Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways
2019-09-25ChunXiaoLiJianGuoGaoXingYongWanYiChenChengFuXuZeMinFengHangZengYiMingLinHanMaPingXuChaoHuiYuYouMingLi
Chun-Xiao Li, Jian-Guo Gao, Xing-Yong Wan, Yi Chen, Cheng-Fu Xu, Ze-Min Feng, Hang Zeng, Yi-Ming Lin,Han Ma, Ping Xu, Chao-Hui Yu, You-Ming Li
Abstract BACKGROUND Allyl isothiocyanate (AITC), a classic anti-inflammatory and antitumorigenic agent, was recently identified as a potential treatment for obesity and insulin resistance. However, little is known about its direct impact on the liver.AIM To investigate the effect and underlying mechanism of AITC in nonalcoholic fatty liver disease (commonly referred to as NAFLD).METHODS To establish a mouse and cellular model of NAFLD, C57BL/6 mice were fed a high fat diet (HFD) for 8 wk, and AML-12 cells were treated with 200 μM palmitate acid for 24 h. For AITC treatment, mice were administered AITC (100 mg/kg/d) orally and AML-12 cells were treated with AITC (20 μmol/L).RESULTS AITC significantly ameliorated HFD-induced weight gain, hepatic lipid accumulation and inflammation in vivo. Furthermore, serum alanine aminotransferase and aspartate aminotransferase levels were markedly reduced in AITC-treated mice. Mechanistically, AITC significantly downregulated the protein levels of sterol regulatory elementbinding protein 1 (SREBP1) and its lipogenesis target genes and upregulated the levels of proteins involved in fatty acid β-oxidation, as well as the upstream mediators Sirtuin 1 (Sirt1) and AMPactivated protein kinase α (AMPKα), in the livers of HFD-fed mice. AITC also attenuated the nuclear factor kappa B (NF-κB) signaling pathway. Consistently,AITC relieved palmitate acid-induced lipid accumulation and inflammation in AML-12 cells in vitro through the Sirt1/AMPK and NF-κB signaling pathways.Importantly, further studies showed that the curative effect of AITC on lipid accumulation was abolished by siRNA-mediated knockdown of either Sirt1 or AMPKα in AML-12 cells.CONCLUSION AITC significantly ameliorates hepatic steatosis and inflammation by activating the Sirt1/AMPK pathway and inhibiting the NF-κB pathway. Therefore, AITC is a potential therapeutic agent for NAFLD.
Key words: Allyl isothiocyanate; Nonalcoholic fatty liver disease; Hepatic steatosis; Liver inflammation
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is currently one of the most epidemic chronic liver diseases worldwide and has become an enormous clinical and economic burden. NAFLD affects approximately one-fourth of adults globally[1]. NAFLD represents a wide spectrum of disease stages, ranging from simple steatosis to nonalcoholic steatohepatitis (commonly known as NASH), which is characterized by hepatocellular injury and inflammation and may eventually progress to cirrhosis and hepatocellular carcinoma[2]. Furthermore, NAFLD is a strong risk factor for type 2 diabetes, atherosclerosis, cardiovascular disease and chronic kidney disease[3-5].However, its pathogenesis remains unclear, and current therapeutic options are relatively limited. No treatments for NAFLD other than lifestyle modifications leading to weight loss and increased physical activity are currently approved[6]. Therefore,there is an urgent need to develop effective medical treatments for NAFLD.
Hepatic steatosis, characterized by excessive triglyceride (TG) accumulation in hepatocytes, is strongly associated with chronic hepatic inflammation and insulin resistance[7]. Furthermore, the IκB kinase (IKK)/nuclear factor kappa B (NF-κB)signaling pathway plays a crucial role in the development of metabolic disorders,including NAFLD, and especially in hepatic inflammation[8-10].
Sirtuin 1 (Sirt1) is a highly conserved nicotinamide adenine dinucleotide-dependent protein deacetylase that regulates a wide variety of biological functions in mammals,including lipid metabolism and energy homeostasis[11,12]. AMP-activated protein kinase (AMPK) functions as an energy switch that controls several cellular processes,such as lipid metabolism, by inhibiting hepatic lipogenesis and stimulating fatty acid oxidation[13]. Previous studies revealed that Sirt1 is an important regulator of hepatic lipogenesis and fatty acid oxidation through multiple nutrient sensors, including sterol regulatory elementbinding protein 1 (SREBP1), peroxisome proliferatoractivated receptor gamma coactivator1α (PGC1α) and peroxisome proliferatoractivated receptor α (PPARα)[14-16]. Furthermore, Sirt1 was shown to regulate AMPK activation in NAFLD, resulting in enhanced lipolysis and β-oxidation, as well as ameliorated hepatic steatosis[17-19].
Allyl isothiocyanate (AITC) is derived from its precursor sinigrin, which is present in many common cruciferous vegetables and is widely consumed by humans[20].Myrosinase in the intestinal microflora catalyzes the hydrolysis of sinigrin to AITC in both humans and animals[20,21]. Previous studies have shown that AITC exhibits antiinflammatory and anticancer activities[22-24]. Recently, AITC was identified as a potential novel treatment for diet-induced obesity and insulin resistance through its modulation of mitochondrial dysfunction[25]. Another study revealed that AITC can augment basal and epinephrine-induced lipolysis in adipocytes and intensify hydrolysis of TG in the blood serum of rats[26]. Moreover, a previous study showed AITC effectively inhibits adipogenic differentiation of 3T3-L1 preadipocytes and suppresses expression of genes up-regulated during adipogenesis[27]. However, little is known about its direct impact on the liver or its underlying mechanism.
Herein, we conducted bothin vivoandin vitroexperiments to explore the effect of AITC on NAFLD, focusing on its role in hepatic steatosis and inflammatory responses, and to elucidate its mechanism of action.
MATERIALS AND METHODS
Animal experiments
All experiments were conducted with approval of the First Affiliated Hospital of Zhejiang University Institutional Animal Care and Use Committee (Permit number:2016-231). Six-week-old male C57BL/6 mice were purchased from B&K Laboratory Animal Corp., Ltd. (Shanghai, China). After acclimatization for 2 wk with free access to food and water, mice were fed a standard chow diet (SCD) or high fat diet (HFD)(60% fat-derived calories, 20% carbohydrate-derived calories, and 20% proteinderived calories; D12492, Research Diets, New Brunswick, NJ, United States). In general, mice were given SCD or HFD feeding for a total of 8 wk, and from the 5thwk,SCD-fed mice began to receive corn oil (control) (n= 10), and HFD-fed mice were randomly divided into two groups to receive 100 mg/kg/d AITC (99.7%; Sigma-Aldrich, St. Louis, MO, United States) (n= 10) or corn oil (n= 9) daily by gavage for an additional 4 wk while remaining on SCD or HFD.
Cell culture and treatments
The established immortalized AML-12 mouse hepatocyte cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai,China). AML-12 cells were cultured in DMEM/F12 (1:1) medium supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, 0.1 µmol/L dexamethasone,and 1% insulin-transferrin-selenium Liquid Media Supplement (I3146; Sigma-Aldrich). To establish a cellular model of NAFLD, palmitate acid (PA) (Sigma-Aldrich) was dissolved in bovine serum albumin (Sangon Biotech, Shanghai, China),and then AML-12 cells were exposed to 200 μM PA for 24 h. To investigate the effect of AITC on lipid depositionin vitro, PA-stimulated AML-12 cells in serum-free conditions were treated with AITC (20 μmol/L) or dimethyl sulfoxide (commonly known as DMSO) (vehicle) for 24 h.
AML-12 cells were transfected withSirt1small interfering RNA (siRNA) #1 (target sequence 5’-GATGAAGTTGACCTCCTCA-3’),Sirt1siRNA #2 (target sequence 5’-CCGATGGACTCCTCACTAA-3’),Sirt1siRNA #3 (target sequence 5’-GGTT GTTAATGAAGCTATA-3’),AMPKαsiRNA #1 (target sequence 5’-GCAGAAGA TTCGGAGCCTT-3’),AMPKαsiRNA #2 (target sequence 5’-GCACACCCTGGA TGAATTA-3’),AMPKαsiRNA #3 (target sequence 5’-GCAGAAGTTTGTAGAGCAA-3’) or the corresponding scrambled control (RIBOBIO, Guangzhou, China) using Lipofectamine RNAiMAX (Invitrogen, Shanghai, China) according to the manufacturer’s protocol. After 48 h, the cells were incubated in medium containing PA with or without AITC for an additional 24 h.
Hepatic and cellular TG assay
Hepatic and cellular TG contents were measured using a commercial kit (Applygen Technologies Inc., Beijing, China) according to the manufacturer’s protocol.
Hematoxylin-eosin and oil red O staining
Mouse liver tissues were rapidly harvested, fixed in 10% formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin (commonly known as H&E) for histological examination. Frozen liver sections (8 μm) and cells in 6-well plates were stained with oil red O (Sigma-Aldrich) to assess lipid accumulation.
Metabolic measurements
Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol and uric acid levels were determined with a Hitachi 7600 autoanalyzer(Hitachi, Tokyo, Japan) according to the manufacturer’s instructions.
Quantitative real-time PCR
Total mRNA was extracted from liver tissues or cultured cells using RNA plus(Takara, Dalian, China) and reverse transcribed into cDNA using a PrimeScript® RT reagent kit (Takara, Japan) according to the manufacturer’s protocol. Real-time PCR was performed on an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, United States) using SYBR Green (Takara) to quantify PCR amplification. Relative mRNA expression levels of target genes were normalized to β-actin or GAPDH mRNA levels for each sample.
Western blot analysis
Liver tissue samples and cells were lysed using RIPA buffer (Applygen Technologies Inc.) supplemented with protease and phosphatase inhibitors (Sigma). Equal amounts of extracted proteins were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Inc., Darmstadt, Germany). Membranes were blocked with 5%nonfat milk in TBST and then incubated overnight at 4°C with the following primary antibodies: Anti-Sirt1 (8469), anti-AMPKα (2603), anti-phosphorylated (p) AMPKα (p-AMPKα; 2535), anti-NF-κB p65 (6956), anti-p-NF-κB p65 (3033), anti-p-IKK (2697),anti-IKKα(2682), anti-IKKβ(8943), anti-p- inhibitor of nuclear factor kappa B alpha(IκBα) (2859), anti-IκBα (4812), anti-glyceraldehyde-3-phosphate dehydrogenase(GAPDH) (2118), and β-actin (3700) (Cell Signaling Technology); and anti-PGC1α(ab54481), anti-PPARα (ab8934), anti-carnitine palmitoyltransferase 1 α (CPT1α)(ab128568), anti-SREBP1 (ab3259), anti-fatty acid synthase (FAS) (ab128856), antistearoyl coenzyme A desaturase 1 (SCD1) (ab19862), and anti-tubulin (ab6160)(Abcam, Cambridge, United Kingdom). Proteins levels were evaluated using an enhanced ECL kit (Lianke, Hangzhou, China).
Statistical analysis
The statistical methods of this study were reviewed by Hong Zhang from the Department of Statistics and Finance, School of Management, University of Science and Technology of China. All statistical analyses were performed with SPSS 22 (IBM,Chicago, IL, United States). Data are presented as the average ± S.D. Statistical analysis was carried out using Student’s two-tailedt-test and one-way ANOVA with Tukey’s post-test.P-values less than 0.05 indicated statistical significance.
RESULTS
AITC reduces body weight, ameliorates hepatic steatosis and attenuates liver injury in an HFD mouse model
Supplementary Figure 1 shows the chemical structure of AITC. To explore the effect of AITC on NAFLD, an HFD model, which is quite similar to but does not completely mirror human NAFLD, was adopted. HFD-fed mice exhibited higher body weight(Figure 1A and B) and liver weight (Figure 1D), more serious lipid accumulation(Figure 1C), much higher TG (Figure 1E), ALT (Figure 1F), AST (Figure 1G),cholesterol (Figure 1H) and uric acid (Figure 1I) levels compared to SCD-fed mice,showing characteristics of NAFLDin vivo. The mice exhibited a significant decrease in body weight upon 100 mg/kg/d AITC administration (Figure 1A and B) and a slight but nonsignificant decrease in liver weight (Figure 1D). Notably, hepatic steatosis was improved after AITC treatment, as demonstrated by decreased hepatic TG levels(Figure 1E) and reduced lipid accumulation (H&E and oil red O staining; Figure 1C).As shown in Figure 1F-I, AITC attenuated HFD-induced liver injury, as evidenced by markedly decreased serum ALT and AST levels. These findings demonstrate that AITC ameliorates body weight, hepatic steatosis and liver injury in HFD-fed mice.
AITC attenuates de novo lipogenesis and promotes fatty acid β-oxidation by activating the Sirt1/AMPK pathway in vivo
To investigate the mechanisms by which AITC ameliorates hepatic steatosis, the expression levels of hepatic lipid metabolism-related genes were measured.SREBP1is the central transcription factor that enhances the expression of genes required for hepatic fatty acid synthesis and TG synthesis[28]. As a result, AITC treatmentin vivosignificantly downregulatedSREBP1protein levels and its target genes, includingSCD1andFAS(Figure 2A). In contrast, AITC increased the expression levels of proteins involved in fatty acid β-oxidation, such asPGC1α,PPARαandCPT1α, in the livers of HFD-fed mice (Figure 2A).
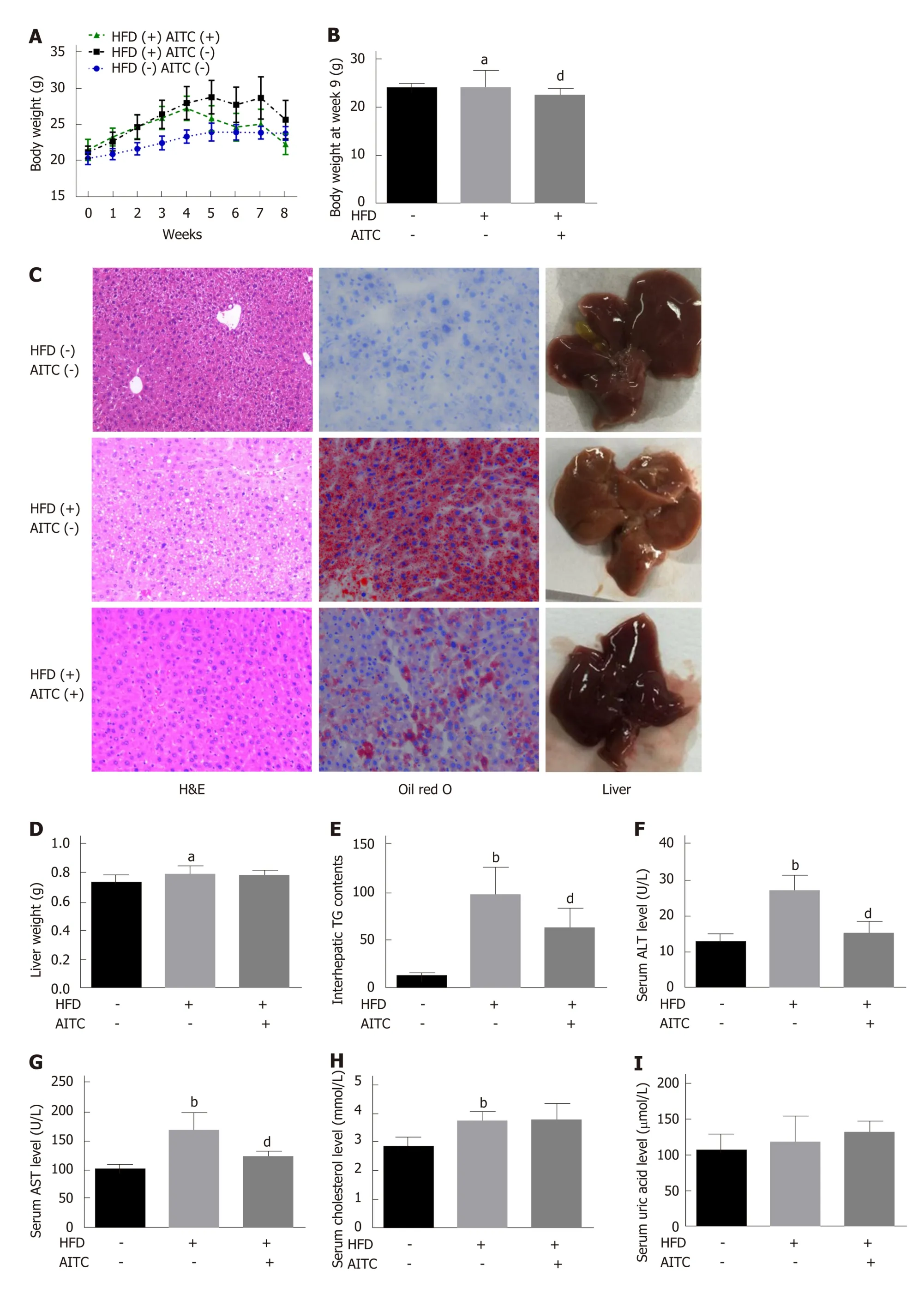
Figure 1 Allyl isothiocyanate reduces body weight, ameliorates hepatic steatosis and attenuates liver injury in high fat diet-fed mice. A: Body weight evaluated weekly; B: Body weight at week 8; C: Representative liver sections stained with hematoxylin and eosin (H&E) (left panel) or oil red O (middle panel) and macroscopic pictures of livers (right panel). D: Liver weight; E: Intrahepatic triglyceride (TG) content. Liver function was evaluated by detecting serum levels of alanine aminotransferase (ALT) (F), aspartate aminotransferase (AST) (G), total cholesterol (H) and uric acid (I). Scale bar in panel represents 100 μm. Data are presented as the mean ± S.D. aP < 0.05, bP < 0.01 vs HFD(-) AITC(-), dP < 0.01 vs HFD(+) AITC(-). HFD: High fat diet; AITC: Allyl isothiocyanate; TG: Triglyceride; H&E:Hematoxylin and eosin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Previous studies revealed thatSirt1activation attenuates hepatic steatosis in mice with diet- or genetics-induced obesity by regulating hepatic lipogenesis and fatty acid oxidation[16,29,30]. To investigate whether Sirt1/AMPK are involved in the amelioration of the HFD-induced dysregulation of hepatic lipid homeostasis by AITC, we evaluatedSirt1and p-AMPKαexpression levelsin vivo. As shown in Figure 2B, AITC treatment markedly elevated hepatic protein levels of Sirt1 and p-AMPKα.Collectively, these results suggest that AITC attenuatesde novolipogenesis and promotes fatty acid β-oxidation by activating the Sirt1/AMPK signaling pathwayin vivoin the liver tissues of HFD-fed mice.
AITC decreases HFD-induced inflammation by inhibiting the NF-κB signaling pathway in vivo
In addition to its protective role in lipogenesis and fatty acid β-oxidation, AITC treatment substantially decreased the transcription of proinflammatory cytokines[tumor necrosis factor α (TNFα), interleukin (IL)-1β, and IL-6] in the liver tissues of HFD-fed mice (Figure 3A).
To investigate whether IKK/NF-κB are involved in the amelioration of hepatic inflammation by AITC, the expression levels of IKK/NF-κB signaling pathway components were detected. As shown in Figure 3B, AITC treatment upregulatedIκBαprotein levels and downregulated IKK, IκBα, and p65 phosphorylation. These results indicate that AITC decreases inflammation by inhibiting the IKK/NF-κB signaling pathwayin vivo.
AITC alleviates PA-induced lipid accumulation in hepatocytes
Our above data revealed that AITC could ameliorate hepatic steatosisin vivo. To investigate the effect of AITC on PA-induced lipid depositionin vitro, we used AML-12 cells, which have been well documented as cellular models of NAFLD[31,32]. First, we evaluated the viability of AML-12 cells after AITC treatment. The cultured hepatocytes were treated with different concentrations of AITC for 24 h, and cell viability was measured using cholecystokinin-8 and lactate dehydrogenase release assays.Finally, we chose 20 μM as the optimal AITC concentration for subsequent experiments because this concentration did not significantly affect cell viability(Figure 4A and B). Then, PA-stimulated AML-12 cells were treated with AITC (20 μmol/L) or vehicle for 24 h. As shown in Figure 4C and D, AITC relieved the PAinduced increases in intracellular TG levels and lipid accumulation in AML-12 cells.These data demonstrate that AITC directly alleviates PA-induced lipid accumulation in hepatocytes.
AITC attenuates de novo lipogenesis and promotes fatty acid β-oxidation by activating the Sirt1/AMPK signaling pathway in vitro
Consistent with thein vivofindings, AITC treatment significantly decreased the protein levels of SREBP1 and its target genes, includingSCD1,FASand acetyl-CoA carboxylase (ACC), in PA-treated AML-12 cells (Figure 5A). In addition, AITC enhancedPGC1αexpression in PA-treated AML-12 cells (Figure 5C).
As shown in Figure 5Bin vitro, AITC upregulatedSirt1and p-AMPKαlevels,consistent with thein vivoresults (Figure 6A). These results indicate that AITC attenuates lipogenesis and promotes fatty acid β-oxidation by activating the Sirt1/AMPK signaling pathwayin vitro.
AITC attenuates inflammation by inhibiting the NF-κB signaling pathway in vitro
Consistent with thein vivofindings, AITC treatment significantly decreasedTNFαandIL-6mRNA levels in PA-treated AML-12 cells (Figure 6A). As shown in Figure 6B,AITC upregulated IκBα protein levels and downregulated IKK, IκBα, and p65 phosphorylation in PA-stimulated AML-12 cells. Taken together, these findings clearly indicate that AITC may attenuate inflammation by inhibiting theNF-κBsignaling pathwayin vitro.
Sirt1 or AMPKα knockdown abolishes the ability of AITC to mitigate TG levels
To investigate whether AITC can alleviate lipid accumulation through the Sirt1/AMPK pathway, Sirt1 expression in AML-12 cells was selectively knocked down by siRNA transfection (Figure 7A). Sirt1 knockdown abolished the ability of AITC to ameliorate TG accumulation and p-AMPKα and PGC1α upregulation induced by PA in AML-12 cells (Figure 7B and C). Consistently, AMPKα knockdown significantly reversed the effects of AITC on PA-induced intracellular TG accumulation in AML-12 cells (Figure 7D and E). Taken together, these findings verify that AITC ameliorates lipid accumulation by activating the Sirt1/AMPK signaling pathway.
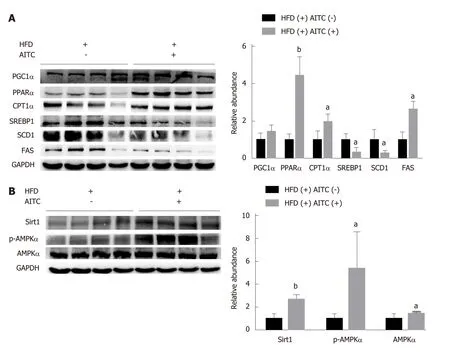
Figure 2 Allyl isothiocyanate upregulates the expression of proteins involved in fatty acid β-oxidation, downregulates the protein levels of lipogenesis genes, and activates the Sirtuin 1/AMP-activated protein kinase signaling pathway in the liver tissues of high fat diet-fed mice. A: The protein expression of sterol regulatory elementbinding protein 1 (SREBP1), its lipogenesis target genes (SCD1 and FAS), and genes involved in fatty acid β-oxidation, such as proliferatoractivated receptor gamma coactivator 1α (PGC1α), peroxisome proliferator-activated receptor α (PPARα) and carnitine palmitoyltransferase 1 α (CPT1α), was detected by western blot analysis. B: Sirtuin 1 (Sirt1), total and phosphorylated AMP-activated protein kinase α (AMPKα) protein expression was detected by western blot analysis. aP < 0.05, bP < 0.01 vs HFD(+) AITC(-). PGC1α: Proliferator-activated receptor gamma coactivator 1α; PPARα: Peroxisome proliferator-activated receptor α; CPT1α: Carnitine palmitoyltransferase 1 α; SREBP1: Sterol regulatory elementbinding protein 1; SCD1: Stearoyl coenzyme A desaturase 1; FAS: Fatty acid synthase; Sirt1: Sirtuin 1; AMPKα: AMP-activated protein kinase α; p-AMPKα: Phosphorylated AMP-activated protein kinase α; HFD: High fat diet; AITC: Allyl isothiocyanate.
DISCUSSION
In the current study, we investigated the effects and mechanisms of AITC on NAFLD development (summarized in Figure 8A). Our results demonstrate that AITC significantly ameliorates hepatic lipid accumulation by activating theSirt1/AMPKpathway and alleviates hepatic inflammatory responses by inhibiting theNF-κBpathway bothin vivoandin vitro.
Some studies suggested that AITC leads to better metabolic outcome. Kim YJ demonstrated that AITC effectively suppresses the expression of genes that are upregulated during adipogenesis, such as PPARγ, C/EBPα and FAS[27]. Miyata S showed that AITC reducesde novosynthesis of both fatty acids and cholesterol in human hepatoma Huh-7 cells[33]. These results indicate a physiological function of AITC in lipid metabolism regulation.
A previous study by Anhet al. demonstrated that AITC protects against HFDinduced obesity and insulin resistance in mice and that the protective effect of AITC may be partly mediated through the modulation of mitochondrial dysfunction in skeletal muscle cells and the liver[25]. However, the mechanism underlying the beneficial effects of AITC on hepatic steatosis and liver inflammation has not been clearly defined. Our results suggested that AITC ameliorates lipid accumulation by activating the Sirt1/AMPKα signaling pathway and improves inflammation in hepatocytes bothin vivoandin vitro.
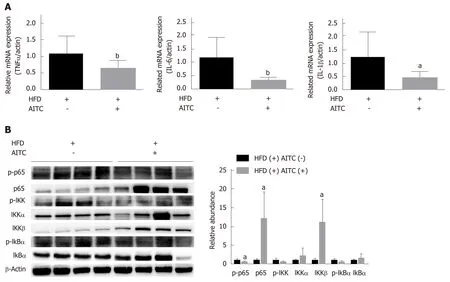
Figure 3 Allyl isothiocyanate attenuates hepatic inflammation and inhibits the IκB kinase /nuclear factor kappa B signaling pathway in the liver tissues of high fat diet-fed mice. A: The mRNA levels of proinflammatory cytokines in the liver of high fat diet (HFD)-fed control (n = 9) and HFD-fed allyl isothiocyanate (AITC)-treated mice (n = 10) were measured by quantitative real-time PCR. B: The protein expression of phosphorylated p65, p65, phosphorylated IκB kinase (IKK), IKKα,IKKβ, total and phosphorylated inhibitor of nuclear factor kappa B α (IκB α) in the liver was detected by western blot analysis. Data are presented as the mean ± S.D.aP < 0.05, bP < 0.01 vs HFD(+) AITC(-). TNFα: Tumor necrosis factor α; IL-6: Interleukin-6; IL-1β: Interleukin-1β; HFD: High fat diet; AITC: Allyl isothiocyanate; p-p65:Phosphorylated p65; IKK: IκB kinase; IκBα: Inhibitor of nuclear factor kappa B α.
A series of recent studies revealed that Sirt1 plays a critical role in various cellular and physiological processes, including lipid metabolism and energy homeostasis. A previous study demonstrated that Sirt1 transgenic mice are protected from HFDinduced metabolic damageviathe upregulation ofPGC1αand the inhibition of the NF-κB pathway[29]. Consistently, another study showed that hepatic overexpression ofSirt1attenuates hepatic steatosis, possibly by inhibiting endoplasmic reticulum stress in the liver of obese mice[30]. On the other hand, liver-specificSirt1-knockout mice develop hepatic steatosis, hepatic inflammation, and endoplasmic reticulum stress,which are associated with decreased hepatic fatty acid oxidation and increased lipogenesis[14,15]. In addition, these findings were supported by later reports that pharmacological activation ofSirt1protects against HFD-induced metabolic disorders[17,34]. Sirt1 regulates lipid homeostasis through multiple nutrient sensors such as SREBP1, AMPK, PGC1α, PPARα and the hepatocyte-derived hormone fibroblast growth factor 21[14-16,35]. In this study, we found that AITC significantly ameliorated hepatocyte lipid accumulation bothin vivoandin vitroby activating the Sirt1/AMPK signaling pathway, resulting in decreased expression of lipogenesis genes, such asSREBP1,SCD1andFAS, and increased expression of fatty acid β-oxidation genes,includingPGC1α,PPARαandCPT1α. Furthermore, another study showed that sulforaphane-inducedSirt1activation inhibits endoplasmic reticulum (commonly referred to as ER) stress and prevents cardiomyocytes from hypoxia/reoxygenation injuryin vitro[36]. As we have detectedSirt1activation after AITC application, further studies should be carried out to explore mechanisms of ER stress in this model.
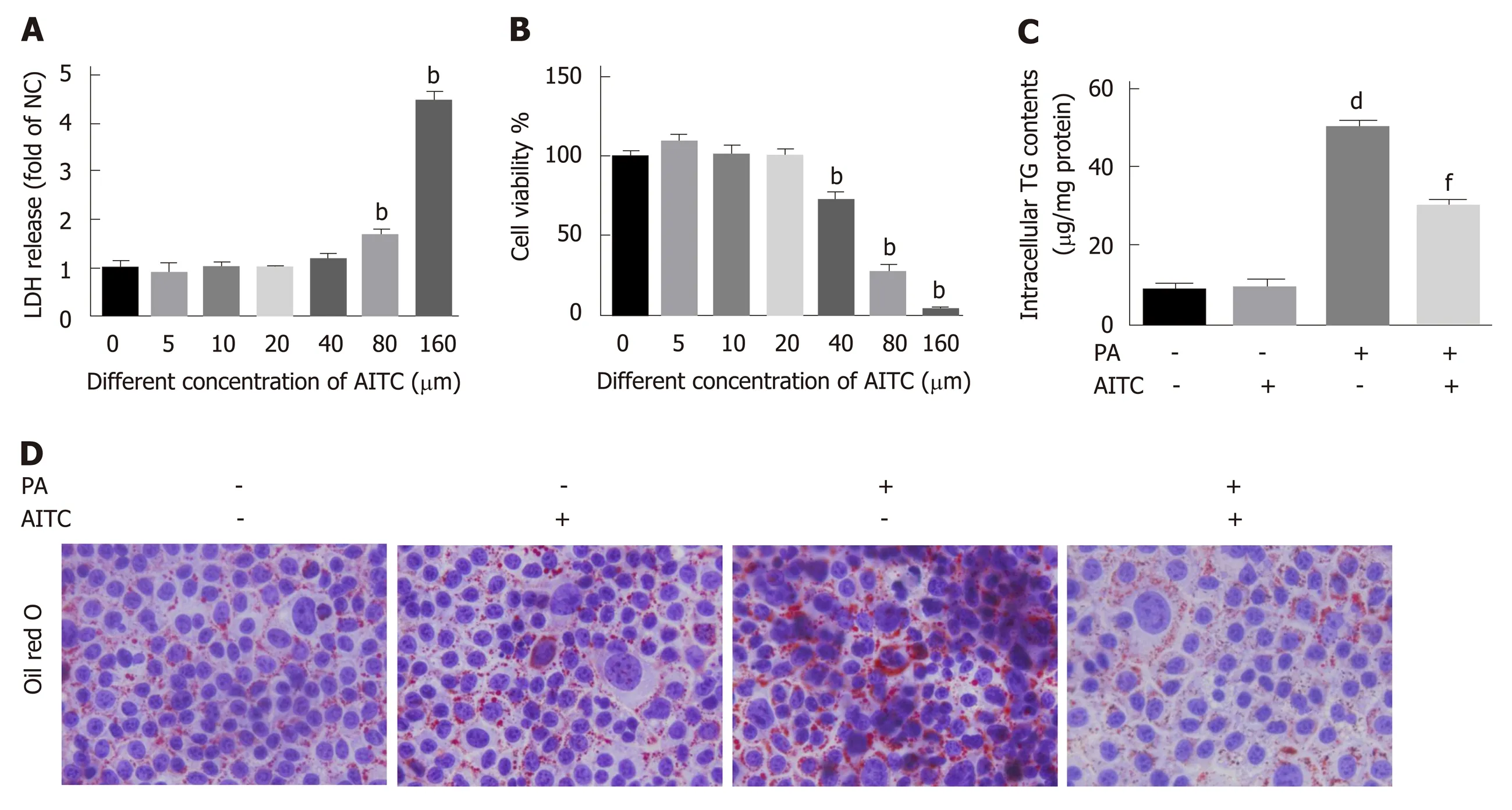
Figure 4 Allyl isothiocyanate alleviates palmitate acid-induced lipid accumulation in vitro. Palmitate acid (200 μmo/L)-stimulated AML-12 cells were treated with allyl isothiocyanate (20 μmo/L) or dimethyl sulfoxide (vehicle) for 24 h. A: Cytotoxicity was measured by the lactate dehydrogenase (LDH) release method in AML-12 cells (n = 3/group). B: Cell viability was measured using the cholecystokinin-8 (CCK-8) assay in AML-12 cells (n = 3/group). C: Intracellular triglyceride (TG) content in AML-12 cells (n = 3/group). And D: Representative image of oil red O staining of AML-12 cells in different groups. Scale bar in panel represents 100 μm. Data are presented as the mean ± SD. bP < 0.01 vs 0 μmol/L AITC, dP < 0.01 vs PA(-) AITC(-), fP < 0.01 vs PA(+) AITC(-). LDH: Lactate dehydrogenase; CCK-8:Cholecystokinin-8; TG: Triglyceride; NC: Negative control; PA: Palmitate acid: AITC: Allyl isothiocyanate.
Chronic inflammation is characterized by the abnormal production of proinflammatory cytokines, including TNFα, IL-1β and IL-6, and the activation of inflammatory signaling pathways, such as the NF-κB and JNK pathways; moreover, it has been proposed to play a crucial role in the pathogenesis of NAFLD[7,37]. A number of recent studies have demonstrated a key role for the IKK/NF-κB signaling pathway in NAFLD development[8,9]. In response to numerous inflammatory stimuli,IKKcomplex activation inducesIκBphosphorylation and subsequent degradation, which releasesNF-κBand allows it to translocate into the nucleus[38]. A previous finding indicated that hepatic lipid accumulation activates the IKK/NF-κB pathway,promoting downstream proinflammatory cytokine production and subacute inflammation[39]. In this study, we demonstrated that AITC treatment upregulated IκBα protein levels and downregulated IKK, IκBα, and p65 phosphorylation in both the NAFLD mouse and cellular models. Furthermore, AITC treatment substantially decreased hepatic proinflammatory cytokine levelsin vivoandin vitro. In addition,HFD-induced liver injury was attenuated in AITC-treated mice, as evidenced by markedly decreased serum levels of ALT and AST.
Although several isothiocyanates have been proposed as chemopreventive agents for cancers, AITC has been reported to exhibit both carcinogenic and anticarcinogenic potential. A previous study demonstrated that AITC has the ability to cause Cu(II)-mediated DNA damage and induce 8-oxo-7,8-dihydro-29-deoxyguanosine (8-oxodG)formation, leading to carcinogenesis in human myelogenous leukemic cell lines[40].Moreover, impaired copper availability in obesity-related NAFLD was shown to predict early atherosclerosis as a main cardiovascular risk[41]. On the other hand,another study demonstrated that AITC could inhibit proliferation of human prostate cancer cells through inducing G2/M arrest and apoptosis[22]. Our studies mentioned above have proposed many different mechanisms of AITC-induced amelioration of hepatic steatosis and inflammation in both in vivoandin vitromodels of NAFLD, but none have explored whether AITC took part in Cu(II)-mediated DNA damage or cancer development. Further studies are required to clarify whether AITC induces oxidative DNA damage in our model and investigate the mechanism of AITC on cancer.
In conclusion, our study demonstrates that AITC ameliorates hepatic steatosis and inflammation by activating the Sirt1/AMPK and inhibiting the IKK/NF-κB signaling pathway, respectively. This study indicates that AITC may be a potential therapeutic agent for the progress of NAFLD.
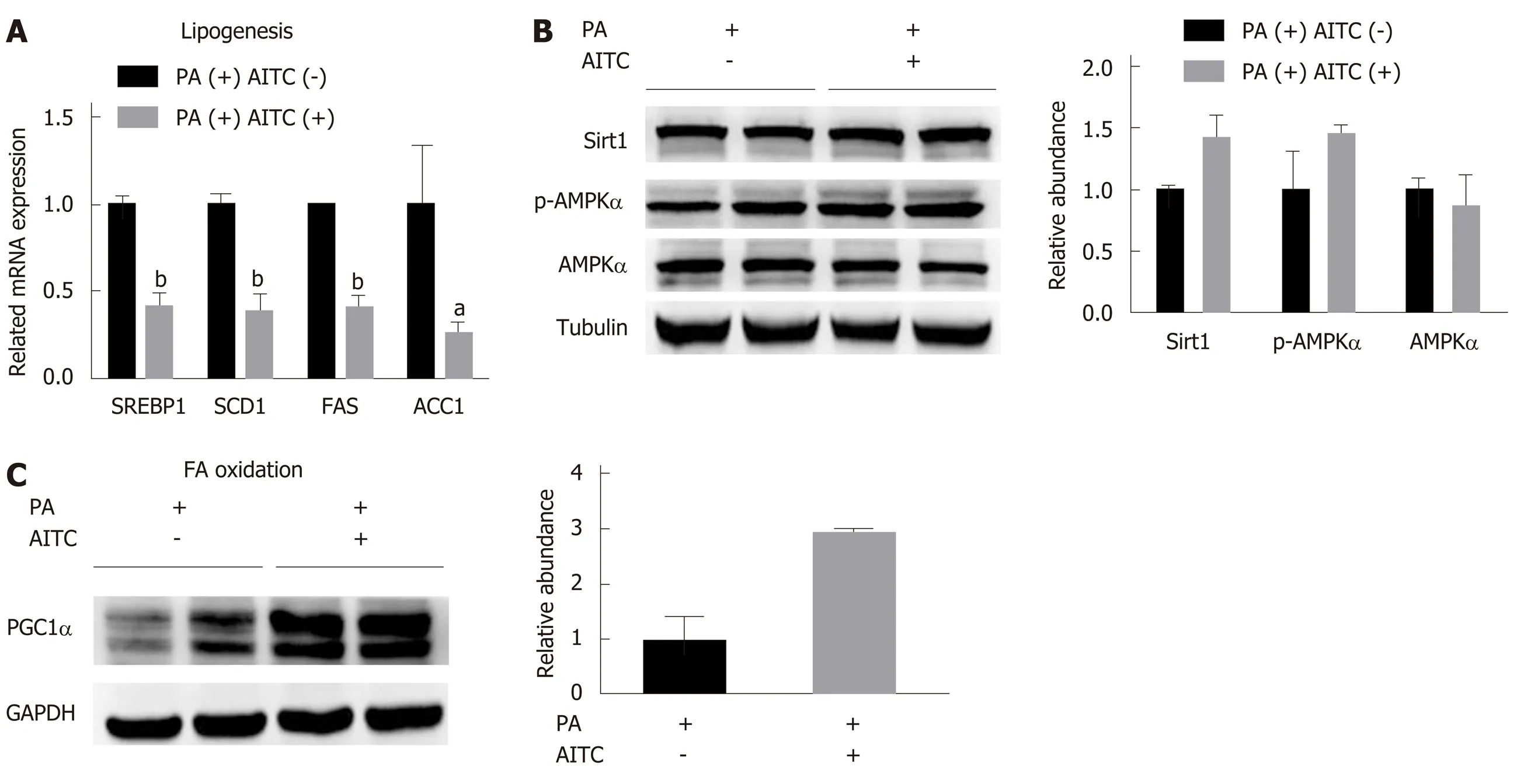
Figure 5 Allyl isothiocyanate downregulates the mRNA levels of genes involved in lipogenesis, upregulates the mRNA levels of genes involved in fatty acid β-oxidation and activates the Sirtuin 1/AMP-activated protein kinase signaling pathway in vitro. A: Palmitate acid (PA) (200 μmol/L)-stimulated AML-12 cells were treated with allyl isothiocyanate (AITC) (20 μmol/L) or dimethyl sulfoxide (DMSO) (vehicle) for 6 h (n = 3/group). The mRNA levels of sterol regulatory elementbinding protein 1 (SREBP1) and its lipogenesis target genes, including stearoyl coenzyme A desaturase 1 (SCD1), fatty acid synthase (FAS) and acetyl-CoA carboxylase 1 (ACC1), were determined. B: PA (200 μmol/L)-stimulated AML-12 cells were treated with AITC (20 μmol/L) or DMSO (vehicle) for 24 h. The protein expression of Sirtuin 1 (Sirt1), total and phosphorylated AMP-activated protein kinase α (AMPKα) was detected by western blot analysis. C: PA (200 μmol/L)-stimulated AML-12 cells were treated with AITC (20 μmol/L) or DMSO (vehicle) for 24 h. The protein level of proliferator-activated receptor gamma coactivator1α(PGC1α), which is involved in fatty acid β-oxidation, was detected by western blot analysis. Data are presented as the mean ± SD. aP < 0.05, bP < 0.01 vs PA(+)AITC(-). PA: Palmitate acid; AITC: Allyl isothiocyanate; PGC1α: Proliferator-activated receptor gamma coactivator 1α; SREBP1: Sterol regulatory elementbinding protein 1; SCD1: Stearoyl coenzyme A desaturase 1; FAS: Fatty acid synthase; ACC1: Acetyl-CoA carboxylase 1; Sirt1: Sirtuin 1; AMPKα: AMP-activated protein kinase α; p-AMPKα: Phosphorylated AMP-activated protein kinase α.
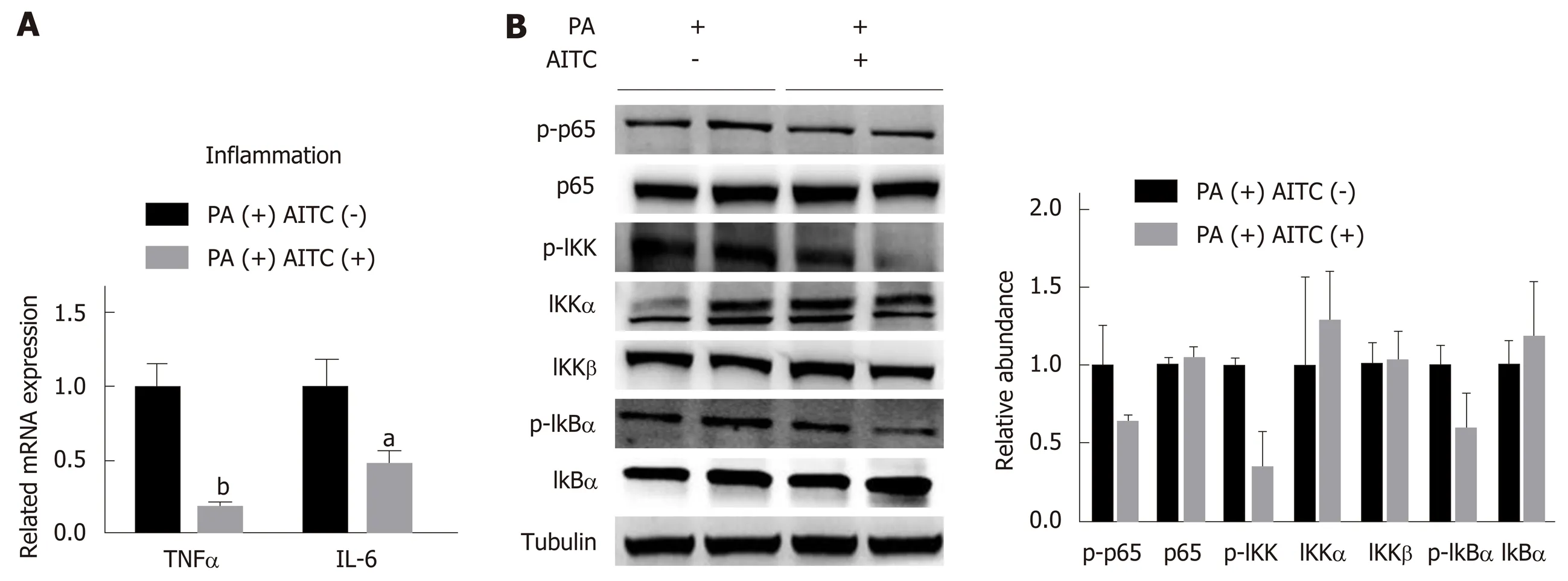
Figure 6 Allyl isothiocyanate downregulates the mRNA levels of proinflammatory markers and inhibits the IκB kinase /nuclear factor kappa B signaling pathway in vitro. A: Palmitate acid (PA) (200 μmol/L)-stimulated AML-12 cells were treated with allyl isothiocyanate (AITC) (20 μmol/L) or dimethyl sulfoxide (DMSO)(vehicle) for 6 h (n = 3/group). The mRNA levels of proinflammatory cytokines were measured by quantitative real-time PCR. (B) PA (200 μmol/L)-stimulated AML-12 cells were treated with AITC (20 μmol/L) or DMSO (vehicle) for 24 h. The protein expression of phosphorylated p65, p65, phosphorylated IκB kinase (IKK), IKKα,IKKβ, total and phosphorylated inhibitor of nuclear factor kappa B α (IκB α) was detected by western blot analysis. Data are presented as the mean ± S.D. aP < 0.05,bP < 0.01 vs PA(+) AITC(-). TNFα: Tumor necrosis factor α; IL-6: Interleukin-6; PA: Palmitate acid; AITC: Allyl isothiocyanate; p-p65: Phosphorylated p65; IKK: IκB kinase; IκBα: Inhibitor of nuclear factor kappa B α.
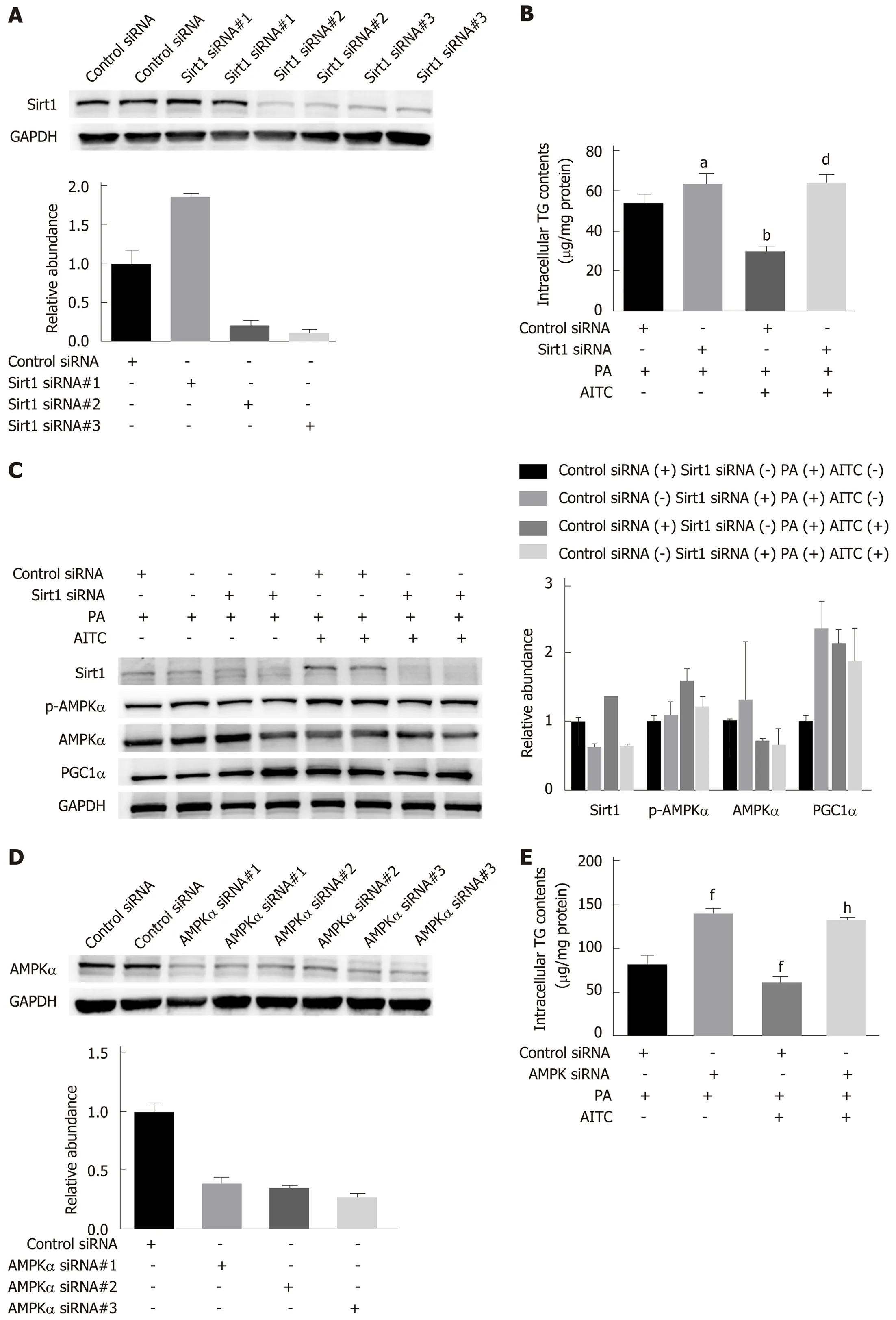
Figure 7 Allyl isothiocyanate ameliorates lipid accumulation by activating the Sirt1/AMPKα signaling pathway. After transfection with Sirtuin 1 (Sirt1) small interfering RNA (siRNA), AMP-activated protein kinase α (AMPKα) siRNA or the corresponding scrambled control for 48 h, AML-12 cells were incubated in normal medium or medium containing palmitate acid (PA) with or without allyl isothiocyanate (AITC) for 24 h. A: Three different Sirt1 siRNA sequences were used, and Sirt1 protein levels were examined by western blot analysis. In the following experiments, we selected Sirt1 siRNA #3. B: Intracellular triglyceride (TG) content in AML-12 cells (n = 4/group). C: Sirt1, phosphorylated AMPKα and proliferator-activated receptor gamma coactivator1α (PGC1α) protein expression was detected by western blot analysis. D: Three different AMPKα siRNA sequences were used, and AMPKα protein levels were examined by western blot analysis. In the subsequent experiments, we selected AMPKα siRNA #3. (E) Intracellular TG content in AML-12 cells (n = 4/group). Data are presented as the mean ± SD. aP < 0.05, bP < 0.01 vs Control siRNA + PA, dP < 0.01 vs Control siRNA + PA + AITC, fP < 0.01 vs Control siRNA + PA, hP < 0.01 vs Control siRNA + PA + AITC. PA: Palmitate acid; AITC:Allyl isothiocyanate; PGC1α: Proliferator-activated receptor gamma coactivator 1α; Sirt1: Sirtuin 1; AMPKα: AMP-activated protein kinase α; p-AMPKα:Phosphorylated AMP-activated protein kinase α; TG: Triglyceride.
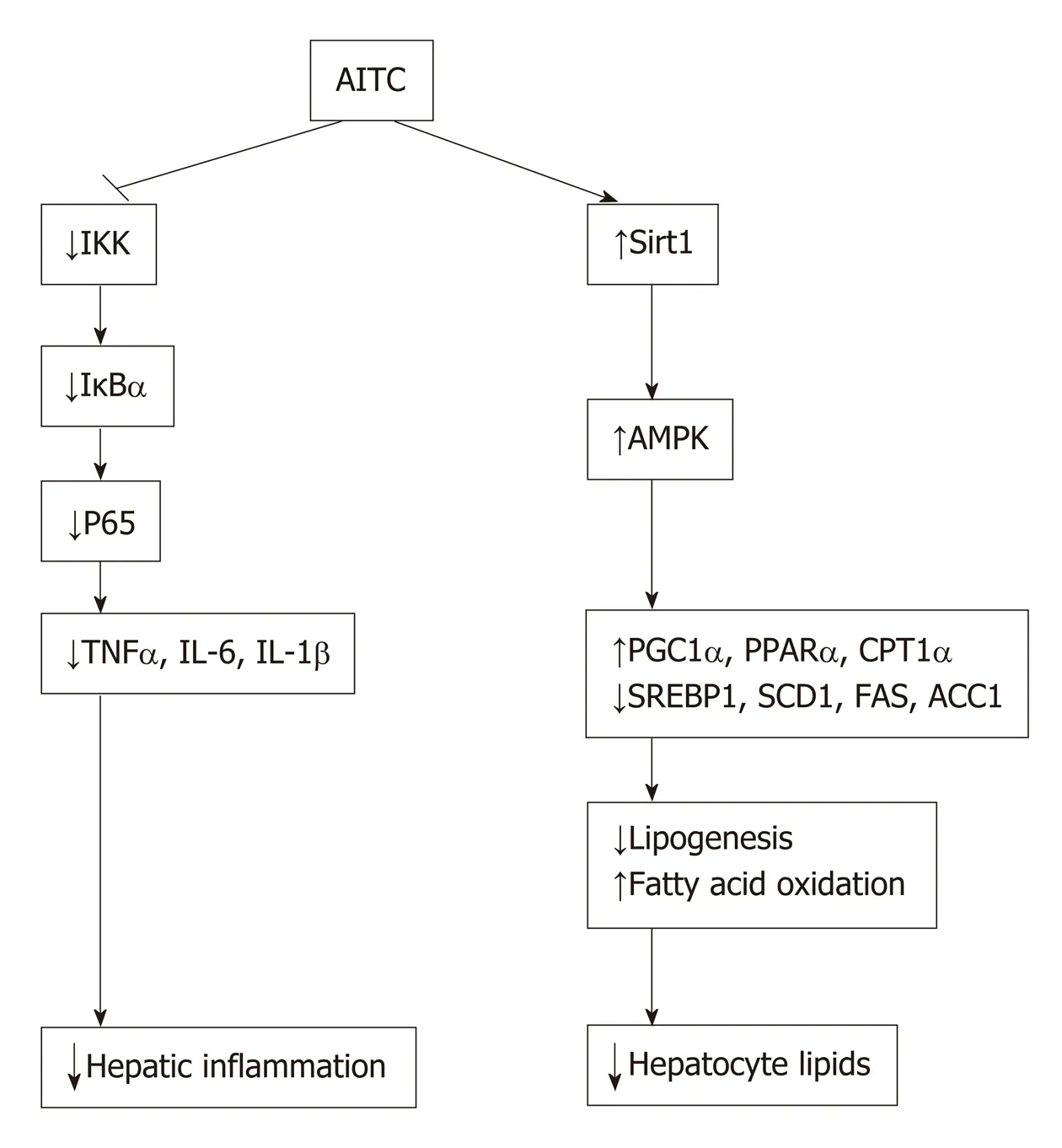
Figure 8 Model of allyl isothiocyanate action. Schematic diagram: allyl isothiocyanate ameliorates hepatic lipid accumulation and hepatic inflammation by activating the Sirt1/AMPK signaling pathway and inhibiting the NF-κB pathway. AITC: Allyl isothiocyanate; IKK: IκB kinase; IκBα: Inhibitor of nuclear factor kappa B α;TNFα: Tumor necrosis factor α; IL-6: Interleukin-6; IL-1β: Interleukin-1β; Sirt1: Sirtuin 1; AMPKα: AMP-activated protein kinase α; PGC1α: Proliferator-activated receptor gamma coactivator 1α; PPARα: Peroxisome proliferator-activated receptor α; CPT1α: Carnitine palmitoyltransferase 1 α; SREBP1: Sterol regulatory elementbinding protein 1; SCD1: Stearoyl coenzyme A desaturase 1; FAS: Fatty acid synthase; ACC1: Acetyl-CoA carboxylase 1.
ARTICLE HIGHLIGHTS
Research background
Nonalcoholic fatty liver disease (NAFLD) is an unmet medical need with no approved therapies.Recent studies have shown that allyl isothiocyanate (AITC) has a potential protective effect on obesity and insulin resistance. The evaluation of the effect of AITC on NAFLD as well as the mechanism of action may provide a new therapeutic trend.
Research motivation
Emerging evidence suggests a beneficial role for AITC in inflammation, cancer, diet-induced obesity and insulin resistance. Enhanced lipolysis in adipocytes and intensified hydrolysis of triglyceride in the serum of rats treated with AITC was also reported. As little is known about its direct impact on liver or its underlying mechanism, it is imperative to characterize the potential effect of AITC on NAFLD.
Research objectives
To validate the effect of AITC on NAFLD and clarify the possible mechanism of action.
Research methods
C57BL/6 mice were fed a high fat diet (HFD) for 8 wk, and AML-12 cells were treated with 200 μmol/L palmitate acid (PA) for 24 h to establishin vivoandin vitromodels of hepatic steatosis.Mice were administered AITC (100 mg/kg/d) orally and AML-12 cells were treated with AITC(20 μmol/L) to detect the effect of AITC on NAFLD.
Research results
Our results show that AITC significantly ameliorates HFD-induced weight gain, hepatic lipid accumulation, inflammation, and PA-induced lipid accumulation as well as inflammation in AML-12 cells, accompanied by activated Sirt1/AMPK and inhibited NF-κB signaling pathways.The curative effect of AITC on lipid accumulation is abolished by siRNA-mediated knockdown of either Sirt1 or AMPKα in AML-12 cells.
Research conclusions
AITC treatment protects against HFD and PA-induced lipid accumulation and inflammationin vivoandin vitro. These effects are associated with Sirt1/AMPK and NF-κB signaling pathways.
Research perspectives
Plant compounds such as AITC should be further explored for their potential effective activity in NAFLD.
杂志排行
World Journal of Gastroenterology的其它文章
- Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors
- Autoantibodies: Potential clinical applications in early detection of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma
- Role of endoscopic ultrasound in the screening and follow-up of high-risk individuals for familial pancreatic cancer
- Hepatic senescence, the good and the bad
- Optimizing proton pump inhibitors in Helicobacter pylori treatment:Old and new tricks to improve effectiveness
- Regulatory effect of a Chinese herbal medicine formula on nonalcoholic fatty liver disease
