Fenton Pre-Oxidation Followed by Microbial Degradation for Removing Crude Oil from Contaminated Soil
2019-10-31GaoChunyangZhangYuHanZhantaoSongQuanwei
Gao Chunyang; Zhang Yu; Han Zhantao; Song Quanwei;
Chen Changzhao2,3,4; Chen Hongkun3,4; Zheng Jin3,4
(1. Hebei and China Geological Survey Key Laboratory of Groundwater Remediation, Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences, Shijiazhuang 050061;2. China University of Geosciences, Beijing 100083;3. State Key Laboratory of Petroleum Pollution Control, Beijing 102206;4. CNPC Research Institute of Safety and Environmental Technology, Beijing 102206)
Abstract: Through the Fenton pre-oxidation followed by microbial degradation, this study gave full play to its advantages while avoiding its shortcomings for the remediation of crude oil contaminated soil. The Fenton reagent coupled with different volumes of H2O2 was applied to the oil contaminated soil and then the microbial agents were introduced to biodegrade the residual oil for 15 days. The correlation between the characteristics of residual oil in soil, the changes in soil physical-chemical property after the Fenton pre-oxidation, and the biodegradation were analyzed in this paper. The results show that the above factors are strongly correlated with the subsequent biodegradation rate, and the order of correlation is as follows: the ratio of TOC to NH4+-N (R2 = 0.9513) > the ratio of light oil components to the heavy oil components (R2 = 0.9095) > the proportion of hydrocarbons with carbon chain number of less than C23 (R2 = 0.8259) > the crude oil content (R2 = 0.7603) > the soil pH(R2 = 0.7492) > the number of microorganisms (R2 = 0.6506). During the biodegradation and pre-oxidation reactions of heavy oil components, an appropriate C:N ratio turns out to be the most critical factor in this study.
Key words: crude oil; Fenton; microbial degradation; soil; correlation analysis
1 Introduction
Soil contamination by petroleum is one of the major environmental problems worldwide[1], when the petroleum gets into soil during oil exploitation, refining, and transportation[2]. Oil in soil is harmful to both plant growth and human health[3]. Compared with gasoline and diesel, crude oil is more difficult to degrade due to its high viscosity, low mobility, low volatility, and low biodegradability[4]. Conventional methods for remediating the crude oil contaminated soil include mainly physical[5],chemical[6], and biological[7]ones. Therein, the biological degradation enjoys great advantages since it is characteristic of low energy consumption, low operational cost, and absence of secondary pollution, and hence it has been the dominant method for the oil contaminated soil remediation over a long period[8-9]. However, efficient biological methods are usually employed at the early stage of degradation for treating heavy components such as resins and asphaltenes in crude oil[10-11].
Previous researches have already successfully applied chemical oxidation process followed by microbial technology to the remediation of oil contaminated soil[12-14],and found that chemical oxidation can enhance biodegradation. And it can be realized because the chemical oxidation can: (1) reduce the concentration of oil in the soil to abate its toxicity[15]; (2) decompose long-chain hydrocarbons that are difficult to biodegrade into biodegradable short-chain hydrocarbons[16]; and(3) release bioavailable nutrient elements in the oil contaminated soil to ensure nutrition[17]. Ιn addition, the Fenton reagent as a pre-oxidant usually does not produce any by-product after reaction[18]. Previous studies[19]have shown that the Fenton reagent has less impact on soil microorganisms than other chemical reagents.
To date, researchers have focused on the impact of Fenton pre-oxidation on soil physicochemical properties and indigenous microorganisms[1,20]. However, there are few researches studying the residual crude oil properties in soils after pre-oxidation and the effects of oil property change on subsequent biodegradation rates. Therefore, this study aims to analyze the correlations among the physical-chemical properties of soil, the number of indigenous microorganisms,the crude oil property change after the Fenton pre-oxidization and subsequent biodegradation rates, in order to provide key information for the optimization of chemical pre-oxidation and subsequent biodegradation process during remediation of the oil contaminated soil.
2 Experimental
2.1 Crude oil contaminated soil
Samples of the crude oil contaminated soil were collected from the Xinjiang Oilfield with a contamination history of more than 10 years. The collected samples were stored in a vehicle refrigerator at a constant temperature of 4 °C and immediately transported to the laboratory. With stones and plant residues removed, the sample soil was sieved through a 2-mm sieve. After that treatment, the physical and chemical properties of the sample soil are presented in Table 1.
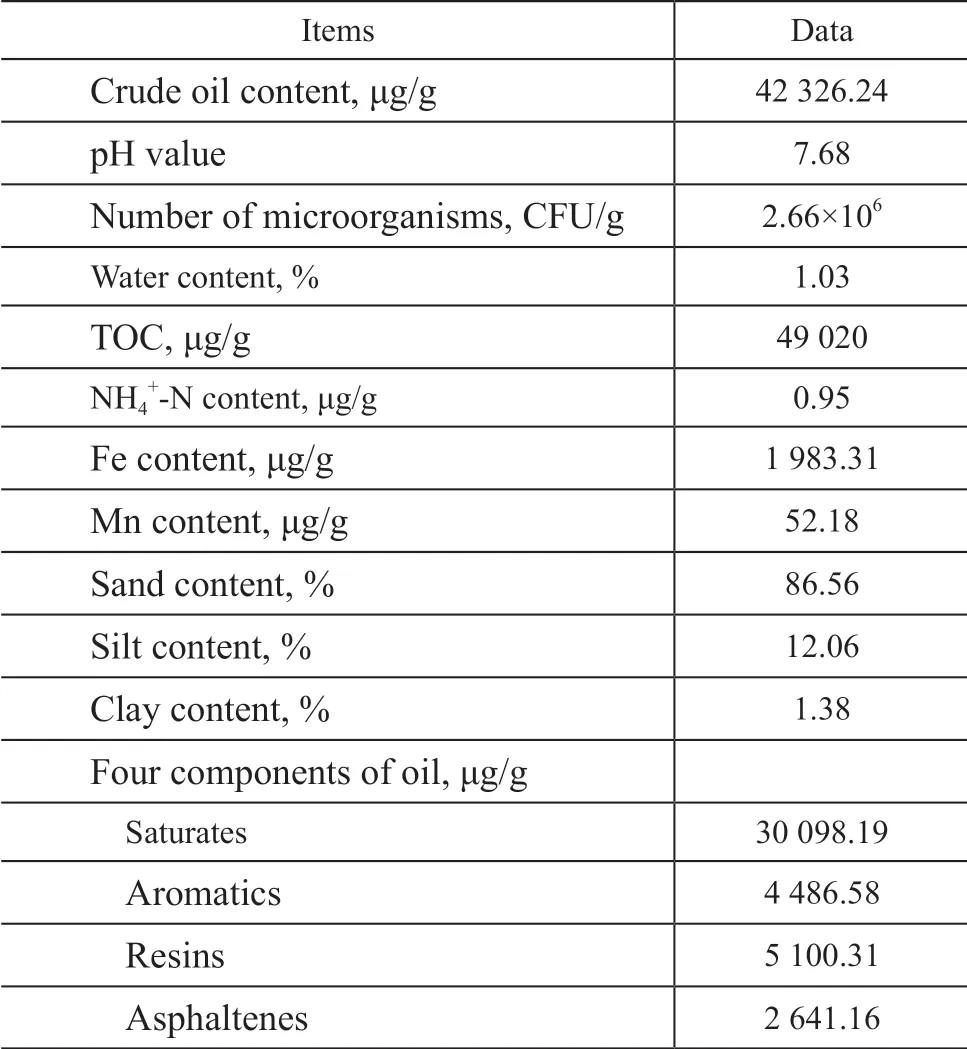
Table 1 Physicochemical properties of oil contaminated soil
2.2 Measurement of residual oil
The oil content in soil was extracted by subsequent method and measured by an infrared oil measuring instrument (Oil 480, Chinainvent, China): 5 g of airdried soil were added into 10 mL of carbon tetrachloride solution, which was subject to extraction for 15 min under 300 W of ultrasonic wave, followed by centrifugation for 5 min at a rotational velocity of 800 r/min. The supernatant was decanted into a Erlenmeyer flask through a glass wool stuffed long-neck glass funnel filled with 1 cm of anhydrous sodium sulfate (being subject to drying at 300 °C for 2 hours), and this step was repeated several times until the extract was colorless. Finally, the collected supernatant was diluted to 50 mL for measurements.
2.3 Determination of four components in crude oil
The four components (saturates, aromatics, resins,asphaltenes) of crude oil were determined through a SPE-10 automatic solid phase extraction instrument (Bonna-Agela, China). The SPE column was activated with 10 mL of n-hexane for 5 min, and then n-hexane was blown out by air.
Separation of saturates: The SPE column was rinsed with 30 mL of n-hexane for 30 min, then was purged with air at a flow rate of 5 mL/min for 2 min. Separation of aromatics: The SPE column was rinsed with 30 mL of mixed solution (with the n-hexane: dichloromethane ratio equating to 1:2) for 30 min, then air was used to purge the column at a flow rate of 5 mL/min for 2 min.Separation of resins: The SPE column was rinsed with 10 mL of ethanol for 10 min, was then rinsed with 15 mL of chloroform for 15 min, and finally the solution was purged with air at a flow rate of 5 mL/min for 2 min.Separation of asphaltenes: the asphaltenes trapped on the sample column sieve plate were washed by carbon tetrachloride. Finally, the obtained extracts were dried by nitrogen and weighed to obtain the content of each component.
2.4 Measurement of n-alkanes
N-alkanes in the separated saturates was measured by the first-time concentrated extracts to 1 mL and was analyzed using a GC-FΙD (Thermo Scientific, USA) equipped with a HP-5 (30 m×0.25 mm×0.25 µm) column (Agilent Technologies). The inlet temperature was 320 °C, the initial temperature was set as 40 °C for 5 min, and then the temperature was gradually raised to 300 °C at a temperature increase rate of 10 °C/ min in 20 min.
2.5 Determination of total organic carbon (TOC)and ammonia nitrogen ()
The total organic carbon content in the soil was determined by a total organic carbon analyzer (MultiN/C2100,Germany). 50 mg of the soil sample were weighed with the injector and then the sample soil was acidified with 10% hydrochloric acid solution. The oxygen pressure was kept at 0.5 MPa, and the furnace temperature was set at 1 200 °C for burning the sample. The content of TOC was calculated automatically by collecting the CO2content.The content of NH4+-N was measured by HACH method via extracting the sample soil in 1 mol/L KCl solution,and finally the NH4+-N in the extract could be measured by a HACH photometer (DRB200, USA).
2.6 Microbial colony count
Put one gram of sample soil into an Erlenmeyer flask containing 9 mL of sterile water and incubate it at 25 °C for 30 min on a constant temperature shaker with a rotational rate of 150 r/min. Then add the nutrient agar medium to the sample and shake it for better mixing. 1 mL of suspension was diluted for coating, and the dilution ratio was specified to be 100, 10-1, 10-2, 10-3, 10-4, 10-5, 10-6, 10-7,and 10-8, respectively. The coated plates were placed in a biochemical incubator which was cultured at 30 °C for 24 h to 48 h, and the microbial colony was then counted using a fully automatic colony counter (Ιnterscience,France).
2.7 Fenton pre-oxidation experiment
The pre-oxidation experiment was carried out in a 150 mL conical flask. 100 g of the contaminated soil were weighed and then were mixed with 0.6 mL of 0.5 mol/L ferrous sulfate solution and 0.6 mL of 0.5 mol/L citric acid solution. Finally, 0.1—1.0 mL of 30% hydrogen peroxide solution was added into the flask in sequence, while different volumes of deionized water were added finally to keep the water-soil mass ratio of 1:1. Blank samples mixed with only deionized water were used as reference,and all soil samples were prepared in triplicate. The reaction continued for 2 days in a shaker operating at a rotational speed of 200 r/min with the temperature controlled at 20 °C.
2.8 Biodegradation experiment
After the Fenton pre-oxidation experiment, the oxidized soil was air-dried for 2—3 days, and the water content was reduced to 30%—50%. 30 g of dried natural soil were weighed for biodegradation experiments. The degrading bacteria used belonged to a degrading strain,which was selected from the contaminated soil by the method of “inducing agent-intermediate product-target contaminant”, and was sequenced by the Beijing Meiji Sangge Biomedical Technology Co., Ltd. using 16s rDNA technology. And it was identified as Pseudomonas aeruginosa strain. During the experiment, the bacteria were cultured to 109CFU/L, and 3 mL of bacterial suspension were added to each soil sample, then 30 mL of sterilized water were added as well. The soil sample was subject to biodegradation for 15 days at a temperature of 30 °C and a rotation speed of 150 r/min.
3 Results and Discussion
3.1 Effects of Fenton pre-oxidation on crude oil
The residual crude oil content in the soil treated with different amounts of H2O2reagent is shown in Figure 1. When the H2O2dosage was increased from 0.0 mL to 0.6 mL, the crude oil content was reduced from 42 326.24 μg/g to 27 689.33 μg/g, and the corresponding degradation rate reached 34.58%. After that, the degradation rate did not increase further with an increasing H2O2dosage used. Ιt can be inferred that this is attributed to the quenching reaction of free radicals.There is an appropriate ratio between H2O2and Fe2+, and hence the increase of H2O2[21]does not result in better degradation of crude oil, which has been confirmed by previous studies[22]. As shown by the formula: H2O2+·OH → H2O+ HO2, the Fenton reagent contributes to the degradation of crude oil in the contaminated soil.Although excessive addition of H2O2will consume ·OH and cause poor degradation, multiple addition of oxidant can easily avoid the quenching effect[23].
All the concentrations of n-alkanes (C8—C40) are shown in Table 2. Ιt can be seen that there are less short-chain (C8—C17) hydrocarbons in the crude oil because they have been degraded or evaporated. Other hydrocarbons with different carbon chain lengths have been degraded by oxidation to varying degrees. Previous study[24]has shown that microorganisms have a good degradation effect on hydrocarbons with a carbon chain length of less than C23. Therefore, we select all hydrocarbons with a carbon chain length of shorter than C23and observe their changes after oxidation. Figure 2 shows that the ratio of hydrocarbons with a carbon chain length of < C23increased from 15.76% to 32.03%, when the amount of H2O2increased to 0.6 mL in the samples.

Figure 2 Content of carbon chain length less than C23 with different dosage of H2O2 added
The change of four components in crude oil after add-ing different oxidants is shown in Table 3. Over all, the degradation rate of saturates, resins, aromatics, and asphaltenes reached the highest values of 22.69%, 56.92%,56.08%, and 90.67%, respectively, when 0.6 mL of H2O2was added. For such components as resins, aromatics, and asphaltenes, which were difficult to degrade, they were oxidized extensively than saturates which were easily biodegradable. This was of great help to subsequent biodegradation. Reasons for degradation differences could be attributed to the following aspects: ①The hydroxyl radicals produced by Fenton’s reagent were more likely to be in contact with macromolecules such as resins and asphaltenes; ②A chain-breaking behavior similar to that of saturates occurred during the reaction, resulting in the formation of saturates after a part of resins and asphaltenes were broken.
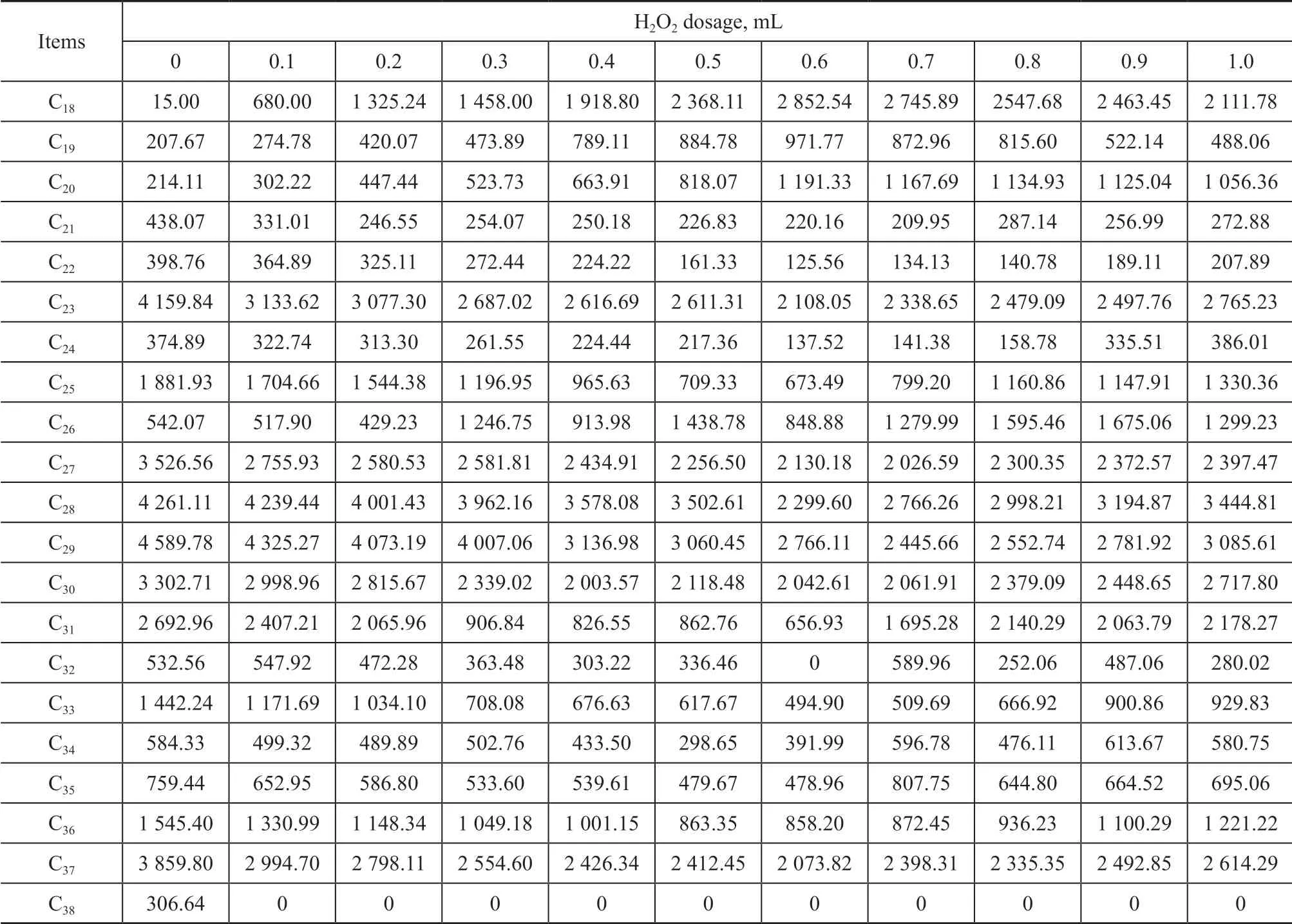
Table 2 N-alkanes content (μg/g) after different amounts of H2O2 added
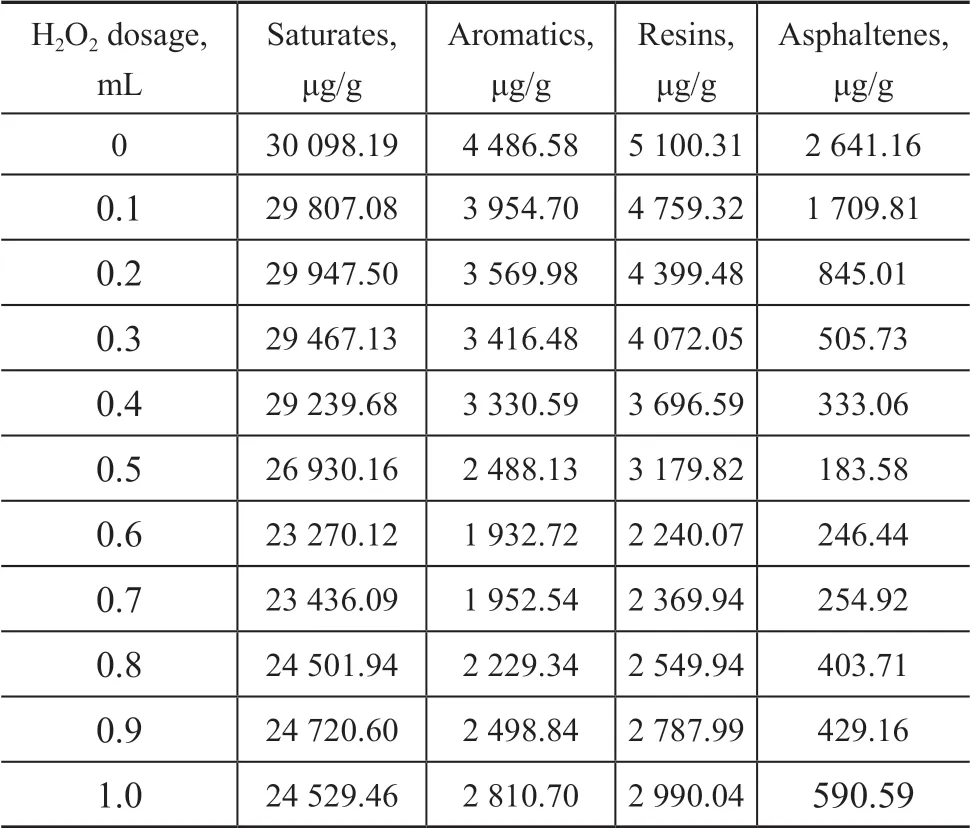
Table 3 Change of four components of crude oil in soil treated with different dosage of H2O2
3.2 Effects of pre-oxidation on soil microorganisms and soil chemical composition
The Fenton pre-oxidation will inevitably change the properties of the soil[25]. The changes of pH value, the number of indigenous microorganisms, TOC, and NH4+-N in the soil are shown in Figure 3. Ιt can be seen that the pH value after the Fenton oxidation was increased,which was consistent with the production of OH-during the Fenton’s reaction: H2O2+ Fe2+→ OH-+ Fe3++·OH,Fe2++·OH→Fe3++OH-[22]. The addition of oxidant would inevitably lead to decrease in the number of indigenous microorganisms, because the oxidant was also a bactericide. To be specific, the number of microorganisms in the soil in this study decreased from 2.66×106CFU/g to 7.88×104CFU/g as the amount of added oxidant increased.Ιn natural setting, microorganisms rely on carbon and nitrogen resources and other nutrients in soil to grow and degrade pollutants. However, oil pollution has greatly increased the carbon content and changed the bioavailable C:N ratio. Studies[26]have shown that the optimum C:N ratio for microorganism was 10:1—15:1, but the C:N ratio in the contaminated soil sample has reached 51 600:1.Ιn this study, the TOC content gradually decreased with the increase of oxidant, and decreased from the initial 49 020 μg/g to 31 280 μg/g when the H2O2dosage reached 0.6 mL. Crude oil is an organic mixture and a part of organic carbon in soil will be mineralized into CO2after the Fenton oxidation, which will reduce the content of organic carbon in soil[27]. Ιn addition, as the amount of the added oxidant increased, the NH4+-N content increased significantly. When the H2O2dosage increased to 0.6 mL, the NH4+-N content increased from 0.95 μg/g to 3.92 μg/g according to previous studies[17,28]. This was mainly ascribed to the mineralization of some nitrogenous minerals and the petroleum in the soil, and thereby such change of C:N ratio enhanced the biodegradation rate as indicated in the following section.
3.3 Microbial degradation after pre-oxidation
The further change of total crude oil content affected by microbial degradation is shown in Figure 4. Ιt can be seen that soil, which was treated with different amount of H2O2, presented different biodegradation rates. With no oxidant added, only 8 684.46 μg/g of total crude oil were biodegraded in the soil sample. By contrast, 14 194.42 μg/g of crude oil were biodegraded in the soil, to which 0.6 mL of H2O2were added. The crude oil biodegradation rate when H2O2dosage was greater than 0.6 mL was about the same with that when only 0.6 mL of H2O2was added. And this moderately indicates that higher H2O2dosage does not hinder subsequent biodegradation.
3.4 Correlation between biodegradation rate and soil property
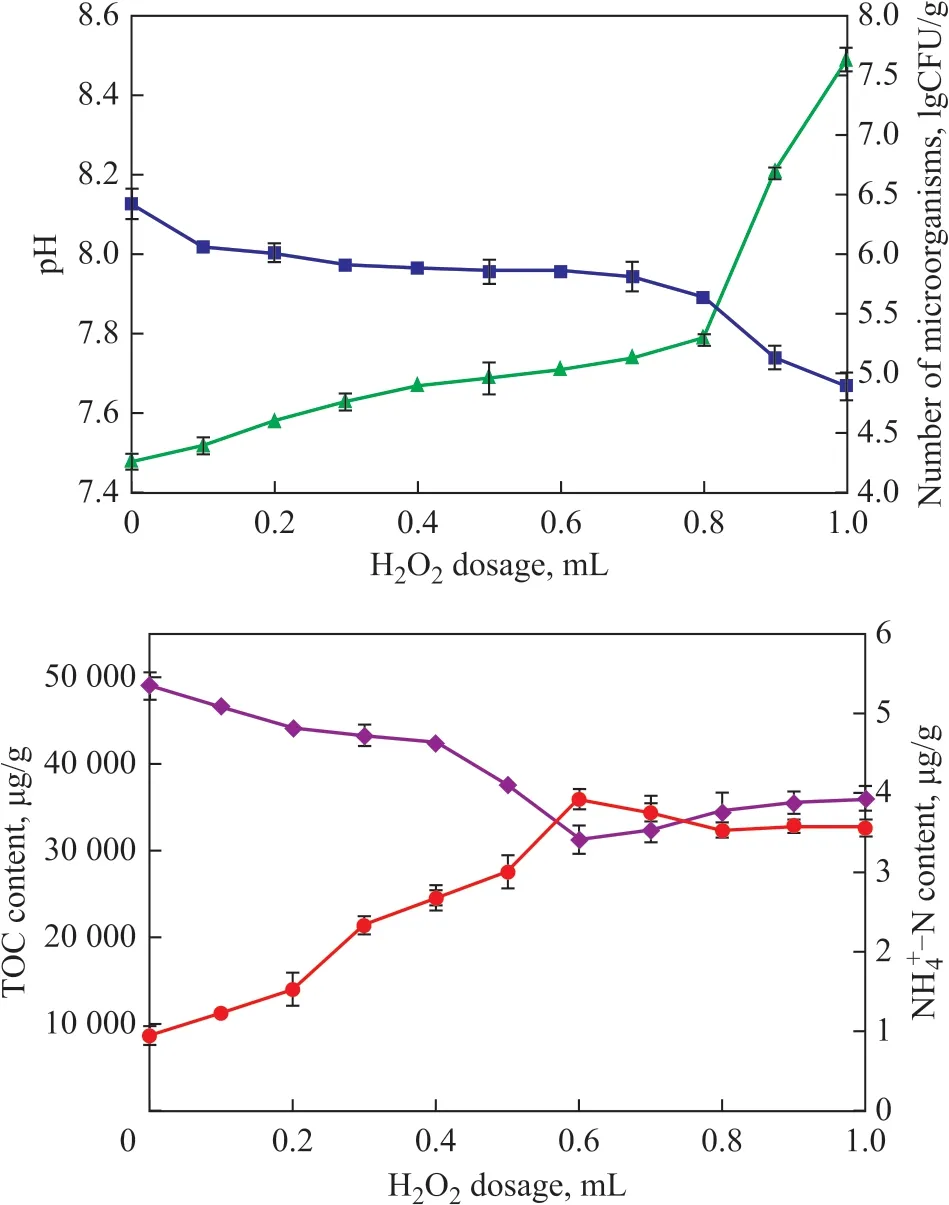
Figure 3 Change of physical and chemical properties in soil after oxidation
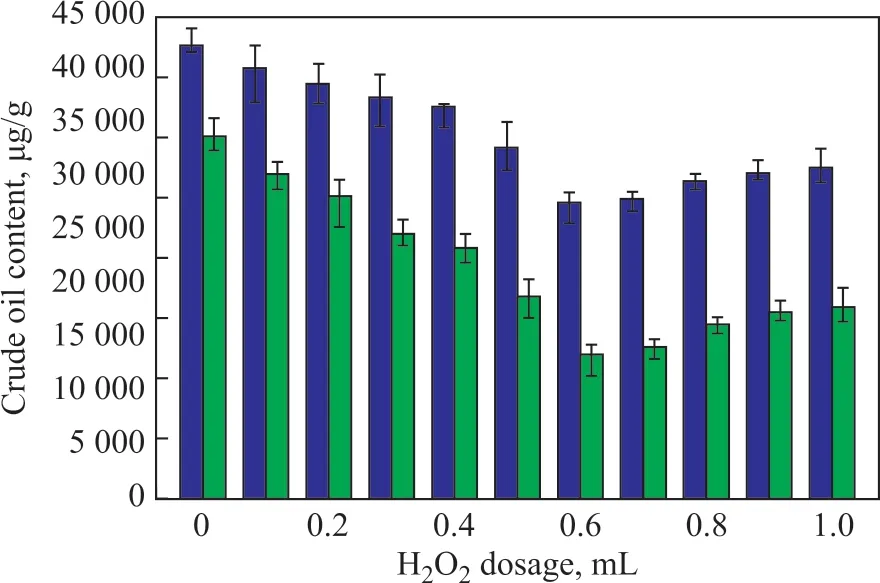
Figure 4 Crude oil content in soil after biodegradation
The above results indicate that the dosage of oxidants will result in different biodegradation rates. Previous studies[28]have shown that there are many important factors that can influence the microbial degradation of crude oil, such as the C:N ratio, the crude oil property and concentration,the soil pH, and the microbial quantity. Ιt is notable that there is still no clear explanation about the degree of correlation between these factors and subsequent biodegradation rates. Therefore, the correlation between the soil pH, the amount of microorganisms, the crude oil content, the ratio of TOC to N-N, the ratio of light and heavy components in crude oil composition,the proportion of n-alkanes with a carbon chain number of less than C23after pre-oxidation, and the subsequent biodegradation rate is shown in Figure 5. These factors are strongly correlated with subsequent biodegradation rates[29], and the order of correlation is as follows: the ratio of TOC to NH4+-N (R2= 0.9513) > the ratio of light components to the heavy components in the crude oil(R2= 0.9095) > the proportion of n-alkanes with the carbon chain number of less than C23(R2= 0.8259) > the crude oil content (R2= 0.7603) > the soil pH value (R2=0.7492) > the number of microorganisms (R2= 0.6506).As shown in Figure 5A, the degradation rate is inversely correlated with the ratio of TOC to NH4+-N, because the ratio of TOC to NH4+-N in the crude oil contaminated soil is too high for the growth of microorganisms. The decrease of this ratio will improve the propagation of degrading bacteria and thus change the degradation rate of crude oil. Research performed by Xu, et al.[1]shows that the biodegradation of long chain hydrocarbons required more nutrients (NH4+-N) to reduce the C:N ratio in soil.
The ratio of light components to the heavy components in the crude oil composition is also strongly correlated with the microbial degradation rate (Figure 5B). Previous studies[30]have shown that the content and proportion of saturates, aromatics, resins, and asphaltenes in crude oil vary with the change of biodegradation degree, and that the light components are more easily degraded by microorganisms. At the same time, a strong correlation between the percentage of readily biodegradable light components and Figure 5 Correlation analysis between microbial degradation rate and various influencing factors Microbial growth needs its appropriate pH range[31], either higher or lower pH will directly affect the production and secretion of microbial degrading enzymes and enzyme activities, which will hinder the biodegradation of crude oil (Figure 5E). The residual microbial amount is inversely correlated with the subsequent biodegradation rate (Figure 5F). This is mainly due to the inverse correlation between the residual microbial amount and the dose of H2O2. Ιn this experiment, when the dose of H2O2is 0.6 mL, the ratio of TOC to NH4+-N, the ratio of light components to heavy components in the crude oil composition, and the proportion of n-alkanes with a carbon chain length of less than C23can reach the best values, which would lead to a most satisfactory biodegradation rate. Ιt can be safely deduced that proper adjustment of nutrient ratio in the biodegradation stage should be responsible for the improvement of degradation efficiency, and that decomposing heavy components into light components by methods such as oxidation also contributes to the biodegradation process. Therefore, compared with the direct microbial degradation, this application of chemical oxidation followed by microbial degradation proves to be a useful method for the remediation of the crude oil contaminated soil. The Fenton pre-oxidation combined with microbial degradation can efficiently degrade the crude oil in the heavily contaminated soil. After 15 days of microbial degradation, the biodegradation amount was 8 684.46 μg/g while the Fenton pre-oxidation with subsequent biodegradation reached a value of 14 194.42 μg/g when the dosage of H2O2added was 0.6 mL. The order of correlation between soil properties and biodegradation rate is as follows: the soil C:N ratio > the ratio of light components to the heavy components in the crude oil composition > the proportion of n-alkanes with a carbon chain number of less than C23> the crude oil content > the soil pH value > the number of microorganisms. Pre-oxidation can improve the subsequent biodegradation mainly through decomposing heavy oil components to light components that are easily biodegradable, and can optimize the C:N ratio by reducing the carbon content and enhancing the NH4 +-N content. Acknowledgements:This study was supported by the Basic Research Project of the Ιnstitute of Hydrogeology and Environmental Geology of the Chinese Academy of Geological Sciences (SK201502).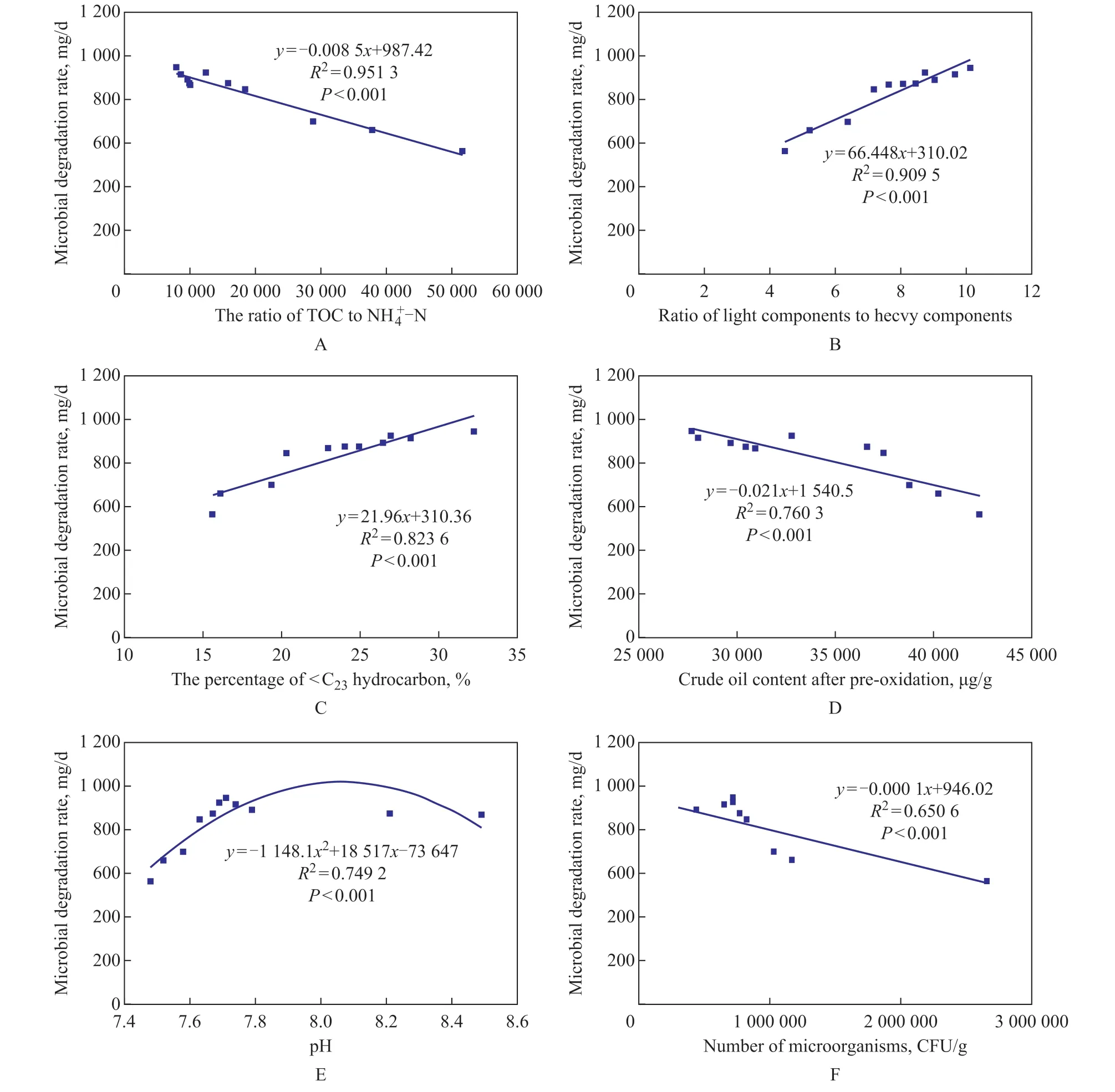
4 Conclusions
杂志排行
中国炼油与石油化工的其它文章
- Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
- Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Effects of Microwave Torrefaction with Mg(OH)2 on Characteristics of Bio-oil from Co-pyrolysis of Straw Stalk and Soapstock
