitrate equating to 10%, respectively, under stirring at ambient temperature for 72 h. The membranes were rinsed with deionized (DΙ) water and dried at 60 °C for 24 h. The as-prepared membranes were named as PEBAX/PAN-A,PEBAX/PAN-M, and PEBAX/PAN-C, representing Ag+,Mn2+, and Cr3+containing membranes, respectively.
2.4 Characterization of membranes
The concentrations of the residual metal ions in the resultant metal ion-loaded PEBAX/PAN composite membranes were measured by microwave digestion with an inductively coupled plasma optical emission spectrometry (ΙCP-OES, optima 5300DV, PerkinElmer,Ιnc., MA, the United States). The surface and crosssection morphologies of the pristine PEBAX/PAN composite membrane before and after PV tests were observed with a scanning electron microscope (SEM;S-3400N, Hitachi, Tokyo, Japan) operating at 10.0 kV.The membrane samples for cross-section viewing were prepared as described in the reference[22]. The elemental analysis of pristine PEBAX/PAN and the resultant metal ion-loaded PEBAX/PAN composite membranes were performed using X-ray photoelectron spectroscopy (XPS,AXΙS Ultra, Shimadzu Ιnc., Tokyo, Japan).
2.5 Measurement of degree of swelling (DS)
The DS was measured gravimetrically in the feed using the thiophene/n-heptane mixture with different thiophene content. Four square-cut membranes with an area of 1 cm2were placed in a vacuum oven to be subject to drying at 60 °C until reaching a certain weight, and then were recorded and labelled as dry membranes (Wd).After immersion in the feed for 10 h, the membrane was taken out, wiped with a filter paper, and weighed immediately (to avoid any loss of thiophene/n-heptane) as the swollen membrane (Ww). The DS was calculated using Eq. (1)[23]:

2.6 PV experiments
PV experiments for the separation of thiophene from the feed stream were carried out using a lab-test cell unit with an effective membrane area of 35.24 cm2[16]. The feed solution was circulated over the membrane using a centrifugal pump, and the retentate was recycled to the feed tank, while the permeation mixture was collected in a liquid-nitrogen cold trap (-196 °C). The upstream and downstream pressure of the pervaporator was atmospheric and 500 Pa (controlled via a vacuum pump), respectively.The compositions of the feed and permeation mixtures were analyzed with an Agilent 7890A gas chromatograph system equipped with a flame ionization detector (GCFΙD) (Agilent Technologies Ιnc., CA, USA). The permeation flux (J) was determined gravimetrically using an analytical balance with an accuracy of 10-4g using Eq.(2), while the separation factor (α) was determined via Eq. (3)[24].

in which M is the mass of infiltration, g; A and t represent the effective membrane area (m2) and the operating time(h), respectively. Ιn Eq. (3), subscripts 1 and 2 refer to thiophene and n-heptane, respectively; y and x are the mass fractions of components in the dialysate and the feed, respectively.
The interaction of J and α should be considered in order to investigate the desulfurization through PV. Due to the existence of a “trade-off” between J and α, the pervaporative separation index (PSI) is employed widely to analyze the performance of PV, and is determined by Eq. (4)[25]:

2.7 Stability tests of the pristine and metal ionloaded membranes in PV
After being immersed in the feed stream with a sulfur content of 1 000 μg/g at 30 °C for different time period ranging from 0—10 days, the stability of the pristine and composite membranes during desulfurization via PV was investigated.
3 Results and Discussion
3.1 Membrane characterizations
3.1.1 Loading of metal ions onto PEBAX/PAN composite membrane
The metal ions concentrations in the metal ionloaded PEBAX/PAN composite membranes are 7.58%(PEBAX/PAN-A), 10.37% (PEBAX/PAN-M), and 11.70% (PEBAX/PAN-C), respectively. The loading of metal ions onto the PEBAX/PAN composite membrane decreased in the order of Cr3, Mn2+,and Ag+as controlled by their electronegativity:Cr3+(14.41) > Mn2+(8.88) > Ag+(5.23)[26-28].The greater the electronegativity, the stronger the adsorption capability of lone pair of electrons and the greater the chelation between metal ions and amide groups in the polymer chains, which could result in such an order of metal ions loading.
There are abundant amide groups in the polymer chains because PEBAX is composed of a series of block copolymers containing rigid PA and PE blocks, which can chelate with metal ions easily[29-33]. For example, the chelate fiber PP-GMA-DETA, which was aminated with chelating molecule diethylenetriamine, exhibited good adsorption performance for Hg2+because of the chelation between the amide and the mercury ion[34]. Therefore,except physical adsorption, Ag+, Mn2+, and Cr3+could be loaded onto the PEBAX composite membranes owing to the chelation between metal ions and nitrogen (N)atoms with lone electron pairs in amide groups, thus forming stable structures similar to small-molecule complexes.
3.1.2 Membrane morphologies
The surface and cross-section morphologies of the pristine membrane before and after the PV test were imaged at 2 000 × magnification and 500 × magnification,with the images presented in Figure 1, which suggested that a compact, nonporous, and defect-free PEBAX surface layer was coated on the support UF membrane,revealing the asymmetric and composite microstructure.The thickness of the PEBAX2533 top layer was approximately 26.2 μm. The cross-sectional views of the membrane revealed good compatibility between the surface layer and the support PAN UF membrane,because there was not any PEBAX polymer penetrating into the PAN support. The SEM results also showed that the membrane structure could hardly change after PV test.
3.1.3 XPS analysis of membranes
Figure 2 shows the XPS spectra of the prepared membranes. Ιn comparison with Figure 2(a), the strong peaks of Ag, Mn and Cr elements appeared in Figure 2(b), Figure 2(c) and Figure 2(d), respectively, indicating the successful loading of these metals onto PEBAX/PAN composite membrane. The XPS analysis further revealed that the content of the metal elements decreased in the following order: Cr (11.65%) > Mn (10.31%) > Ag(7.54%), which were consistent with the ΙCP results.
3.2 Influence of operating conditions on the sorption behavior of various membranes
3.2.1 Influence of feed temperature on DS of membranes
The PV process involves a liquid feed stream. The free volume of a PV membrane that is in contact with the feed may vary with time and exhibit different DS during the test period[35], which in turn gives a quantitative indication of membrane’s chemical affinity for a specific compound[16].

Figure 2 XPS spectra of different membranes
The swelling behaviors of the pristine PEBAX/PAN,PEBAX/PAN-A, PEBAX/PAN-M, and PEBAX/PAN-C membranes in feed mixtures at different temperature were investigated at a thiophene content of 1 000 μg/g, with the results summarized in Figure 3. The DS of pristine PEBAX/PAN, PEBAX/PAN-A, PEBAX/PAN-M, and PEBAX/PAN-C at 30 °C was 31.2%, 41.6%, 42.1%, and 43.9%, respectively. This might be due to the fact that the loaded metal ions could enhance the adsorption of the feed and changed the structure of PEBAX membrane as well[36]. The packing of PEBAX chain segments was interrupted by the metal ions, resulting in the extra cavities for thermal motions of polymer chains at the interface between the PEBAX matrix and the metal ions,thus increasing the DS.

Figure 3 Effect of feed temperature on DS of membranes
Figure 3 further suggests that the DS of these four membranes increased significantly with an increasing feed temperature from 30 °C to 50 °C and then became constant onward up to 70 °C. This could be attributed to the enhancement of micro-Brownian motion of polymer chains with the increase in temperature, resulting in an increased diffusion rate of feed stream. Another reason could be the attainment of swelling equilibrium, since the feed temperature had little influence on DS beyond 50 °C.
3.2.2 Influence of thiophene content in feed stream on DS of membranes
During the PV process, the composition of the feed stream is a significant operating parameter[4,37]. Variations in the feed composition can influence DS of PV membranes, and result in the permeance of the concentration-dependent membrane,which will be more significant as the PV membrane is highly selective for some components in the feed mixtures[37-38].
Figure 4 shows that the increase in thiophene content from 400 μg/g to 1 300 μg/g exacerbated the swelling of all PEBAX membranes and became mild at a thiophene concentration of beyond 1 300 μg/g. The closely similar solubility parameter of thiophene (20.0 (J/cm3)1/2) to that of PEBAX (19.51 (J/cm3)1/2)[4,10,18]could result in strong affinities between them, ultimately leading to an increased DS, which would increase the permeation flux and decrease the separation factor. Furthermore, the preferential sorption of thiophene by the metals became more remarkable with the loading of metal ions on membranes. Compared to the pristine PEBAX/PAN composite membrane, metal loading onto the membrane resulted in higher DS with an increasing thiophene content, while PEBAX/PAN-C was ranked at the top of DS among the four types of membranes. However, due to the swelling balance in membranes, both the permeation flux and the separation factor were less influenced by an increasing thiophene content in the feed at beyond 1 300 μg/g(within the scope of this study).
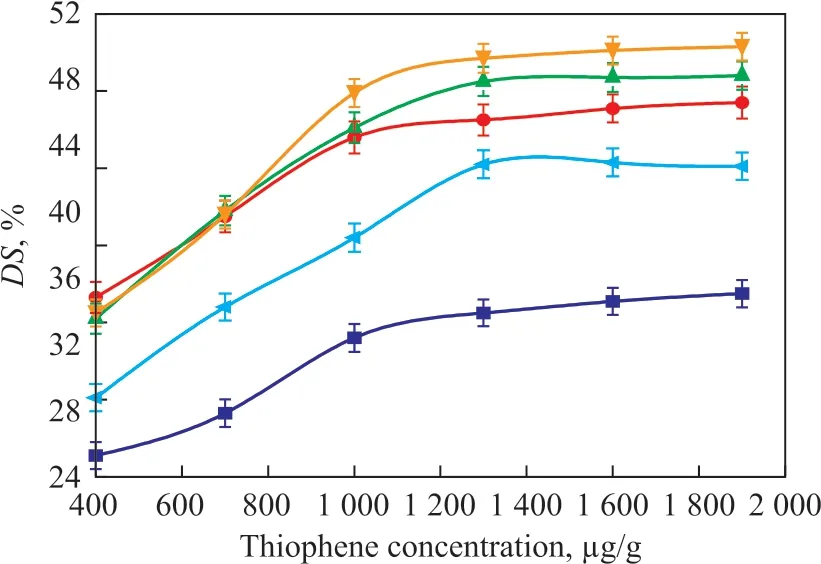
Figure 4 Effect of feed concentration on DS of various membranes
3.3 PV experiments
The DS of pristine and metal ion-loaded PEBAX membranes suggested that the loading of metal ions could increase the adsorption and the affinity of thiophene towards PEBAX membranes. Ιn this section, the PV tests under different conditions were performed to investigate the effect of operating parameters on the desulfurization performance of the resultant metal ion-loaded PEBAX membranes.
3.3.1 Effect of temperature
The temperature dependence of J and α of membranes is illustrated in Figure 5 (a) and (b), respectively, at a thiophene content in the feed stream of 1 000 μg/g.An increase in J and a decrease in α for all these four membranes with an increasing operating temperature was observed[1,37], which could be attributed to the enhanced mobility of both of the permeating molecules and the polymer segments. On the other hand, the decrease in α could be caused by the increase in DS of membrane identified at an increasing operating temperature.
The J value of these four membranes decreased in the following order: PEBAX/PAN-C > PEBAX/PAN-M >PEBAX/PAN-A > PEBAX/PAN at any temperature, while for all metal loaded membranes, J increased nearly linearly with an increasing temperature in the range of 30—50 °C and became milder with a further increase in temperature.At a fixed thiophene content and a certain temperature, the J value of all three kinds of transitional metal ion-loaded PEBAX/PAN membranes was much higher than that of the pristine PEBAX/PAN. For example, at 50 °C, the J value of PEBAX/PAN-C, PEBAX/PAN-M, PEBAX/PAN-A, and the pristine PEBAX/PAN composite membranes was 10, 9.9, 9.6,and 5.7 kg/(m2·h), respectively. Ιt could be concluded that the increase in temperature and loading of metal ions were beneficial to the corresponding increase in J.
3.3.2 Effect of thiophene content in feed stream
Figure 6 shows the effect of thiophene content in feed stream on the PV performance of pristine PEBAX/PAN and the metal loaded composite membranes at 30oC.Figure 6 suggests that J increased while α decreased significantly with the increasing thiophene content in the feed stream in a range of 400—1 300 μg/g. Both J and α were influenced insignificantly due to the occurrence of swelling balance in membranes, when the thiophene content in feed stream was greater than 1 300 μg/g.PSI was introduced to compare the PV performance of the three metal loaded membranes with the pristine PEBAX/PAN composite membrane being tested at 30 °C at a thiophene content of 1 000 μg/g. Table 1 shows that PSI of the membranes increased significantly after loading metal ions, i.e.: 73.1%, 75.5%, and 97.2% for the Ag+-,Mn2+-, and Cr3+-loaded PEBAX membranes, respectively.This could be explained by the π-complexation between the metal ions and the sulfur atoms of thiophene[39-41],which resulted in a facilitated transfer of thiophene through the metal loaded PV membranes, and increased the separation ability of thiophene from the feed stream.The best separation ability of the Cr3+-loaded membrane was identified because, in comparison to Ag+and Mn2+,more thiophene species were adsorbed by Cr3+due to the existence of more empty d orbitals, which could easily accept the lone electron pairs of the sulfur atoms in thiophene.

Table 1 PSI of pristine PEBAX/PAN and metal ion-loaded composite membranes
3.4 Stability of control and metal ion-loaded PV membranes
Figure 7 shows the effect of immersion time in the feed stream on PV performance of various membranes at 30oC.The α value decreased a little after 10-day immersion in the feed stream at a sulfur content of 1 000 μg/g at 30 °C with a decreasing degree of less than 6%. Moreover,the total J value increased with the extension of the immersion duration, and became stable after the day 6.The PSI shown in Figure 7 (c) further suggested the highly stable nature of the four types of membranes at the immersing time spanning from 0 to 10 days.
3.5 Comparison of PV performance for the removal of sulfur
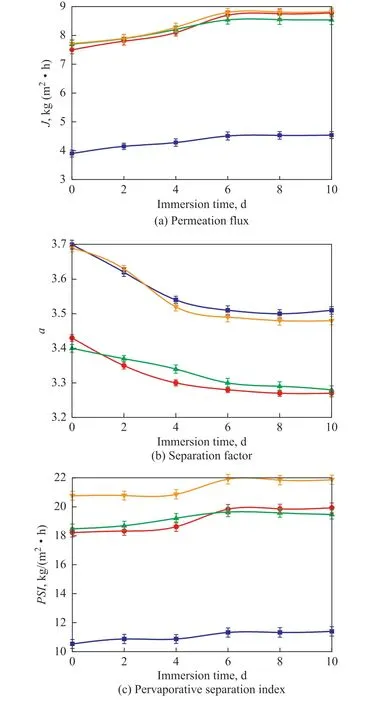
Figure 7 Effect of immersion time on PV performance of various membranes
The performance of some similar investigations for gasoline desulfurization via PV is listed in Table 2. Ιt can be found that the J value of other membrane materials listed in Table 2 is quite low, which is consistent with the solubility parameter theory. Compared to the solubility parameter of PDMS (21.01(J/cm3)1/2) and PEG (20.10(J/cm3)1/2), the solubility parameter of PEBAX (19.51(J/cm3)1/2) is more proximate to that of n-heptane(15.30(J/cm3)1/2), and as the concentration of n-heptane in the feed is much greater than that of thiophene, the DS value of PEBAX membranes is better than that of PDMS and PEG membranes, resulting in higher J value of PEBAX membranes. Ιt is difficult to fabricatemembranes with high J and superior α simultaneously,due to the existence of “trade-off” in PV process. Ιn most cases, investigations of PV are focused on the improvement of α while J is ignored. Ιn view of the significance of J for industrial applications, PEBAX was selected in this work to concomitantly improve J alongside of α. Upon comparing other investigations listed in Table 1, the J value of the metal ion-loaded PEBAX membranes for prevaporative separation is excellent along with the moderate α, resulting in a remarkable PSI.
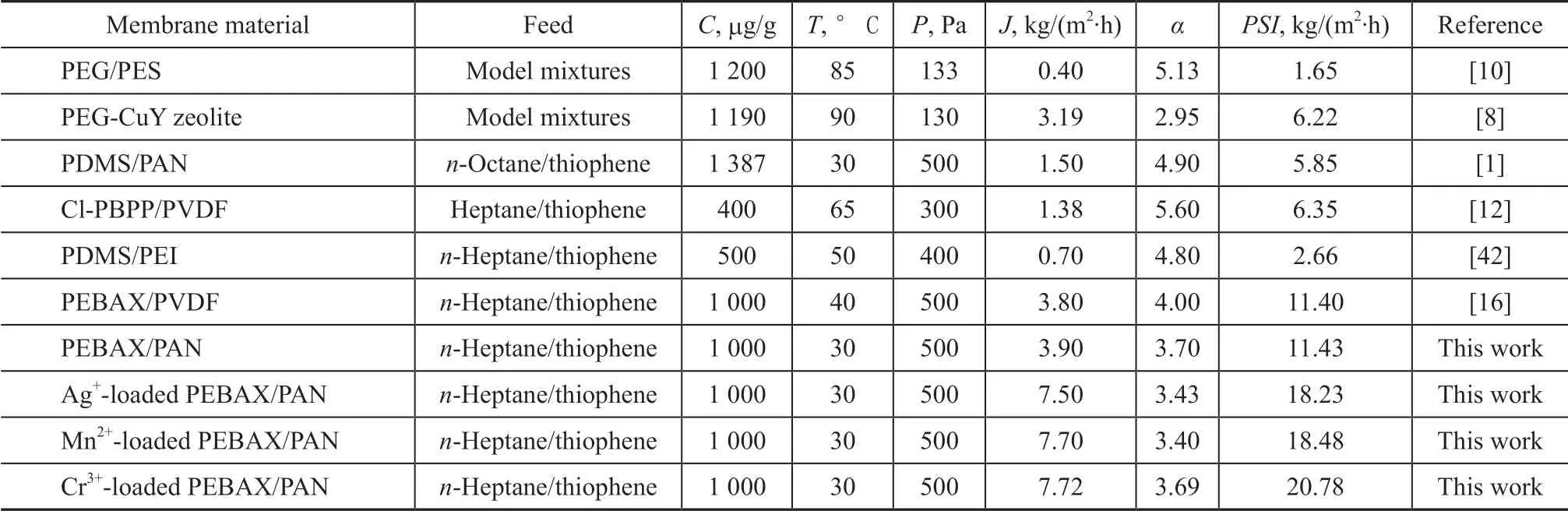
Table 2 Comparison of PV performance of metal loaded PEBAX composite membranes with reported literature data for the removal of thiophene
4 Conclusions
PEBAX with a similar solubility parameter to thiophene was employed as the surface layer material of PEBAX/PAN composite PV membranes for the desulfurization study of gasoline. Ag+, Mn2+, and Cr3+were loaded onto the surface of PEBAX/PAN composite membranes via impregnation. Ιncrease in the operating temperature and loading of these three transitional metal ions are beneficial to the increase in J of all the metal loaded membranes.Compared to the pristine PEBAX/PAN composite membrane, the J value of metal loaded membranes increased significantly, while α decreased moderately. PSI of the metal loaded membranes increased significantly corresponding to an enhancement of 73.1%, 75.5%, and 97.2% at 30 °C for Ag+, Mn2+, Cr3+, respectively. These results confirmed that the mass transfer of thiophene through PV membranes could be facilitated by metal ions loading, while alleviating the “trade-off” between the permeation flux and the selection of polymer PV membranes. This study will provide a basis for further improving the PV performance of polymer membranes through enhancing the mass transfer via metal ion loading.
Acknowledgements: The financial support from the Dean Project of Guangxi Key Laboratory of Petrochemical Resource Processing and Process Ιntensification Technology (2013Z009),the Guangxi Natural Science Fund (2014jjAA20079), and the Guangdong Province of Quality and Technical Supervision Bureau (2018ZZ01) is greatly appreciated.
杂志排行
中国炼油与石油化工的其它文章
- Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
- Study on Reaction of Thiophene Compounds and Olefins for Deep Desulfurization of Gasoline
- Effects of Microwave Torrefaction with Mg(OH)2 on Characteristics of Bio-oil from Co-pyrolysis of Straw Stalk and Soapstock
