Extractive Desulfurization from Simulated Sulfur-Rich Naphtha
2019-10-31ShenXizhouLiZhiqiangFangLiuyaSongHaoGanFengShenZhi
Shen Xizhou; Li Zhiqiang; Fang Liuya; Song Hao; Gan Feng; Shen Zhi
(Lab of Petroleum Refining, Wuhan Institute of Technology, Wuhan 430070)
Abstract: Upon extractive desulfurization of sulfur-rich oil obtained after recycling raffinate, the effects of single and compound extractants, temperature, and extractant / oil ratio on desulfurization rate, oil loss rate, extractant loss rate, and selectivity coefficient were investigated. The results showed that the thiophene existing in the simulated sulfur-rich oil could be further removed by extraction. The single-stage desulfurization rates achieved by dimethyl sulfoxide (DMSO) and sulfolane(SF) were 53.51% and 47.36%, respectively, while the selectivity coefficient of SF was 1.13, which was higher than that of DMSO (with a selectivity coefficient of 1.04). For the compound extractant, a single-stage desulfurization rate of 49.59%and a selectivity coefficient of 1.40 were obtained by using DMSO+10%MEA as the extractant, which was higher than that of SF. Upon using 50%SF+50%DMSO as the extractant, the single-stage desulfurization rate reached 50.92% with a selectivity coefficient of 1.32. Based on the comprehensive consideration, the DMSO + 10% MEA extractant was selected as the best extractant for treating the sulfur-rich oil.
Key words: extractive desulfurization; simulated sulfur-rich oil; dimethyl sulfoxide; monoethanolamine
1 Introduction
Stringent regulations have been put forward on the sulfur content in fuel oils by most countries. How to produce low sulfur fuel has become a major concern for the refining industry[1-4]. At present, hydrodesulfurization (HDS)as a mature conventional desulfurization technology can effectively reduce the sulfur content. However, the traditional HDS process consumes hydrogen and needs high investment cost. Moreover, the octane number of gasoline is reduced due to the saturation of olefins during hydrogenation[5-7]. Therefore, the non-hydrodesulfurization technologies such as oxidation, extraction, adsorption, and biological desulfurization have attracted great attention,especially the extractive desulfurization (EDS) process.The EDS process with low investment cost can be carried out under mild conditions with less octane number loss of the product and the extractant can be recycled[8-9]. Wang Xinsheng, et al.[10]used N-formylmorpholine (NFM) to remove sulfides from the simulated catalytic cracking(FCC) gasoline. Mokhtar, et al.[11]compared the effect of DMF, acetonitrile (MeCN) and N-methyl pyrolidone(NMP) serving as extractants and found that DMF was the best extractant in term of recyclability and environmental aspects. Sana, et al.[12]claimed that DMF exhibited the most efficient characteristics of extractant by addition of ethylene glycol. Kianpour, et al.[13]utilized a green solvent polyethylene glycol as the extractant to remove benzothiophene from the simulated gasoline. Wang Junmin,et al.[14]compared the extractive performance of glycol for removing thiophene from the simulated FCC gasoline.
The process for extractive desulfurization of FCC gasoline has been applied at some refineries. By regenerating the solvents from gasoline extraction unit, a large amount of sulfur-rich oils are produced. Thiophene compounds have the highest proportion among all sulfides in the sulfurrich oil. Refineries usually adopt hydrogenation to treat the sulfur-rich oil, but there are some shortcomings such as high hydrogen consumption, high energy consumption, and inefficient utilization of thiophene. Therefore, the extractive desulfurization technology featuring simple operation,low cost, and recovery of thiophene may be more suitable for the desulfurization of sulfur-rich oil. The sulfur-rich oil has a strong polarity because of its high content of aromatics, olefins, and sulphides, along with low alkanes content. Therefore, since it is more difficult to extract sulfides from the sulfur-rich oil, it is necessary to explore different extractants compared to the case for FCC gasoline desulfurization. Although the extractive desulfurization technology has been gradually applied in industry, there are few studies on extractive desulfurization of the sulfurrich oil referred to in the literature. According to the particularity of chemical composition of sulfur-rich oil, this paper attempts to find one or several extractants to remove thiophene from the simulated sulfur-rich oil and to further study the desulfurization rate, oil loss rate, extractant loss rate, and selectivity of extractants.
2 Experimental
2.1 Materials
(1) Experimental instrument: The electronic balance (with an accuracy of 0.0001 g) was purchased from the Mettler Toledo Ιnstruments Co., Ltd. The ultraviolet fluorescence sulfur detector ST0689-2A was purchased from the Wuhan Yanrun Technology Development Co., Ltd.
(2) Chemical reagents: Benzene (AR grade), toluene (AR grade), xylene (AR grade), isooctane (AR grade), octane(AR grade), 1-octene (AR grade), cyclohexane (AR grade),absolute ethanol (AR grade), dimethyl sulfoxide (DMSO,AR grade), monoethanolamine (MEA, AR grade), sulfone(SF, AR grade) were all purchased from the Sinopharm Chemical Reagent Co., Ltd. NFM (AR grade) was obtained from Aladdin. Thiophene (99%) was acquired from Mclean.
2.2 Extractive desulfurization of simulated sulfurrich oil
The sulfur-rich oil, originated from extractive desulfurization unit of the Shandong Jingbo Petrochemical Co., Ltd., was analyzed by the Agilent 7890-5977A GC-MS, with the chemical composition shown in Table 1. A sulfur content of 2 051 mg/L was detected by the ultraviolet fluorescence sulfur detector.The simulated sulfur-rich oil was prepared according to Table 1. Benzene (12.8%), toluene (30.79%), xylene(24.2%), isooctane (15.24%), n-octane (2.53%), 1-octene(3.09%), cyclohexane (11.35%), and thiophene were used to simulate aromatics, isoalkanes, n-alkanes, olefins,cycloalkanes, and sulfides, respectively. Ιn order to facilitate detection, the sulfur content of the simulated sulfur-rich oil was reduced by about 90%.
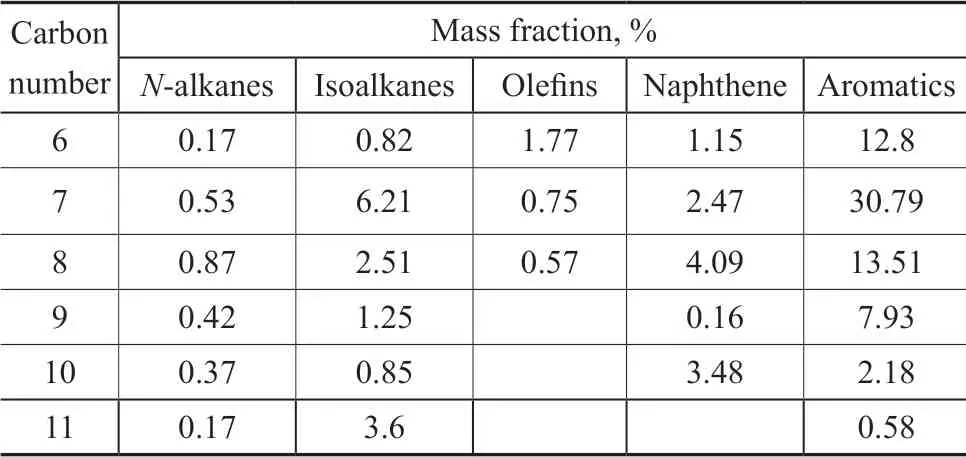
Table 1 The chemical composition of sulfur-rich oil
20 g of oil and 20 g of extractant were added into a dry 250-mL flask and then were stirred for half an hour at room temperature. Then, the liquid was poured into a dry 125-mL separating funnel for settling for an hour and then the lower solvent was separated and weighed. The upper oil was poured into a clean 125-mL separating funnel,and washed with 20 g of distilled water. After settling and layering, the lower liquid was released and weighed.Then the upper oil was continually washed three times with distilled water. After settling and layering, the upper oil was taken for analysis.
3 Results and Discussion
3.1 Extractive desulfurization performance of single solvent
The results of the extraction of the simulated sulfur-rich oil by NFM, DMSO and sulfolane (SF) are shown in Table 2. The initial sulfur content of the simulated sulfur rich oil was 240.22 mg/L.
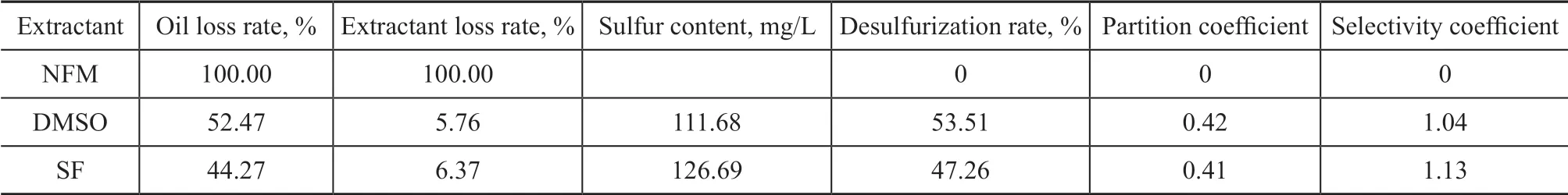
Table 2 Results of extractive desulfurization by three single extractants, NFM, DMSO, and SF
Ιt can be seen from Table 2 that the oil loss rate and the extractant loss rate obtained during extraction with NFM were 100%, which demonstrated that NFM and the simulated sulfur-rich oil were completely soluble after stirring for 30 min. This could occur due to the fact that the simulated sulfur-rich oil had high content of aromatics and olefins, while the six-membered ring of NFM was similar to the benzene ring of aromatics, and the similar polarity between NFM and sulfur-rich oils led to the complete miscibility of two phases. The single-stage desulfurization rates achieved by DMSO and SF were 53.51% and 47.26%, respectively, while the selectivity coefficient of SF was 1.13, which was higher than that of DMSO having a selectivity coefficient of 1.04.
3.2 Extraction effect of three desulfurizers with lossreducing extractant
The results of the extraction treatment of the simulated sulfur-rich oil by NFM+MEA and NFM+water are shown in Table 3. The initial sulfur content of the oil was 286.12 mg/L.Ιt can be seen from Table 3 that with an increasing dosage of MEA or water in NFM, the selectivity coefficientincreased gradually, but the desulfurization rate, oil loss rate, and extractant loss rate decreased regularly. Ιt could be assumed that the hydrogen bonds between the hydrogen atoms of MEA or H2O and the oxygen atoms of the six-membered ring in NFM led to the reduction of π-electron cloud density and the decrease of NFM polarity. The reduction of π-π complexation could cause the decrease of desulfurization rate and oil loss rate.The selectivity achieved by adding water in NFM was higher than that by adding MEA, but the desulfurization rate decreased by nearly 20%. A desulfurization rate of 42.88% and a selectivity coefficient of 0.44 achieved by the NFM + 10% MEA system were lower than that of DMSO and SF. Therefore, judging from the perspectives of desulfurization rate and selectivity coefficient, both W+NFM and MEA + NFM were not suitable for being used as compound extractants.
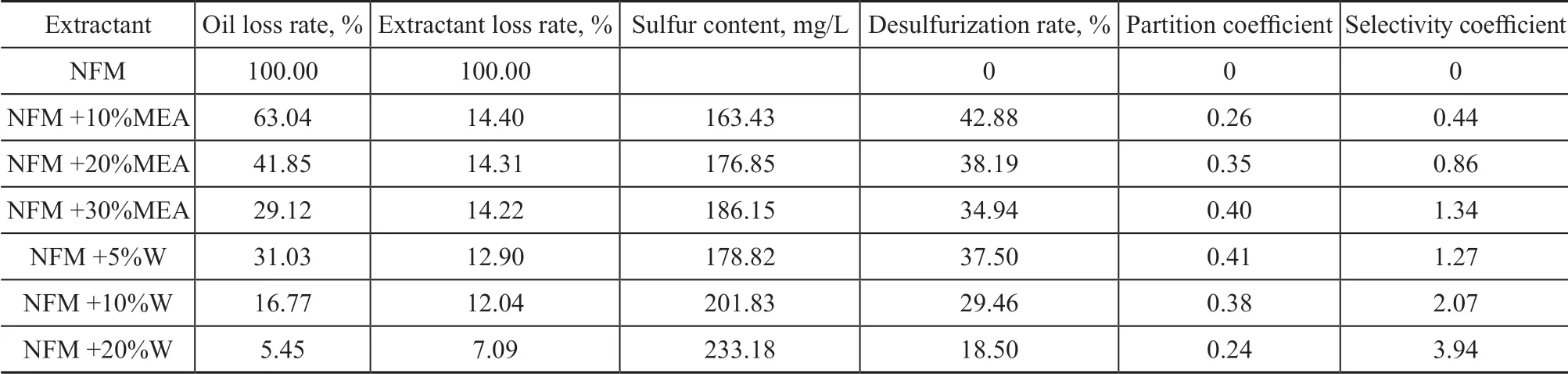
Table 3 Results of extractive desulfurization by complex solvent NFM+MEA or NFM+water (W)
The results of the extraction of the simulated sulfur-rich oil by DMSO+MEA and DMSO+W are shown in Table 4.The initial sulfur content of the simulated sulfur-rich oil was 274.62 mg/L.
As illustrated in Table 4, with the increase of MEA or water content in DMSO, the desulfurization rate, oil loss rate, and extractant loss rate decreased to some extent,while the selectivity coefficient increased. The possible reason for the above results was that the hydrogen atoms of MEA or H2O could combine with lone pair electrons of S atoms in DMSO to occupy the vacant orbital.However, the main mechanism of DMSO extraction is the complexation between the lone pair electrons of S atom and π bond of aromatic sulfides. Since the vacant orbital of S atoms has been occupied, the complexation reaction between the saturated DMSO and aromatics or thiophene rings is reduced, which can directly cause the decrease of desulfurization rate and oil loss rate. Upon considering the desulfurization rate and selectivity coefficient,DMSO+10% MEA had the best extraction effect with a desulfurization rate of 49.59% and a selectivity coefficient of 1.40, which were higher than those of SF.Compared with DMSO, although the desulfurization rate decreased slightly, the selectivity coefficient achieved by DMSO+10% MEA increased from 1.04 to 1.40 with an improvement of 34.62%.
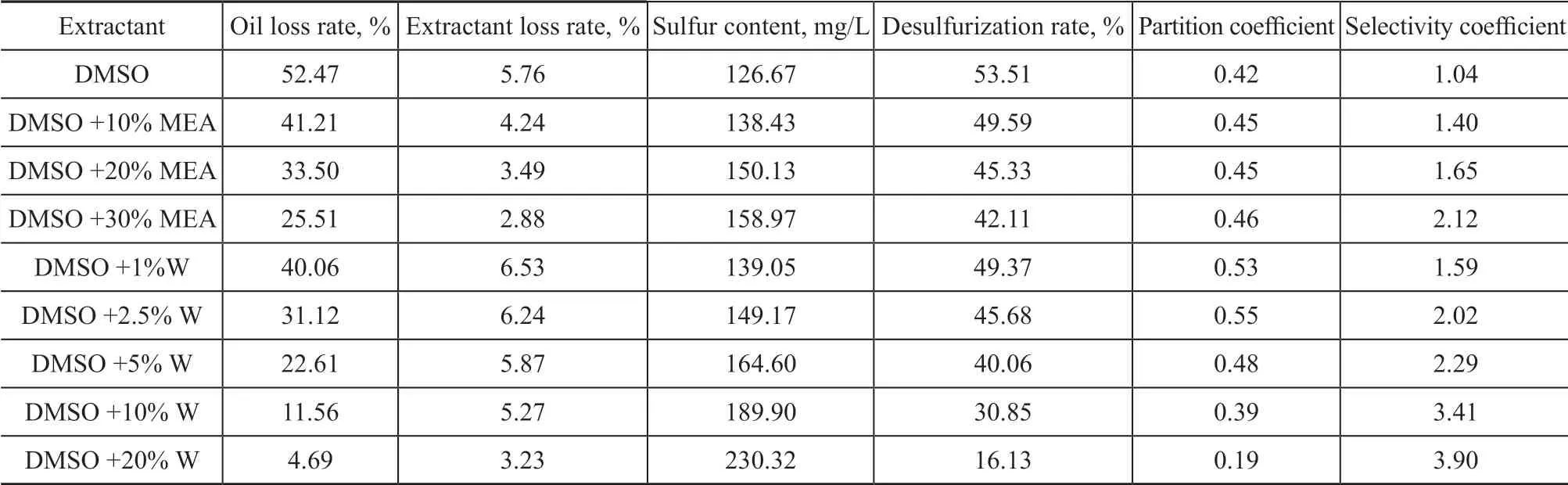
Table 4 Results of extractive desulfurization by complex solvent DMSO+MEA or NFM+W
The results of the extraction of the simulated sulfur-rich oil by SF+MEA and SF+W are shown in Table 5. The initial sulfur content of the oil was 259.43 mg/L.
Table 5 shows that with the increase of MEA or W content in SF, the desulfurization rate, oil loss rate,and extractant loss rate gradually decreased, while the selectivity coefficient increased. This could occur due to the hydrogen bond association between the H atoms in MEA or water and the O atoms which can provide common electron pairs with the S atom of SF,resulting in the decrease of π-electron cloud density of SF quinary ring. Therefore the π-π complexation effect between SF and aromatic ring or thiophene ring weakens, leading to the decrease of the desulfurization rate and the oil loss rate. The single-stage desulfurization rate of SF + 10% MEA could reach 43.53% and the selectivity coefficient was 1.58. Compared with SF,the desulfurization rate decreased by 7.89%, while the selectivity coefficient increased by 43.11%. Ιn contrast with DMSO, the desulfurization rate decreased by 9.98%and the selectivity coefficient increased by 54.81%, when SF + 10% MEA was used as the compound extractant.Compared with DMSO + 10% MEA, the desulfurization rate decreased by 6.06%, and the selectivity coefficient increased by 19.62%, when SF + 10% MEA was used as the compound extractant. Therefore, DMSO + 10% MEA was selected as the best complex extractant.
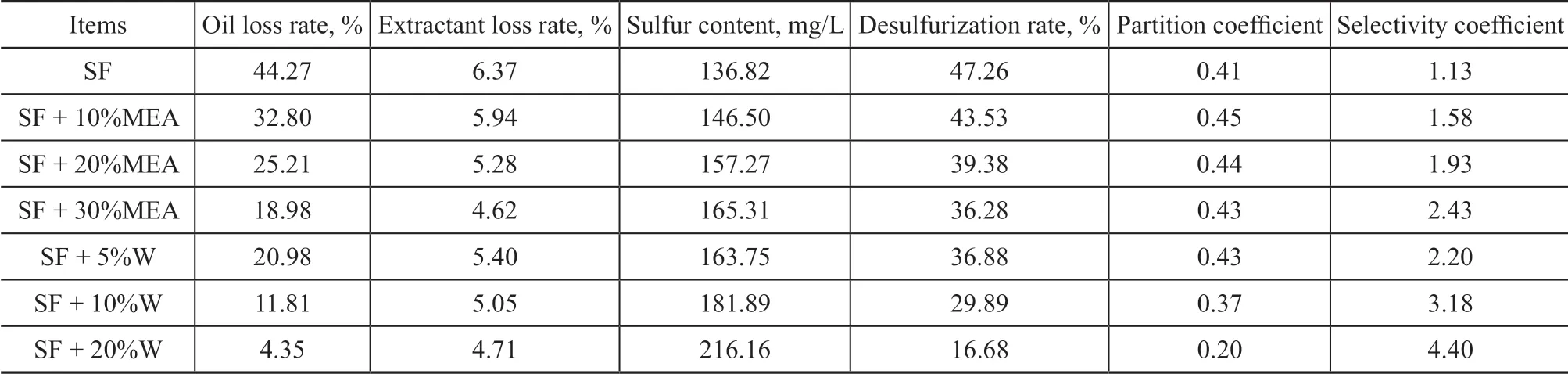
Table 5 Results of extractive desulfurization by complex solvent SF+MEA or SF+W
3.3 Compounds of different single extractants
The results of the extraction of the simulated sulfur-rich oil by the compounds NFM and DMSO are shown in Table 6. The initial sulfur content of oil was 278.22 mg/L.Ιt can be seen from Table 6 that with an increasingproportion of NFM in DMSO, the oil loss rate increased gradually, and the desulfurization rate changed little, while the selectivity coefficient decreased greatly. The obvious decrease of selectivity coefficient might be attributed to the similarity in polarity between the six-membered ring of NFM and the aromatic rings in the sulfur-rich oil. The desulfurization rate and selectivity coefficient of NFM+DMSO were lower than that of DMSO, no matter to what proportion they were compounded. Hence, the compound extractant DMSO+NFM was not suitable for being used as the compound extractant.

Table 6 Results of extractive desulfurization by complex solvent NFM+DMSO
The results of the extraction of the simulated sulfur-rich oil by the compounds of NFM and DMSO are shown in Table 7. The initial sulfur content of the oil was 254.14 mg/L.
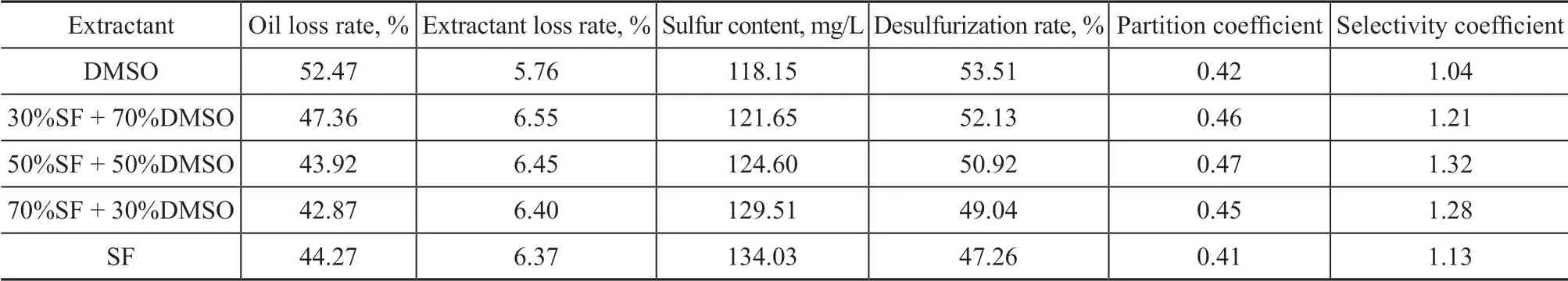
Table 7 Results of extractive desulfurization by complex solvent SF+DMSO
Ιt can be seen from Table 7 that the oil loss rate gradually decreased, and the desulfurization rate also slightly dwindled with an increasing proportion of SF in DMSO,while the selectivity coefficient increased firstly and then decreased. Upon using the compound extractant containing 50%SF+50%DMSO, the single-stage desulfurization rate was 50.92% and the selectivity coefficient was 1.32, which were higher than those achieved by SF. Compared to DMSO,although the desulfurization rate achieved by the compound extractant decreased somewhat, the selectivity coefficient increased from 1.04 to 1.32 with a growth rate of 26.92%. The reason could be that the lone pair electrons of oxygen atoms in SF combined with the solitary lone pair electrons on S atom of DMSO to form an eight-electron stable structure which could then reduce the polarity of DMSO, resulting in the decrease of desulfurization ability of DMSO. On the other hand,the five-membered ring of SF has a certain desulfurization ability, causing a slight decrease of the desulfurization rate.According to the principle of “like dissolves like”, this may occur because SF and thiophene have similar five-membered ring structures and can achieve high desulfurization rate, but the similarity between six-membered rings of aromatics and five-membered ring of SF is relatively poor, resulting in a high selectivity of SF. However, NFM is just the opposite to the above understanding.
The results of desulfurization achieved by DMSO+10% MEA and 50%SF+50%DMSO with different ratios of extractant to oil are shown in Figure 1 and Figure 2, respectively. The initial sulfur content of the oil was 262.97 mg/L.

Figure 1 Oil loss, extractant loss and desulfurization rate vs. mass ratio of extractant/oil for DMSO+10% MEA,50%DMSO+ 50%SF and SF
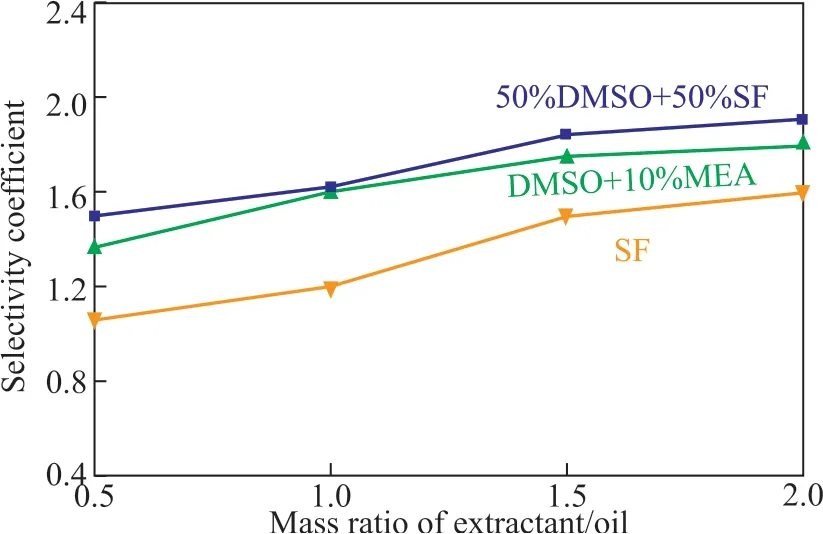
Figure 2 Selectivity coefficient vs. mass ratio of extractant/oil for DMSO+10%MEA, 50%DMSO + 50%SF, and SF
Ιt can be found from Figure 1 and Figure 2 that the oil loss rate and the desulfurization rate increased gradually with the increase of solvent-oil ratio, and the selectivity coefficient also showed a gradual upward trend, but the extractant loss rate decreased regularly. Ιt can be concluded that the increasing extractant/oil ratio could improve the desulfurization rate, but the oil loss rate also increased substantially. Fortunately, when the ratio was increased from 1:1 to 1.5:1, the oil loss rate increased only slightly. Especially, the extractant loss rate decreased by more than 50%, and the desulfurization rate could increase by 12%. Therefore, a ratio of 1.5:1 could be chosen as the best solvent/oil ratio in this paper. Under these conditions,the selectivity coefficient and the desulfurization rate obtained by using DMSO+10% MEA were slightly higher than those achieved by 50%SF + 50%DMSO. Compared with SF, the desulfurization rate increased a little, while the selectivity coefficient increased significantly. Finally,the extractant DMSO+10% MEA was selected as the optimum compound extractant.
The extraction performance of DMSO+10% MEA at different temperatures are shown in Figure 3. The initial sulfur content of the oil was 262.42 mg/L.
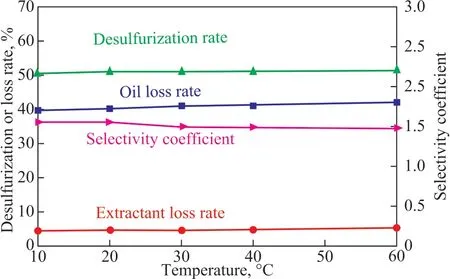
Figure 3 Selectivity coefficient of DMSO+10% MEA at different temperatures
Ιt can be clearly observed from Figure 3 that with a rising temperature in the range of 10 - 60 °C, the oil loss rate,the extractant loss rate, the desulfurization rate and the selectivity coefficient did not change significantly.
4 Conclusions
The extractive desulfurization of simulated sulfur-rich oil was carried out using single or several solvents as the extractant. The single-stage desulfurization rates achieved by DMSO and SF were 53.51% and 47.36%, respectively.NFM should not be used as extractant. Compared with using DMSO alone, the desulfurization rate achieved by DSMO+10%MEA decreased slightly, while the selectivity coefficient increased from 1.04 to 1.40. Upon using 50%SF+50%DMSO as the compound extractant, the single-stage desulfurization rate was 50.92% along with a selectivity coefficient of 1.32. The selectivity coefficient and the desulfurization rate achieved by DMSO+10%MEA were slightly higher than those achieved by 50%SF+50%DMSO at an optimum extractant/oil ratio of 1.5:1. Based on the comprehensive consideration, the DMSO + 10% MEA was selected as the best extractant for desulfurization of the sulfur-rich oil.
杂志排行
中国炼油与石油化工的其它文章
- Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
- Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Effects of Microwave Torrefaction with Mg(OH)2 on Characteristics of Bio-oil from Co-pyrolysis of Straw Stalk and Soapstock
