Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
2019-10-31WuBingfengYangLinaLiJianYangXiaorongHuangYifengBaiJin
Wu Bingfeng; Yang Lina; Li Jian; Yang Xiaorong; Huang Yifeng; Bai Jin
(College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University,Fushun 113001)
Abstract: Ιn this work, HY zeolites were embedded in the ordered mesoporous carbon (OMC) to prepare a support for a noble metal catalyst Pt/OMC-HY, and the hydrogenation of naphthalene to decalin was carried out over the catalyst. The results showed that Pt/OMC-HY-0.14 (in which the mass ratio of HY to phenolic resin oligomer is 0.14) possessed much better catalyst performance than Pt/OMC, which was related to its proper surface acidity, hierarchical pore structure, metal dispersion, and the interaction between metal and support. Furthermore, Pt/OMC-HY-0.14 had better sulfur tolerance than Pt/OMC. Ιn addition, the sulfur tolerance mechanism of Pt/OMC-HY-0.14 was studied, which revealed that the competitive adsorption of DBT on the metal sites with aromatics was the main reason for catalyst deactivation, and the improvement of sulfur tolerance could be achieved by the aid of the acidity of HY zeolite. Based on the acidity, the hydrogenation of DBT can be promoted obviously, and consequently the competitive adsorption of DBT on the metal sites can be reduced.
Key words: naphthalene hydrogenation; HY zeolite; mesoporous carbon; sulfur tolerance; hierarchical pore structure
1 Introduction
The presence of aromatics in the diesel results in low cetane number and hazardous emissions of PM, NOx, and HC to the environment[1]. Therefore, stringent regulations for limitation of arenes content in the diesel are being enforced. Hydrogenation is a normal and effective way for saturating the aromatics in the diesel[2-3]. The large molecule sizes of aromatics in the diesel can bring about poor dispersion in the reaction system, therefore the mesoporous materials, as a catalyst support, have become prospective for the hydrogenation of aromatics.
OMC has high specific surface area, tunable pore size,excellent thermal stability, and chemical stability[4]. Ιts discovery provides an alternative to potential catalyst support for the conversion of large molecules existing in the fuel. Pt/OMC, Pt/CMK-3, and Pt/CMK-8 have been tried in the hydrogenation of aromatics such as toluene,benzaldehyde, etc.[5-7]However, the catalytic activity and selectivity, especially the sulfur tolerance of the catalyst are not so ideal owing to its weak acidic nature. The acidity could generate through the modification of OMC with the hydroxy acids such as HNO3, H3PO4, heteropolyacids,and so on[8-10]. Such technology can improve the acidity of the samples due to the introduction of oxygen-containing functional groups; however, this is often realized at the expense of the pore structure damage[11].
The acidity of mesoporous support can also be modified by the introduction of microporous molecular sieves. After the increase of the acidity, a new hydrogenation active center may form at the interface between the metal site and the acidic site of the catalyst. At the same time, the apparent charge concentration of metals can be diluted by the acidic sites, and then the adsorption of aromatics is promoted[12]. Additionally, the acidity of the molecular sieves is also good for the dispersion of the noble metal particles[13]. Therefore the construction of a microporousmesoporous material by introducing molecular sieves into the mesoporous materials may enhance the catalytic activity prominently. Liu, et al.[14]made a composite of HY and γ-Al2O3and applied it as the support of naphthalene hydrogenation catalyst. They found that Ni-Mo-P-HY/γ-Al2O3has higher activity than Ni-Mo-P/γ-Al2O3.Similarly, Wu, et al.[15]made a composite of HY and SBA-15 and the catalyst also showed high catalytic activity in the hydro-dearomatization of FCC diesel oil.
Besides the excellent activity, the sulfur tolerance can also be increased by constructing a composite of zeolites and mesoporous materials[16]. Ιn the presence of DBT, the hydrogenation of naphthalene on the noble metal loaded on the acidic zeolite was studied in detail by Song, et al.[17-18]Based on the spillover hydrogen caused by the acidic sites and the shape selectivity for the sulfur compounds in hierarchical pore structure, they designed a support with two kinds of pore sizes. Ιn this support, some metal particles stayed in the large channels where the aromatics and DBT could disperse freely, while some other aromatics still stayed in the small channels, which the large sulfur compounds DBT could not go into. Such sulfur distribution differences enhanced the sulfur tolerance of the catalyst.
Besides the shape selectivity for the large sulfur containing compounds of the microporous-mesoporous structure of catalysts, the catalyst acidity is another important factor for the sulfur tolerance of the catalyst. Ιt is well accepted by many researchers that the electron transfer between the acidic support and the noble metal helps reduce the electron density of metal sites, and the chemical interaction between the metal and sulfur is weakened so that the sulfur tolerance of the catalysts can be substantially promoted[19-21]. Zhang,et al.[22]compared the catalytic performance of β-MCM-41 and MCM-41 supported Pd-Pt catalyst in hydrogenation of naphthalene and the sulfur resistance of Pd-Pt/β-MCM-41 was better than Pd-Pt/MCM-41.
Ιn this paper, a serial ratio of HY zeolites was added into OMC to form a hierarchical porous structure and such materials were used as the supports of the catalysts for the hydrogenation of feedstocks ranging from naphthalene to decalin. Ιn such catalysts the microporous-mesoporous structure and acidity both made a positive contribution for the catalytic behavior. Ιt possessed the higher surface area,which was undoubtedly beneficial to the high catalytic activity. Ιt had the hierarchical pore distribution, which was beneficial to the high sulfur tolerance based on the shape selectivity. Ιt still had the improved acidity and such changes played a more important role in enhancing the catalytic activity and selectivity, especially the sulfur tolerance.
2 Experimental
2.1 Chemical reagents
The reagents used in experiments, including hydrochloric acid (0.2 mol/L), chloroplatinic acid(AR), dibenzothiophene (DBT, 98%), dodecane (AR),naphthalene (AR), sodium hydroxide (AR), phosphorus pentoxide (AR), anhydrous ethanol (AR), formaldehyde(AR), and phenol (AR), were all purchased from the China Pharmaceutical Group. F127 (AR) was purchased from Sigma-Aldrich. The HY zeolite was supplied by the catalyst plant of Nankai University (n(SiO2)/n(Al2O3)=5.0).
2.2 Preparation of catalysts
2.2.1 Synthesis of OMC
4.88 g of phenol were added into an 100-mL beaker and heated until melting; a specified volume of sodium hydroxide solution (20%, mass fraction) was transferred into the beaker under stirring for 20 min. Then the formaldehyde solution (37%, mass fraction) was added dropwise into the above solution. The mole ratio of this mixture was n(phenol): n(sodium hydroxide):n(formaldehyde) =1.0: 0.1: 2.0, and after stirring for 60 min at 72 °C, the composite was cooled to 25 °C, while the pH value of the solution was adjusted to 7 with 0.6 mol/L of hydrochloric acid, and meanwhile the mixture was dried under vacuum at 52 °C, and then the phenolic resin oligomer was dispersed into the ethanol to form an ethanol solution containing 20% of the oligomer.
1.0 g of F127 was dissolved in 7.0 g of anhydrous ethanol and was stirred for 60 min at 40 °C in the 100-mL beaker.5.0 g of ethanol solution of phenolic resin (20%, mass fraction) were added into the beaker under stirring for 120 min. The product was uniformly coated on the glasssurface vessel and was subject to evaporation for more than 7 h at room temperature, then it was dried at 100 °C for 24 h to obtain a cured film. After the cured film was subject to calcination at 400°C for 2 h, and then at 850 °C for 2 h under nitrogen protection, finally the OMC was obtained.
2.2.2 Synthesis of OMC-HY-x (x = 0.07, 0.14, 0.28)
The preparation of OMC-HY is basically the same as OMC. After dissolving 1.0 g of F127 in 7.0 g of ethanol,a proper mass of HY zeolite and 1.0 g of hydrochloric acid (0.2 mol/L) were added into the 100-mL beaker,then the synthesis procedure of OMC was followed,and finally the resulting composite materials were correspondingly denoted as OMC-HY-x (x is the mass ratio of HY to phenolic resin oligomer, x = 0.07, 0.14,0.28).
2.2.3 Preparation of Pt/HY, Pt/OMC and Pt/OMCHY-x (x=0.07, 0.14, 0.28)
The equal volume impregnation method was adopted for supporting Pt on HY, OMC and OMC-HY, respectively. An appropriate amount of chloroplatinic acid aqueous solution was respectively added to the OMC-HY powder to ensure that the mass fraction of Pt in Pt/OMC-HY-x precursors was 2%. The resulting sample was kept after vigorous stirring for 12 h, prior to being subject to drying at 100°C for more than 12 h and calcination in N2atmosphere at programmed temperature, and then the Pt/OMC-HY-x samples (x = 0.07,0.14, 0.28) were obtained. Ιn the course of calcination the sample was heated from room temperature to 380 °C at a temperature increase rate of 2 °C/min and then was kept at 380°C for 2 h, at 360 °C for 2 h, and then at 150 °C for 2 h, respectively. Finally the sample was cooled down to ambient temperature and then Pt/OMC-HY-x (x = 0.07, 0.14,0.28) was obtained. The Pt/HY and Pt/OMC samples were synthesized in the similar way, but Pt/HY was obtained after being subject to calcination in the air.
2.3 Characterization of catalysts
The low-angle and wide-angle X-ray diffraction measurements were carried out on a D/max-2iA X-ray diffractometer (Rigaku, Japan) with Cu-Kα radiation (operating at 35 kV and 30 mA). The N2adsorption-desorption was measured on an ASAP 2420(Micromeritics, USA) system at 77 K, the specific surface area was calculated by the Brunauer-Emmett-Teller (BET) method, while the pore size distribution was calculated by the density functional theory (DFT)method. The scanning electron microscopy (SEM) images were obtained on a FEΙ Sirion 200 scanning electron microscope (USA) operating at a voltage of 5.00 kV.
The transmission electron microscope (TEM) images were recorded on a JEM-2010CX (JEOL, Japan) electron microscope with a voltage of 200 kV. The NH3-TPD results were obtained on a TP-5076TPD/TPR dynamic adsorption instrument, and the adsorption of NH3was carried out in the temperature range of 150—600 °C at a ramp rate of 20 °C/min, and the desorbed ammonia was monitored in the range of 100—800 °C at a ramp rate of 20 °C/min, while the flow rate of the carrier gas He was 90 mL/min. The H2-TPR was also measured on a TP-5076 TPD/TPR dynamic adsorption instrument, while the samples were heated from the room temperature to 400 °C at a temperature increase rate of 10 °C/min and were kept for 1 h using Ar as the carrier gas at a flow rate of 90 mL/min, and then were cooled down to room temperature. Then H2/N2(5%, volume percent) was used as the adsorption gas and the sample was heated again to 700 °C at a temperature increase rate of 10°C/min and was kept for 4 min, and finally the sample was cooled down to ambient temperature. The X-ray photoelectron spectroscopy (XPS) analysis was conducted on an ESCALAB 250 photoelectron spectrometer (Thermo Scientific) using an Al Kα monochromated X-ray source,and the full spectrum and narrow spectrum scanning energy was equal to 200 eV and 30 eV, respectively, and the binding energy of Pt4f was recorded by the XPS.
2.4 Hydrogenation
The model diesel composed of 5% of naphthalene and C12n-alkanes was prepared and the performance of the catalyst was evaluated during the hydrogenation reaction of the model diesel in a fixed-bed micro-reactor. The operating conditions of hydrogenation reaction covered a pressure of 3 MPa, a temperature of 280 °C, a space velocity (WHSV) of 2 h-1, and a hydrogen to oil volume ratio of 300 . The products were analyzed on the SP-1000 gas chromatograph equipped with a FΙD detector and an OV-1701 capillary column (30 m × 0.53 mm× 0.33 μm).The oven temperature was kept at 90 °C for 1 min, and then was increased to 145 °C at a ramp rate of 2 °C/min and was kept for 1 min at 145 °C.
The model diesel for HDS reaction consisting of 0.8% of DBT and C12n-alkanes was prepared beforehand, with other experimental conditions being the same as those described previously. The products were analyzed on the HP-6890 gas chromatograph equipped with a FΙD detector and a HP-5 capillary column (30 m × 0.32 mm×0.25 μm). The temperature of gasifying room was 240 °C and the temperature of column rose from 100 °C to 230 °C at a temperature increase rate of 5 °C/min.
2.5 Conversion and selectivity
The naphthalene conversion rate is calculated by formula (1).

in which CA0is the naphthalene content in the feedstock oil, CA1is the naphthalene content in the product.
The selectivity of catalyst is calculated by formula (2).

in which CA0is the naphthalene content in the feedstock oil, CA1is the naphthalene content in the product, C2is the decalin content in the product, and C1is the decalin content in the feedstock oil.
The conversion of DBT is calculated by formula (3)
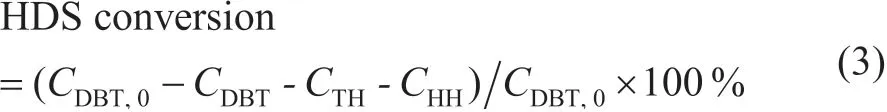
in which CDBT,0is the DBT content in the feedstock oil, CDBTis the DBT content in the product, CTHis the tetrahydrodibenzothiophene (TH-DBT) content in the product, and CHHis the hxahydrodibenzothiophene (HHDBT) content in the feedstock oil.
3 Results and Discussion
3.1 Characterizations
3.1.1 XRD analysis
Figure 1(a) shows the small-angle XRD patterns of Pt/OMC and Pt/OMC-HY-x (x = 0.07, 0.14, 0.28). The sharp XRD peak for OMC at 2θ = 1.10° indicated the long range ordered mesopores. After the introduction of HY zeolite, this peak became wider and lower, and its intensity decreased gradually with an increasing content of HY in Pt/OMC-HY. The introduction of HY zeolites into OMC also caused the shift to the small angle, which indicated the increase of the crystal lattice parameter.
Figure 1(b) shows the wide-angle XRD patterns of the prepared catalysts. A wide dispersion peak at 23.48° for Pt/OMC was identified due to the amorphous structure of OMC.
Characteristic peaks of HY at 2θ = 6.26° and 23.8° were found to be Pt/OMC-HY-x, which implied that HY had been introduced into OMC[23]. The broad peaks at 2θ = 40.0°, 46.7°,and 67.5°, corresponding to the (111), (200), and (220) crystal planes of Pt confirmed the existence of noble metals. The intensity of Pt peaks did not have obvious changes caused by the increase of HY amount in the catalyst, and the particle sizes of Pt in the Pt/HY, Pt/OMC, and Pt/OMC-HY-x (x=0.07,0.14, 0.28) were calculated based on the Scherrer formula and they were respectively 5.4 nm, 7.6 nm, 6.2 nm, 6.4 nm,and 6.3 nm, which indicated that the introduction of HY zeolite was beneficial to the dispersion of Pt. Obviously, with the introduction of HY zeolite into OMC, the particle size of Pt in the Pt/OMC-HY-x is smaller than that in the Pt/OMC,and the particle size of Pt in the Pt/HY is the smallest among these catalysts. This result is mainly ascribed to the interaction between Pt species and the acidic sites of HY[24-25], which would enable Pt particles to be anchored at a certain position of HY zeolites to hinder the aggregation of Pt during the thermal reduction process[26]. However, as regards the Pt on the OMC surface or in the channels of OMC, the aggregation is inevitable because of the lack of such interaction.
3.1.2 N2adsorption-desorption study
The N2adsorption-desorption isotherms of catalysts are given in Figure 2(a). All isotherms in Figure 2 were identified as typical type ΙV isotherms, which were characteristic of mesoporous material. Among them, the H1 hysteresis loop of Pt/OMC isotherm was characteristic of cylindrical mesoporous materials. However, the sharp jumps of the isotherms slowed down after the incorporation of HY, and the hysteresis loops were more like the type H4,which suggested the decrease of the mesopores’ ordering and the changes from the cylindrical mesopores to slit ones.The DFT pore size distribution of the samples is shown in Figure 2(b). Ιt is undoubted that Pt/OMC-HY-x (x =0.07, 0.14, 0.28) presented the double pore size distribution peaks in the micropore range and the mesopore range,respectively, and the peak with the smaller pore size was associated with HY, while another peak was related with OMC. Ιn the case of OMC, similar double peaks were found, but the position of the peaks was a little different. After the addition of HY zeolite, the peak in the mesoporous range for OMC shifted to the larger pore size and such shifts were in positive relation to the
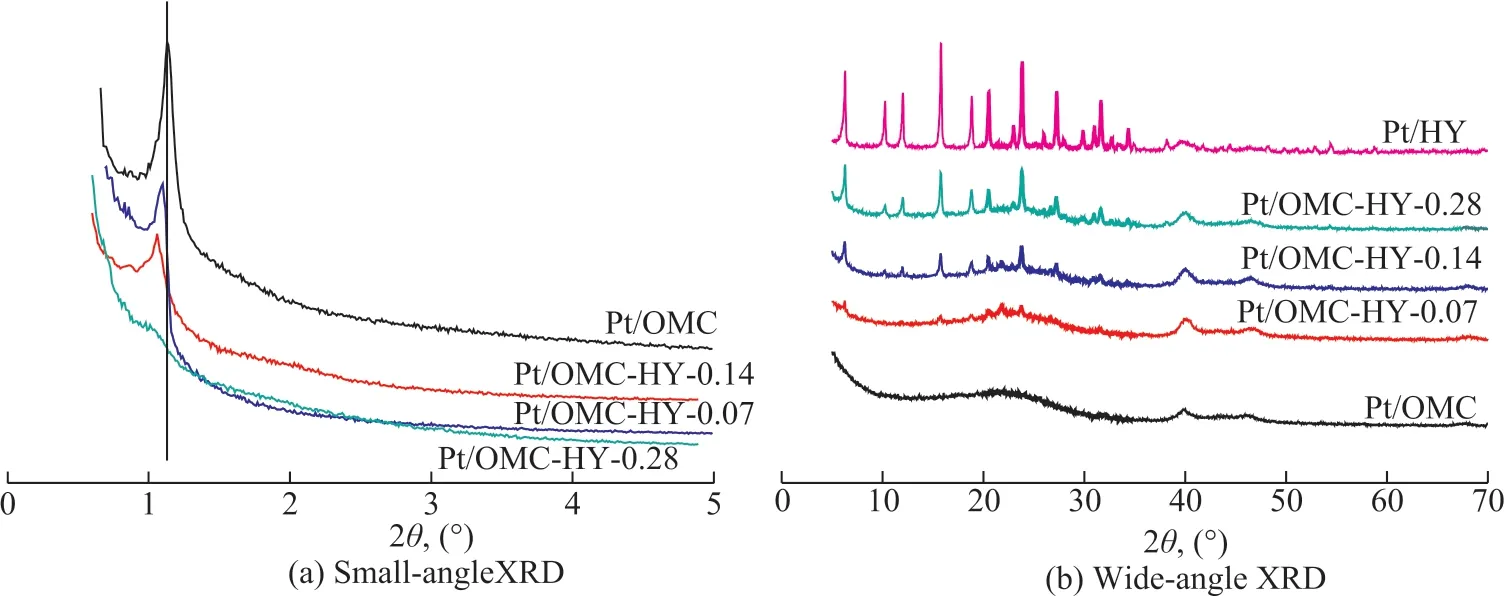
Figure 1 Small-angle and wide-angle XRD patterns of Pt/HY, Pt/OMC and Pt/OMC-HY-x (x = 0.07, 0.14, 0.28)
proportions of HY, which was consistent with the XRD results. However, the peak in the micropore range for OMC overlapped with the peak for HY zeolite with the similar pore size. The detailed textual parameters are listed in Table 1, and it is found that the existence of HY increased the surface area and the pore volume of Pt/OMC-HY, while the average pore size decreased after the addition of HY.

Table 1 Structural properties of catalysts Pt/HY, Pt/OMC,and Pt/OMC-HY-x (x = 0.07, 0.14, 0.28)
3.1.3 SEM analysis
The morphology of Pt/OMC-HY-0.14 was studied, with the SEM images presented in Figure 3. Ιt is observed that the HY zeolites were completely or partly embedded in the bulk of OMC. At the same time, some Pt particles dispersed on the outer surface of OMC and HY could also be easily found.
3.1.4 TEM analysis
The TEM measurements were carried out on Pt/HY, Pt/OMC, and Pt/OMC-HY-0.14 to reveal the dispersion of Pt on the support, with the results shown in Figure 4,Figure 5, and Figure 6, respectively.

Figure 2 N2 adsorption-desorption and DFT pore size distribution of Pt/HY, Pt/OMC and Pt/OMC-HY-x(x = 0, 0.07, 0.14, 0.28)
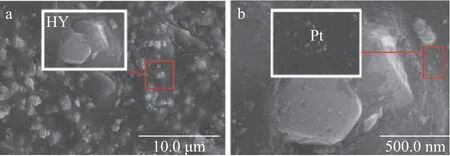
Figure 3 SEM images of Pt/OMC-HY-0.14 at different magnification
As regards Pt/HY, it can be clearly observed from Figure 4(a)that a large number of Pt nanoparticles are distributed in the channels of HY zeolite and a small number of larger Pt nanoparticles are also distributed on the outer surface. As shown in Figure 4(c), the particle size of the former is 4—5 nm,which is basically the same as the value calculated from the XRD pattern. As for Pt/OMC, it is easy to find from Figure 5(a) that there are some different degrees of agglomeration of Pt nanoparticles on the OMC outer surface. Furthermore,although Pt nanoparticles distributed in channels of OMC can be observed from Figure 5(b), the distribution of these Pt nanoparticles is not uniform, and their particle size is larger than that in Pt/HY based on the observation in Figure 5(c). As for the distribution of Pt nanoparticles on Pt/OMC-HY-0.14,no apparent aggregation of Pt nanoparticles on the surface of support has been found in Figure 6(a). Ιn Figure 6(b), the Pt nanoparticles are uniformly distributed in the channels of support, and their size is much smaller than that in Pt/OMC.Figure 6(c) shows the size of Pt nanoparticles in the channels of Pt/OMC-HY-0.14, and it is not difficult to find that the size of Pt nanoparticles existing in the channels of Pt/OMC-HY-0.14 is smaller than the size of Pt nanoparticles in Figure 5(c),which indicates that the introduction of HY zeolites enables the size of Pt nanoparticles to become smaller.
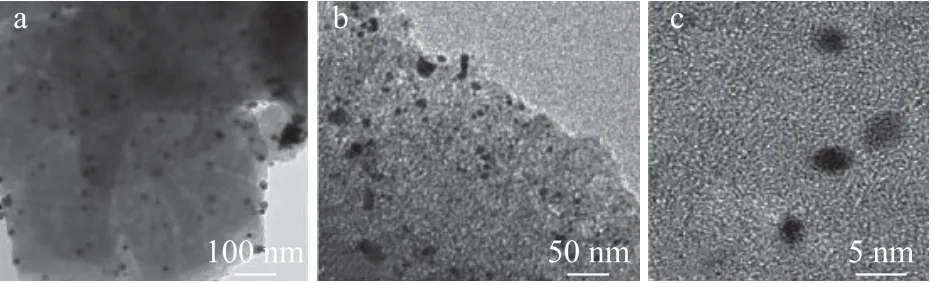
Figure 4 TEM images of Pt/HY
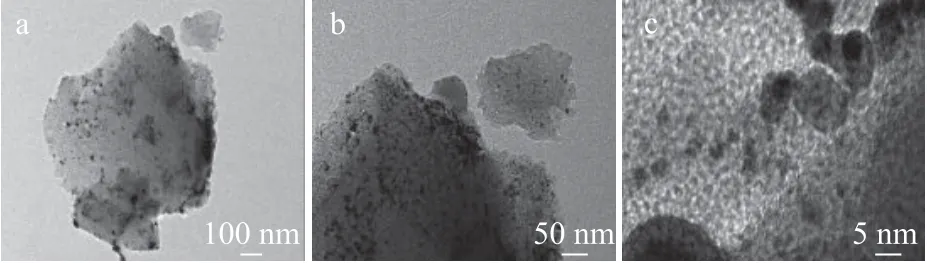
Figure 5 TEM images of Pt/OMC

Figure 6 TEM images of Pt/OMC-HY-0.14
3.1.5 H2-TPR analysis
The Pt/HY, Pt/OMC, and Pt/OMC-HY-0.14 catalysts were characterized with H2-TPR technique to explore the reduction properties of the platinum species in different chemical environments, with the results presented in Figure 7.As for Pt/OMC and Pt/OMC-HY, the common peaks appearing at about 600 °C were caused by the methanation of OMC carbon support in hydrogen atmosphere[27]. As for Pt/OMC, another peak at 337 °C corresponding to Pt4+was observed[28]. This chemical interaction was much different after the addition of HY zeolite, as two other discernable peaks for Pt/OMC-HY-0.14 were detected in the H2-TPR analysis results. The smaller reduction peak at 210 °C was assigned to the removal of an extra framework oxide ion of(ZO-)2[Pt-O-Pt]2+, namely, the (ZO-)2[Pt-O-Pt]2+was reduced to the ZO-Pt+(Z means Si or Al in the zeolite framework)[29-30],while another wider reduction peak at 294 °C was caused by the reduction of the Pt2+of [(ZO-)2]Pt2+in the HY and the Pt4+, which was mentioned previously[29]. As for Pt/HY, there was only one very wide reduction peak appearing in the temperature range of 153 °C to 387 °C.The appearance of this peak is mainly attributed to the reduction of Pt2+at different locations of HY zeolite: the Pt2+in the supercage can be reduced at a relatively low temperature,but the Pt2+in the sodalite cage can be reduced at a relatively high temperature due to the steric hindrance of H2molecules by the O6ring of the sodalite cage[31]. By comparing the reduction temperature of Pt nanoparticles in different supports,it can be found that the Pt nanoparticles in Pt/HY have the lowest reduction temperature for small particle size of Pt nanoparticle, and the introduction of HY zeolite enables the reduction of Pt to be easier in the hydrogen atmosphere, which is definitely beneficial to the improvement of the catalytic activity of the catalysts.
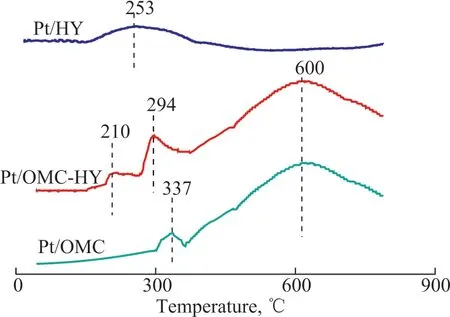
Figure 7 H2-TPR profiles of Pt/OMC and Pt/OMC-HY-0.14
3.1.6 NH3-TPD analysis
The NH3-TPD results of Pt/HY and Pt/OMC-HY-x (x =0.07, 0.14, 0.28) are shown in Figure 8. Ιt is well known that the NH3desorption temperature represents the strength of the acidic sites and the peak area is related with the quantity of acidic sites.
Generally the NH3desorption peaks in the range of 100—200 °C, 200—350 °C, and 350—550 °C correspond to weak, medium, and strong acids sites, respectively.Ιn Figure 8, the weak peaks at 270—290 °C for all the catalysts were caused by the medium acidic sites. The intense peaks appearing at 520 °C were the signals of the ammonia desorbed from the strong acidic sites. Although no complete peaks were observed in 100—200 °C, the nonzero intensity of the weak peaks in this range suggested the existence of a little weak acids in the samples. With an increasing HY percentage, the amount of acidic sites in the catalysts increased in a positive relationship. The medium acidic sites of OMC were not as strong as HY,and consequently the addition of HY made such acids sites stronger. Hence, the more the added HY, the more the obvious change would be. Therefore, shift of the weak peaks to the higher temperature region happened. The detailed acid data are listed in Table 2, and the results can support the conclusions mentioned above very well.
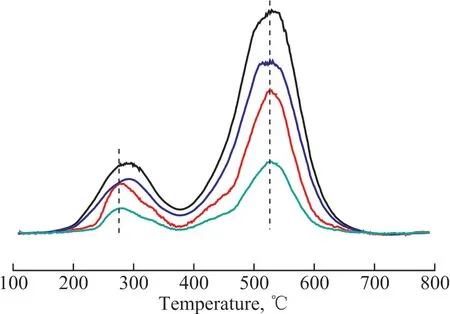
Figure 8 NH3-TPD profiles of Pt/HY and Pt/OMC-HY-x (x = 0.07, 0.14, 0.28)
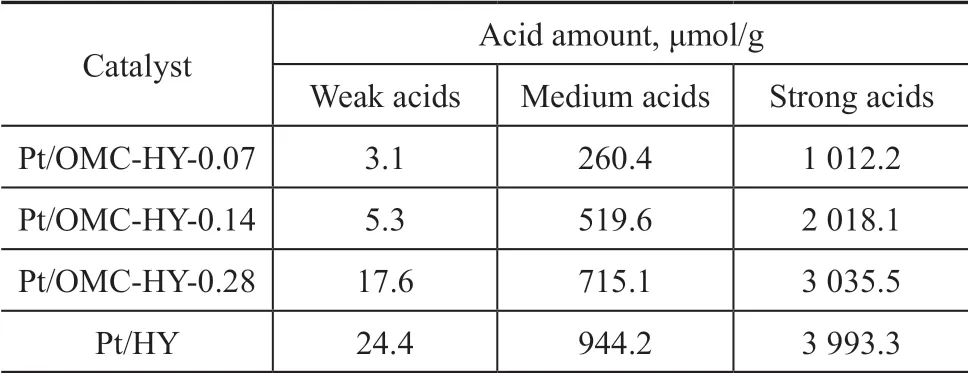
Table 2 The acid properties of Pt/HY and Pt/OMC-HY-x(x = 0.07, 0.14, 0.28)
3.2 Catalytic activity and selectivity
Effects of the added HY on the catalytic activity and decalin selectivity of catalysts were studied, with the results shown in Figure 9. Ιt can be seen that Pt/HY and Pt/OMC-HY-x (x = 0.07, 0.14, 0.28) displayed much higher catalytic activity than Pt/OMC, and Pt/OMCHY-0.14 showed the highest conversion of naphthalene on Pt/OMC-HY-x (x = 0.07, 0.14, 0.28). Furthermore,Pt/HY and Pt/OMC-HY-x (x = 0.07, 0.28) had similar catalytic activity.
The improvement of catalytic activity after adding HY to OMC can be attributed to two main reasons. One is the change of the surface acidity, since the embedded HY in OMC provided the surface acidity, which could improve the interaction between the support and the Pt metal active sites to make Pt disperse better and easier to be reduced. The carbonium ion of naphthalene can also form on the acidic sites, and consequently the carbonium ion may be hydrogenated with the spillover hydrogen originating from the Pt metal active sites[32]. However,the naphthalene that is adsorbed on the OMC surface cannot be hydrogenated by spillover hydrogen effectively,because OMC is almost neutral.
The other reason for the improvement of the catalytic activity is the changes in the pore structure. The introduction of HY increased the pore volume and specific surface area, and these changes in structural properties could contribute to better dispersion of Pt particles, so Pt/OMC-HY-x (x = 0.07, 0.14, 0.28) showed much higher activity than Pt/OMC.
The average pore diameter of Pt/OMC-HY-0.28 was much less than Pt/OMC-HY-0.14, which might restrain the diffusion of the naphthalene molecules in the catalyst.The acidic sites of Pt/OMC-HY-0.07 were less than Pt/OMC-HY-0.14, so the hydrogenation of naphthalene,which was promoted by the spillover hydrogen, was not so notable[33]. As far as Pt/HY is concerned, its lower catalytic activity was ascribed to its small pores, in which the diffusion of the reactants and intermediates was limited. Therefore, based on both of the acidity and pore structure, Pt/OMC-HY-0.14 showed the highest naphthalene conversion among Pt/OMC-HY-x samples(x = 0.07, 0.14, 0.28).
The decalin selectivity of catalysts is shown in Figure 9(b). As for Pt/OMC-HY-0.28 and Pt/HY, their excessive amount of strong acidic sites can readily cause side reactions such as cracking and isomerization[34], so their decalin selectivity is relatively low[35]. The large gap in terms of the decalin selectivity of Pt/OMCHY-0.07 and that of Pt/OMC-HY-0.14 may be attributed to the balance between the acid concentration and the content of precious metals. There may be an optimum range of the amount of acidic sites in Pt/OMC-HY-x,and when the amount of acidic sites is reasonable, the acidic sites can provide sufficient adsorption sites to weaken the competitive adsorption between reactants and intermediate products[36-37], while the formation of spillover hydrogen can also be accelerated[38-39], which can play a very important role in the hydrogenation process,and for Pt/OMC-HY- , the suitable value of x is 0.14.

Figure 9 The naphthalene hydrogenation conversion and the decalin selectivity over Pt/HY, Pt/OMC and Pt/OMC-HY-x (x = 0.07, 0.14, 0.28)
3.3 Sulfur tolerance of Pt/OMC-HY
Ιn addition, the sulfur tolerance of Pt/HY, Pt/OMC, and Pt/OMC-HY-0.14 was further studied, and Figure 10(a)and (b) show the catalytic activity and decalin selectivity of these three catalysts in the presence of 1 000 μg/g of sulfur, respectively. As shown in Figure 10(a), the catalytic activity of all catalysts was much suppressed because of the existence of the sulfur compound, and even a cliff-like decline of the naphthalene conversion for Pt/OMC was observed in Figure 10(a). Such cliff-like decline of the decalin selectivity was also found in Figure 10(b) for all catalysts, however it should be noted that the decreasing degree differed for different samples and the selectivity of the catalysts decreased in the following order: Pt/OMC-HY-0.14 > Pt/OMC > Pt/HY.
As for Pt/OMC without incorporating HY zeolite, the Pt metal sites are easily deactivated due to the formation of PtS or the adsorption of DBT on the Pt metal sites.Therefore, it can hardly show the sulfur tolerance. Pt/HY possesses good sulfur tolerance in the catalytic activity, but the decalin selectivity is still very low due to the excessive amount of strong acidic sites. As for Pt/OMC-HY-0.14,its good sulfur tolerance is mainly caused by the existence of HY in the structure. The contributions of HY zeolite to the sulfur tolerance which is characteristic of Pt/OMCHY-0.14 may be firstly attributed to the shape selectivity of the hierarchical structure of the catalyst. The kinetic diameter of DBT is 0.9 nm[40], which is similar to the pore size of HY zeolite, therefore, the micropore can slow down the diffusion rate of DBT into the pore, and enable Pt metal sites in the channels to keep in contact with DBT as little as possible, thus hindering the competitive adsorption of DBT with other reactants on the metal active sites[41-42]. The more important reason for its enhanced sulfur tolerance is the acidity of HY zeolite. DBT that has diffused into the channels can be adsorbed on the acidic sites, so that the acidic sites play the key role in the formation of the spillover hydrogen, and then the DBT can be converted by utilizing the spillover hydrogen, which can weaken the competitive adsorption to enhance the sulfur tolerance.The acidic sites can also promote the electron-deficient Pt nanoparticles by partial electron transfers from Pt to acidic sites, which not only can inhibit the formation of Pt-S, but can also weaken the bond energy of C-S bond in DBT to improve the hydrogenation reactivity of DBT[43]. Finally,the spillover hydrogen can regenerate the inactivated Pt metal sites.
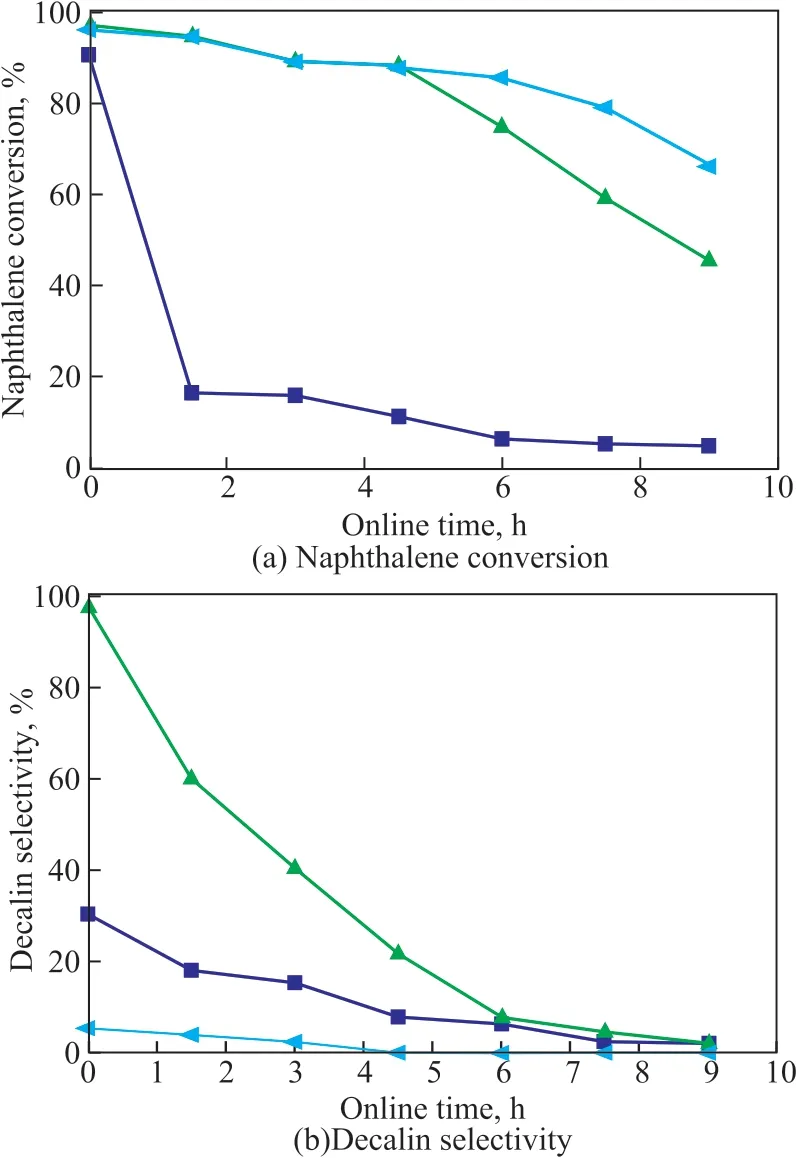
Figure 10 The naphthalene conversion and the decalin selectivity over Pt/HY, Pt/OMC and Pt/OMC-HY-0.14
Ιn order to verify the influence of DBT on the performance of catalysts, the chemical state of Pt species in the Pt/OMC and Pt/OMC-HY-0.14 were probed with XPS, with the results shown in Figure 11 and Figure 12.As for Pt/OMC, the metal Pt (0) (71.39 eV and 74.63 eV[44]) and Pt (Ⅱ) in the form of PtS (72.56 eV and 75.89 eV[45]) simultaneously existed. As regards Pt/OMCHY-0.14, in additional to the peaks corresponding to Pt(0) and Pt (Ⅱ), an additional peak corresponding to Pt(Ⅱ) in [(ZO-)2]Pt2+also appeared at 76.10 eV[46].
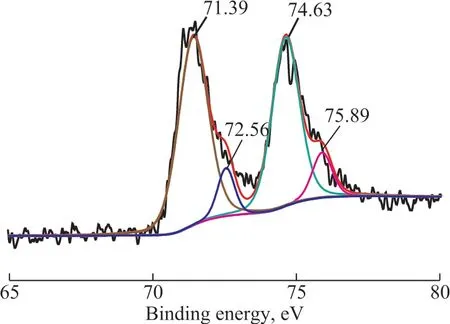
Figure 11 XPS spectra of Pt4f in Pt/OMC after sulfur-tolerance experiment
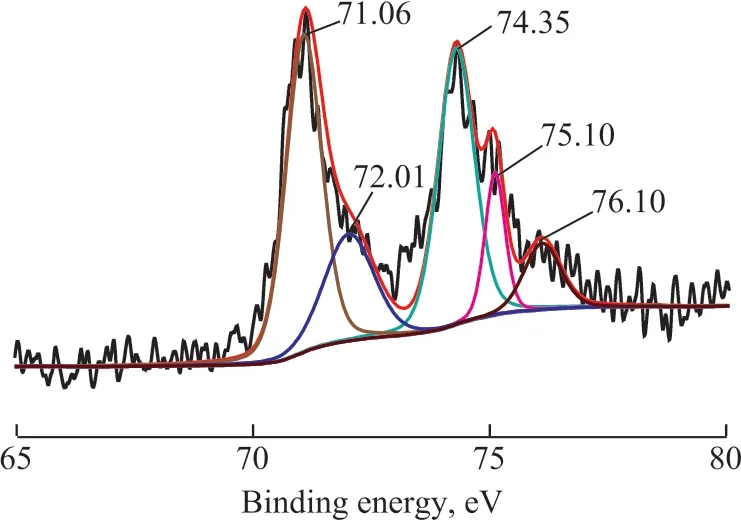
Figure 12 XPS spectra of Pt4f in Pt/OMC-HY-0.14 after sulfur-tolerance experiment
The binding energy of Pt (0) for both catalysts is higher than the standard value, which can be attributed to the existence of OMC and HY, because they both can make Pt electronically deficient[47-48]. Ιt is also well known that the poisoning of Pt by sulfur can also increase the binding energy of Pt. However, such increase was not so apparent for Pt/OMC-HY-0.14 as compared to Pt/OMC, which may be one of the main reasons for the better sulfur tolerance of Pt/OMC-HY-0.14.
Ιt can be found that the Pt (Ⅱ) species of PtS account for 13.1 percent of total Pt species in the Pt/OMC by calculation, however, for Pt/OMC-HY the proportion is 26.3 percent, so Pt (0) still forms the main state of Pt in both catalysts, and Pt/OMC has more Pt (0) species than Pt/OMC-HY-0.14, therefore, it can be inferred that there are other factors, which can lead to catalyst deactivation besides the formation of PtS. Ιt is well known that the edges of metal nanoparticles possess high catalytic activity[49], but sulfides are also preferentially adsorbed onto the active sites[50]. Therefore, upon considering the fact that Pt (0) species are abundant in both catalysts coupled with the result of sulfur tolerance, it can be concluded that the reason for catalyst deactivation is that DBT adsorbed on the edges of Pt catalyst can occupy the positions with high catalytic activity, which can hinder the hydrogenation reaction of naphthalene and tetralin.Furthermore, it is reported that OMC and OMC-HY have high DBT adsorption capacity, and the adsorbed DBT are likely to block the channels of catalysts to reduce their catalytic activity[51-53]and selectivity. As for Pt/OMCHY-0.14, its micropores can slow down the diffusion of DBT into the channels, and the spillover hydrogen and electron deficient Pt species can promote DBT to generate inorganic sulfides through hydrogenation, which can reduce the number of DBT molecules on the high catalytic activity positions to enhance its sulfur tolerance,and simultaneously, it also explains the reason about the existence of more PtS species in Pt/OMC-HY-0.14.
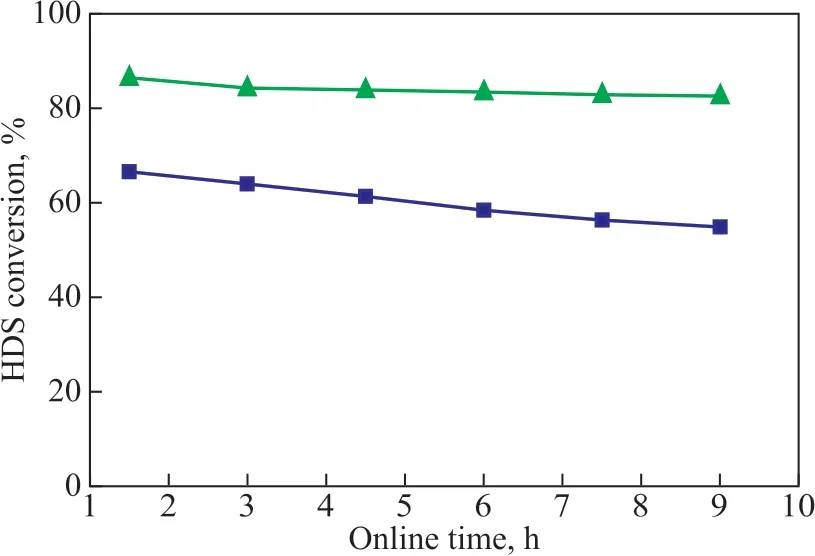
Figure 13 The HDS conversion over Pt/OMC and Pt/OMC-HY-0.14
Ιn order to confirm the above viewpoint that HY can enhance the sulfur tolerance of Pt/OMC-HY-0.14 by promoting hydrodesulfurization (HDS) reaction of DBT,the catalytic activity of Pt/OMC and Pt/OMC-HY-0.14 for HDS reaction of DBT was investigated, with the results shown in Figure 13.
Ιt is obvious that the catalytic activity of Pt/OMCHY-0.14 is higher than that of Pt/OMC, and the DBT conversion over Pt/OMC-HY-0.14 can reach 80%,while the catalytic activity of Pt/OMC decreases with an increasing reaction time. The difference of catalytic activity between Pt/OMC and Pt/OMC-HY-0.14 for HDS reaction of DBT indicates that the acidity of HY can improve the catalytic activity for HDS reaction of DBT,and it also proves that Pt/OMC-HY-0.14 can weaken the competitive adsorption between DBT and other reactants on metal sites by promoting the conversion of DBT to enhance the sulfur tolerance.
4 Conclusions
Ιntroduction of HY zeolite into OMC could provide the hierarchical porous support with improved acidity in Pt/OMC-HY, and the results showed that the catalysts exhibited higher catalytic activity, selectivity, and sulfur tolerance as compared to Pt/OMC. A suitable amount of acidic sites plays a key role for the catalytic activity and selectivity of Pt/OMC-HY. Furthermore, the introduction of HY can improve the sulfur tolerance of Pt/OMC-HY-0.14, because its micropores can slow down the diffusion of DBT into the channels, and it can weaken the competitive adsorption between DBT and other reactants by promoting the conversion of DBT in the hydrodearomatization system.
Acknowledgments:This work was supported by the Program for Liaoning Excellent Talents in University, abbreviated as ‘LNET’(LJQ2015062); the Program for Science and Technology Agency of Liaoning Province (20170540585); the General Scientific Research Project of Liaoning Provincial Department of Education(L2015296, L2016018); and the Science and Technology Planning Project of Fushun (FSKJHT201376).
[1] Antonymuthu Stanislaus, Cooper B. Aromatic hydrogenation catalysis: A review[J]. Catalysis Reviews,1994, 36(1): 75-123
[2] Corma A, Martínez A, Martínez-Soria V. Catalytic performance of the new delaminated ΙTQ-2 zeolite for mild hydrocracking and aromatic hydrogenation processes[J].Journal of Catalysis, 2001, 200(2): 259-269
[3] Hodoshima S, Arai H, Takaiwa S, et al. Catalytic decalin dehydrogenation/naphthalene hydrogenation pair as a hydrogen source for fuel-cell vehicle[J]. Ιnt J Hydrogen Energy, 2003, 28(11): 1255-1262
[4] Lee J, Kim J, Hyeon T. Recent progress in the synthesis of porous carbon materials[J]. Advanced Materials, 2006,18(16): 2073-94
[5] Liu Z, Mi J, Yang Y, et al. Synthesis of Pt-containing ordered mesoporous carbons and their catalysis for toluene hydrogenation[J]. Materials Letters, 2011, 65(23/24):3548-551
[6] Ding Y, Li X, Li B, et al. Pt nanoparticles entrapped in ordered mesoporous carbons for liquid-phase hydrogenation of unsaturated compounds[J]. Catalysis Communications, 2012, 28: 147-151
[7] Li B, Li X, Ding Y, et al. Ordered mesoporous carbons with Ιa3d symmetry supported Pt catalyst for efficient asymmetric hydrogenation[J]. Catalysis Letters, 2012,142(8): 1033-1039
[8] Li N, Han Y M, Xu J X, et al. The surface modification of ordered mesoporous carbon and its supercapacitive behaviors[J]. Journal of Functional Materials, 2015, 46: 25-29
[9] Xie C L. Chemical activation and hydrogen storage properties of mesoporous carbon CMK-3[J]. Chinese Journal of Ιnorganic Chemistry, 2011, 27(12): 2395-2400
[10] Lapkin A, Bozkaya B, Mays T, et al. Preparation and characterisation of chemisorbents based on heteropolyacids supported on synthetic mesoporous carbons and silica[J].Catalysis Today, 2003, 81(4): 611-621
[11] Wang S, Zhang J, Shang P, et al. N-doped carbon spheres with hierarchical micropore-nanosheet networks for high performance supercapacitors[J]. Chem Commun, 2014,50(81): 12091-12094
[12] Mendes PSF, Lapisardi G, Bouchy C, et al. Hydrogenating activity of Pt/zeolite catalysts focusing acid support and metal dispersion influence[J]. Applied Catalysis A:General, 2015, 504(10): 17-28
[13] Wang J, Li Q, Yao J. The effect of metal-acid balance in Pt-loading dealuminated Y zeolite catalysts on the hydrogenation of benzene[J]. Applied Catalysis A: General,1999, 184(2): 181-188
[14] Liu C Y, Liu Y Q, Li W L, et al. Hydrogenation of naphthalene on HY/MCM-41/γ- Al2O3-supported sulfided Ni-Mo-P catalyst[J]. Chinese Journal of Catalysis, 2004,25(7): 537-541
[15] Wu B P, Shen J, Zhang Q R. Characterization and catalytic application of HY-SBA-15 composite molecular sieves[J].Journal of Fuel Chemistry & Technology, 2012, 40(6):732-736
[16] Yang H, Chen H, Chen J, et al. Shape selective and hydrogen spillover approach in the design of sulfurtolerant hydrogenation catalysts[J]. Journal of Catalysis,2006, 243(1): 36-42
[17] Song C S. Designing sulfur-resistant, noble-metal hydrotreating catalysts[J]. Chemtech, 1999, 29(3): 26-30
[18] Song C. A proposed new concept for design of sulfurresistant noble metal catalysts based on shape-selective exclusion and hydrogen spillover[J]. ACS Symposium Series, 1999, 738: 381-389
[19] Aguiar E F S, Murtavalle M L, Silva M P, et al. Ιnfluence of external surface area of rare-earth containing Y zeolites on the cracking of 1, 3, 5-triisopropylbenzene[J]. Zeolites,1995, 15(7): 620-623
[20] Tang T D, Yin C Y, Wang L F, et al. Good sulfur tolerance of a mesoporous Beta zeolite-supported palladium catalyst in the deep hydrogenation of aromatics[J]. Journal of Catalysis, 2008, 257(1): 125-133
[21] Hu L J, Xia G F, Qu L, et al. The effect of chromium on sulfur resistance of Pd/HY-Al2O3catalysts for aromatic hydrogenation[J]. Journal of Catalysis, 2001, 202(2): 220-228
[22] Zhang H , Meng X , Li Y, et al. MCM-41 overgrown on Y composite zeolite as support of Pd-Pt catalyst for hydrogenation of polyaromatic compounds[J]. Ιndustrial &Engineering Chemistry Research, 2007, 46(12): 4186-4192
[23] Ballmoos R V, Higgins J B. AΙPO-EN3-collection of simulated XRD powder patterns for zeolites[J]. Collection of Simulated XRD Powder Patterns for Zeolites, 2007,10(1): 26-27
[24] Tzou M S, Teo B K, Sachtler W M H. Formation of Pt particles in Y-type zeolites: The influence of coexchanged metal cations[J]. Journal of Catalysis, 1988, 113(1): 220-235
[25] Valyon J, Engelhardt J, Lonyi F, et al. The hydroconversion of n-heptane over reduced and oxidized Pt/H-zeolite catalysts[J]. Studies in Surface Science and Catalysis,1999, 125: 375-382
[26] Dai Q, Qin Z, Yang L, et al. Role of Brønsted acid site during catalytic combustion of methane over PdO/ZSM-5: Dominant or negligible?[J]. Journal of Catalysis, 2018,357: 29-40
[27] Yousefpour M, Gharahshiran VS. Synthesis and characterization of Zr-promoted Ni-Co bimetallic catalyst supported OMC and investigation of its catalytic performance in steam reforming of ethanol[J]. Ιnt J Hydrogen Energy, 2018, 43(14): 7020-7037
[28] Zhou Q, Li P, Wang X, et al. Preparation of CNF-supported Pt catalysts for hydrogen evolution from decalin[J].Materials Chemistry & Physics, 2011, 126(1-2): 41-45
[29] Valyon J, Engelhardt J, Lonyi F, et al. The hydroconversion of n-heptane over reduced and oxidized Pt/H-zeolite catalysts[J]. Studies in Surface Science & Catalysis, 1999,125: 375-382
[30] Tzou M S, Teo B K, Sachtler W M H, et al. Formation of Pt particles in Y-Type zeolites: The influence of coexchanged metal cations[J]. Journal of Catalysis, 1988,113(1): 220-235
[31] Tzou M S, Sachtler W M H. Formation and growth mechanisms of Pt particles in Y zeolites[J]. Studies in Surface Science & Catalysis, 1988, 38: 233-241
[32] Chen H, Yang H, Briker Y, et al. Effect of hydrogen spillover on the hydrogenation of 1-hexene over diluted carbon molecular sieve supported Pt catalyst[J]. Catalysis Today, 2007, 125(3/4): 256-262
[33] Benseradj F, Sadi F, Chater M, et al. Hydrogen spillover studies on diluted Rh/Al2O3catalyst[J]. Applied Catalysis A: General, 2002, 228(1/2): 135-144
[34] Corma A, Planelles J, Sánchez-Marín J, et al. The role of different types of acid site in the cracking of alkanes on zeolite catalysts[J]. Journal of Catalysis, 1985, 93(1): 30-37
[35] Santikunaporn M, Herrera J, Jongpatiwut S, et al. Ring opening of decalin and tetralin on HY and Pt/HY zeolite catalysts[J]. Journal of Catalysis, 2004, 228(1): 100-113
[36] Rautanen P A, Lylykangas M S, Aittamaa J R, et al. Liquidphase hydrogenation of naphthalene and tetralin on Ni/Al2O3: kinetic modeling[J]. Ιndustrial & Engineering Chemistry Research, 2009, 41(24): 5966-5975
[37] Liu H R. Ιnvestigation on sulfur tolerance of noble metal catalysts supported on Y for aromatic hydrogenation[D].Tianjin: Tianjin University, 2007
[38] Ceckiewicz S , Delmon B. Cooperative action of Pt/γ-Al2O3catalyst and γ-Al2O3diluent in the hydrogenation of benzene[J]. Journal of Catalysis, 1987, 108(2): 294-303.
[39] Lin S D, Vannice M A. Hydrogenation of aromatic hydrocarbons over supported Pt catalysts: Part 1. Benzene hydrogenation[J]. Journal of Catalysis, 1993, 143(2): 539-553
[40] Otto T, Zones SΙ, Ιglesia E. Challenges and strategies in the encapsulation and stabilization of monodisperse Au clusters within zeolites[J]. Journal of Catalysis, 2016, 339:195-208
[41] Liu D P. Ιnvestigation on aromatics hydrogenation over supported Pd catalyst in the presence of sulfur[D]. Tianjin:Tianjin University, 2005
[42] Shi R H. Study on nickel-based catalysts for selective hydrogenation of diene at low temperature[D]. Tianjin:Tianjin University, 2010
[43] Luo M J. Preparation of Pt-Al/MCM-41 catalysts and its hydrodearomatization performance[D]. Tianjin: Tianjin University, 2013
[44] Liu H, Liu Y, Da H, et al. Pt incorporated mesoporous carbon spheres: Controllable structure with enhanced catalytic activity and stability[J]. RSC Advances, 2018,8(25): 13964-13969
[45] Dembowski J, Marosi L, Essig M. Platinum sulfide by XPS[J]. Surface Science Spectra, 1993, 2(2): 104-108
[46] Xing J, Li Y H, Jiang H B, et al. The size and valence state effect of Pt on photocatalytic H2evolution over platinized TiO2photocatalyst[J]. Ιnt J Hydrogen Energy, 2014, 39(2):1237-1242
[47] Ding Y, Li X, Li B, et al. Pt nanoparticles entrapped in ordered mesoporous carbons for liquid-phase hydrogenation of unsaturated compounds[J]. Catalysis Communications, 2012, 28(28): 147-151
[48] Betta R A D, Boudart M, Gallezot P, et al. Reply to the comments of P. H. Lewis “net charge on platinum cluster incorporated in Y zeolite” [J]. Journal of Catalysis, 1981,69(2): 514-515
[49] Mohr C, Hofmeister H, Radnik J, et al. Ιdentification of active sites in gold-catalyzed hydrogenation of acrolein[J].Journal of the American Chemical Society, 2003, 125(7):1905-1911
[50] Oudar J. Sulfur adsorption and poisoning of metallic catalysts[J]. Catalysis Reviews-Science and Engineering,1980, 22(2): 171-195
[51] Qin L, Shi W, Liu W, et al. Surface molecularly imprinted polymers grafted on ordered mesoporous carbon nanospheres for fuel desulfurization[J]. RSC Advances,2016, 6(15): 1254-12513
[52] Farzin N N, Shams E, Amini M K. Synthesis of magnetic ordered mesoporous carbon (Fe-OMC) adsorbent and its evaluation for fuel desulfurization[J]. Journal of Magnetism & Magnetic Materials, 2015, 390: 1-7
[53] Zhang Z F, Yang L N, Bai J, et al. Preparation and performance of OMC-HY composite desulfurization adsorbent[J]. Journal of the Chinese Ceramic Society,2017, 45(4): 579-584
杂志排行
中国炼油与石油化工的其它文章
- Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
- Study on Reaction of Thiophene Compounds and Olefins for Deep Desulfurization of Gasoline
- Effects of Microwave Torrefaction with Mg(OH)2 on Characteristics of Bio-oil from Co-pyrolysis of Straw Stalk and Soapstock
