Autoantibodies: Potential clinical applications in early detection of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma
2019-09-25YiWeiXuYuHuiPengLiYanXuJianJunXieEnMinLi
Yi-Wei Xu, Yu-Hui Peng, Li-Yan Xu, Jian-Jun Xie, En-Min Li
Abstract Esophageal squamous cell carcinoma (ESCC) and esophagogastric junction adenocarcinoma (EGJA) are the two main types of gastrointestinal cancers that pose a huge threat to human health. ESCC remains one of the most common malignant diseases around the world. In contrast to the decreasing prevalence of ESCC, the incidence of EGJA is rising rapidly. Early detection represents one of the most promising ways to improve the prognosis and reduce the mortality of these cancers. Current approaches for early diagnosis mainly depend on invasive and costly endoscopy. Non-invasive biomarkers are in great need to facilitate earlier detection for better clinical management of patients. Tumor-associated autoantibodies can be detected at an early stage before manifestations of clinical signs of tumorigenesis, making them promising biomarkers for early detection and monitoring of ESCC and EGJA. In this review, we summarize recent insights into the iden-tification and validation of tumor-associated autoantibodies for the early detection of ESCC and EGJA and discuss the challenges remaining for clinical validation.
Key words: Esophageal squamous cell carcinoma; Esophagogastric junction adenocarcinoma; Biomarker; Autoantibody; Diagnosis
INTRODUCTION
Esophageal cancer is the eighth leading malignant disease and the sixth most common cause of cancer-related death worldwide. It represents a serious health problem globally[1]. Esophageal cancer is mainly composed of two epidemiologically and histopathologically distinct sub-types designated as esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. In China, esophageal cancer is the third leading cause of cancer death with an estimated 246000 new cases and 188000 deaths in 2015[2]. Although ESCC, which accounts for 70% of cases, remains the most prevalent form of esophageal cancer, the prevalence of ESCC has declined substantially in recent years. In contrast to the decreasing prevalence of ESCC, an alarming rise of the incidence in esophagogastric junction adenocarcinoma (EGJA) has been observed in both developed and developing countries with 260000 new cases diagnosed in 2012[2-4]. Interestingly, in China the incidence of EGJA appears to be high in areas where the prevalence of ESCC is also high[5]. This similar geographic distribution suggests similar environmental factors, similar dietary habits and even similar molecular alterations are involved in both ESCC and EGJA[6].
The prognosis of ESCC is poor with an overall 5-year incidence of survival ranging from 15% to 25%[7,8]. The high mortality in ESCC and EGJA mostly results from diagnosis at late stages due to the lack of specific symptoms of patients in early stage disease, but the prognosis is substantially better for patients diagnosed in the early stage (e.g., 5-year survival of more than 85% for ESCC patients diagnosed in early stage and more than 90% for EGJA patients with node-negative T1 tumors)[9,10].However, effective strategies are lacking for screening or detection of pre-cancerous lesions in early-stage ESCC and EGJA. Although endoscopy is used as a primary screening technique and can identify ESCC and EGJA at an early stage, its extensive utilization is limited by the invasive nature, serious side effects and dependence on the skill of the endoscopist. Moreover, some individuals are unwilling to undergo endoscopy, whereas a simple blood test might be more acceptable. Thus,identification and validation of novel non-invasive, blood-based biomarkers can fulfill a great need for early detection of ESCC and EGJA.
Tumor-associated (TA) autoantibodies are emerging as strong candidates for clinically useful cancer biomarkers because they are produced early in tumorigenesis and can be detectable up to five years before the clinical manifestations of cancer[11-13].Moreover, autoantibodies are also reported as biomarkers used in cancer prognosis and therapeutic monitoring (Table 1)[11,14-21]. A large number of articles have evaluated the potential use of TA autoantibodies for early ESCC detection. Therefore, a systematic review is warranted to assess the current potential of TA autoantibodies for the early diagnosis of patients with ESCC. Considering the similarity of the etiology and epidemiology in EGJA and ESCC, we believe that it would be much desirable to provide together a review of TA autoantibodies in EGJA and ESCC at this current time. We focus on the key aspects of the study designs and participant characteristics, the sensitivity, specificity and area under the receiver operating characteristic curve of the TA autoantibody biomarkers to help identify the most promising candidates for future clinical screening tests.
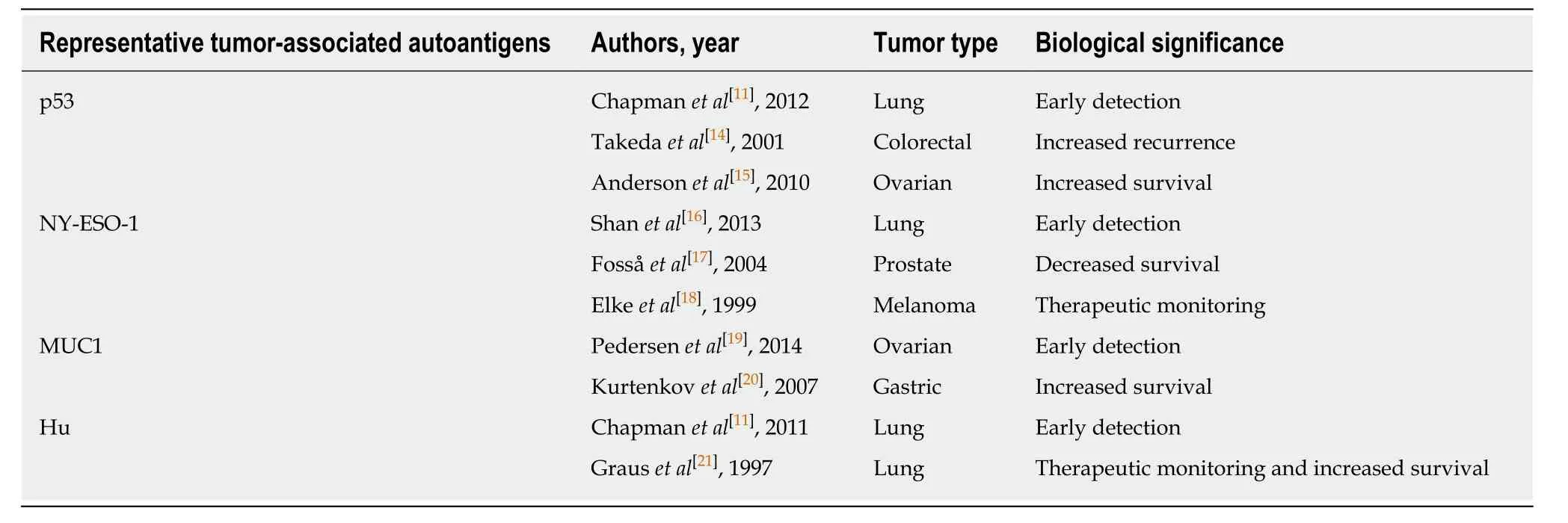
Table 1 A brief summary of the biological significance of some common tumor-associated autoantibodies
PROPOSED ORIGINS OF AUTOANTIBODY PRODUCTION IN CANCER
As early as the 1960s, Robert W. Baldwin showed that the immune system could react to a developing tumor[22-24]. Most studies have mainly focused on evaluating TA autoantibodies as early cancer biomarkers since their discovery. On the other hand,investigation of the underlying causes of TA autoantibody production may contribute to a clearer understanding of mechanisms concerning the rendering of autologous proteins immunogenic and also reveal novel therapeutic targets for potential clinical use. It is commonly accepted that autologous cellular antigens expressed in tumors,also referred to as TA antigens (TAA), can be recognized early by the immune system and thus trigger a reaction known as cancer immunoediting, which consists of three phases: Elimination, equilibration and escape[25,26]. Immunosurveillance occurs during the elimination phase when the first few transformed cells are recognized by the immune system and targeted by natural killer cells that secrete certain cytokines to let other immune cells convene to the tumor[27]. The ensuing disruption of certain transformed cells and the uptake and disposal of the corresponding fragments by the recruited immune cells activate the appropriate immune response. A cascade of dynamic events further boosts the activation of innate immunity and facilitates the expansion and generation of T and B cells (the latter produces antibodies)[28]. Tumor cells that escape elimination and are permitted to grow will enter into the equilibrium phase during which tumor cell variants emerge with increasing ability to survive an immune attack. The equilibrium phase is the longest among these three phases and may persist for many years. Escape eventually occurs if the host immune defenses are breached and tumor cell variants grow and proliferate in an uncontrolled manner[29].
It is clear that the generation of many abnormal antigens during tumorigenesis can induce the host immune response to produce autoantibodies. However, how factors exactly facilitate an enhancement or disorder of immune surveillance in cancer resulting in the TA autoantibody production response is still unclear. The generation of TA autoantibodies is thought to occur in response to mutations[30,31], overexpression[32,33]or abnormal processing[34,35], which lead to the formation of altered or novel epitopes, aberrantly high expression levels resulting in loss of tolerance and abnormal post-translational modifications, such as acetylation, glycosylation and phosphorylation, all of which could create a neoepitope, enhance self-epitope presentation or expose antigens normally located in immune-privileged sites (e.g.,cancer-testis antigens). With these mechanisms, extracellular and intracellular host proteins could be recognized by B cells to produce TA autoantibodies. Recent research has estimated that most TA autoantigens are mutated or overexpressed proteins among which 42% are cytoplasmatic, 26.1% are expressed predominantly in the nucleus, 21.4% are membrane-bound and 10.3% are extracellular[36]. It is surprising that TA autoantibodies seem to be more specific to intracellular molecules rather than their more common cell surface targets. This may be explained by greater vascular permeability for cytoplasmic proteins and enhancement of autoantibody generation by the proinflammatory environment[37,38]. Although the exact role of TA autoantibodies in cancer is largely undefined, that secreted TA autoantibodies reflect tumor burden makes them attractive and promising biomarkers.
DIAGNOSTIC PERFORMANCE OF SINGLE AUTOANTIBODIES IN ESCC
Although TA autoantibodies have been described in a wide variety of human malignancies in the past several decades and have shown early diagnostic relevance,most studies evaluating autoantibodies in patients with ESCC and normal controls have emerged within the last 20 years. At least 49 individual TA autoantibodies have been assessed with diagnostic parameters in ESCC. Table 2 represents a list of single TA autoantibodies reported in the literature that could serve as potential serum/plasma biomarkers for ESCC. The diagnostic value of the TA autoantibodies, whether for the same autoantibody or for different types of autoantibodies, shows large variation for ESCC in terms of sensitivity and specificity, which might be due to sample size, the ethnic group studied or the test method of evaluation.
In general, the majority of TA autoantibody biomarkers show relatively low sensitivity but high specificity. The sensitivities and specificities for ESCC range from 3.9% to 93.7% and from 78.7% to 100%, respectively (Table 2). Receiver operating characteristic curves as a summary measure will not set cutoff values artificially but rather considers sensitivity and specificity simultaneously. Nevertheless, only a few studies used receiver operating characteristic curve analysis and area under the curve values to evaluate the diagnostic performance of TA autoantibodies (Table 2). The graphical representation of the sensitivities and specificities for autoantibodies in ESCC evaluated in more than one study is shown in Figure 1. Of note, the diagnostic ability of the great majority of TA autoantibodies lack independent validation, and the numbers of cases in some of the studies are very small. It is also noteworthy that most of the single TA autoantibodies lack diagnostic assessment in patients with early stage ESCC, which is one of the most important elements for biomarker development and application in early cancer diagnosis.
The most comprehensively investigated TA autoantibodies in ESCC have been p53 autoantibodies followed by autoantibodies against P16 and c-Myc. Given the prominent feature of p53 in cancers it is not unexpected that this is the most widely studied autoantibody in ESCC. Autoantibodies against p53 in the diagnosis of ESCC have been evaluated in 17 studies (Table 2), and the sensitivities vary largely between reports (7%-60%) while less variance is observed in the specificity (range 89.5%-100%,Table 2). A meta-analysis by Zhanget al[39]showed the overall sensitivity and specificity of p53 autoantibody for esophageal cancer are 29.6% and 97.9%,respectively. Autoantibodies against P16 and c-Myc were each analyzed in five studies, and both exhibited high specificity but poor sensitivity (Table 2). Therefore,despite the high specificity, all studies show that use of a single autoantibody provides low sensitivity indicating limited clinical application. The sensitivity and specificity for Hsp70 autoantibodies reported by Fujitaet al[40]can be up to 93.7% and 100%, respectively. However, the very small sample size of this study reduces the stability and power of the results. Overall, quite apparent is the fact that the diagnostic value of individual TA autoantibody biomarkers in ESCC is quite limited.
DIAGNOSTIC PERFORMANCE OF SINGLE AUTOANTIBODIES IN EGJA
Very few clinical or translational studies have treated EGJA as a separate entity,which have been generally divided between those targeting esophageal cancer and those targeting gastric cancer. Likewise, a similar phenomenon has been observed in the studies on autoantibodies for the diagnosis of EGJA. As can be seen from Table 3,a total of 13 autoantibodies were investigated in two studies[41,42], all of which were initially assessed in ESCC by Xuet al[43]and Zhouet al[44]. As anticipated, the presence of TA autoantibodies indicates early diagnostic potential for EGJA. The sensitivity of single TA autoantibody biomarkers for EGJA ranged from 11.0% to 54.3% with generally high specificity ranging from 86.3% to 97% (Table 3). From the list of autoantibodies shown in Table 3, there is no good way of forecasting which TA autoantibodies may work. Like ESCC, the most commonly tested TA autoantibody in EGJA is the p53 autoantibody, which has the highest area under the curve value(0.799) with moderate sensitivity and specificity in the diagnosis of early stage EGJA(Table 3). However, it remains fact that the capability of a single TA autoantibody biomarker to identify EGJA patients is limited. It also should be pointed out that research on autoantibodies is still in its infancy. Thus, more autoantibody biomarkers need to be identified and evaluated to enlarge the autoantibody pool for EGJA.
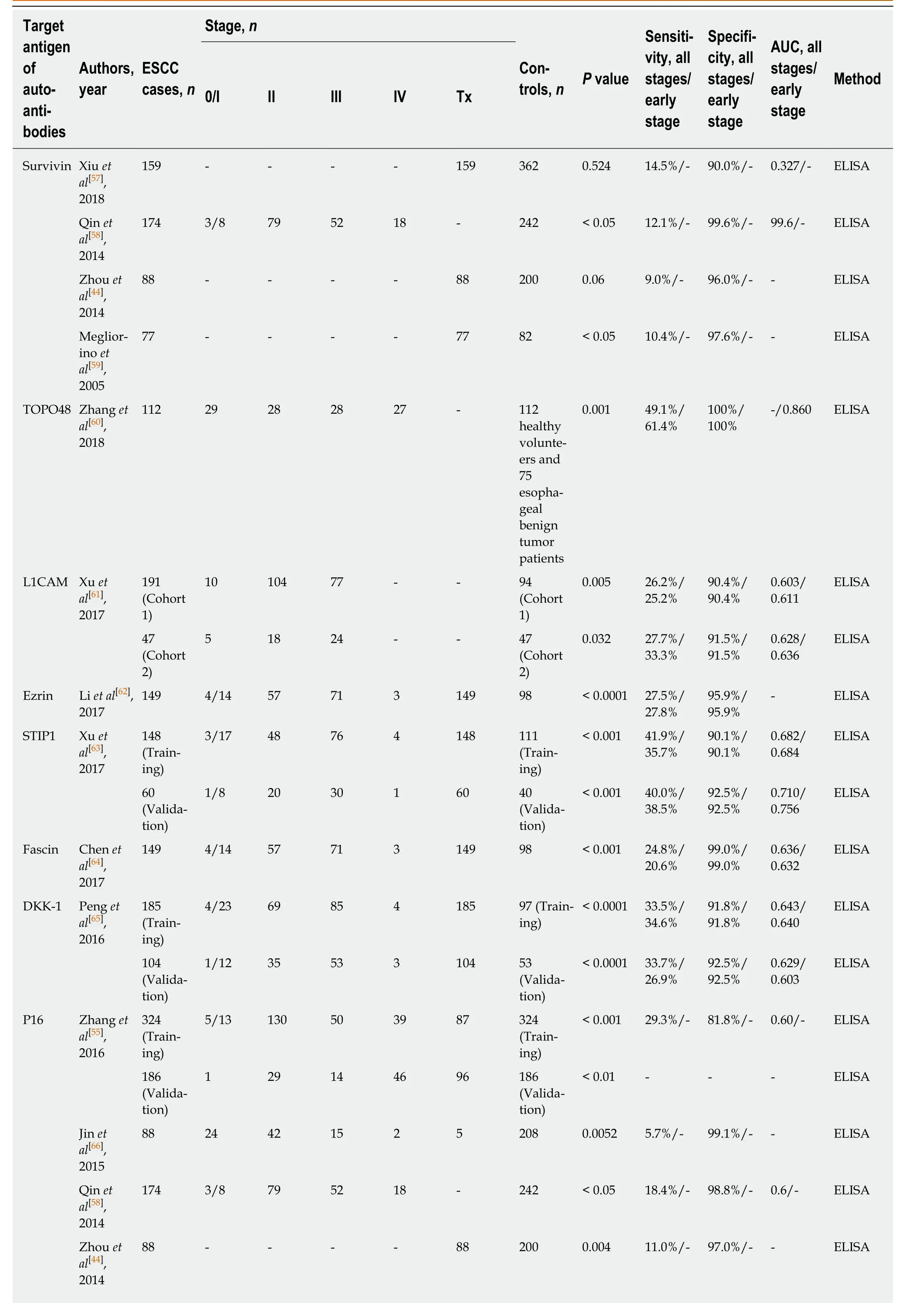
Table 2 Diagnostic performance of single tumor-associated autoantibody biomarkers in esophageal squamous cell carcinoma
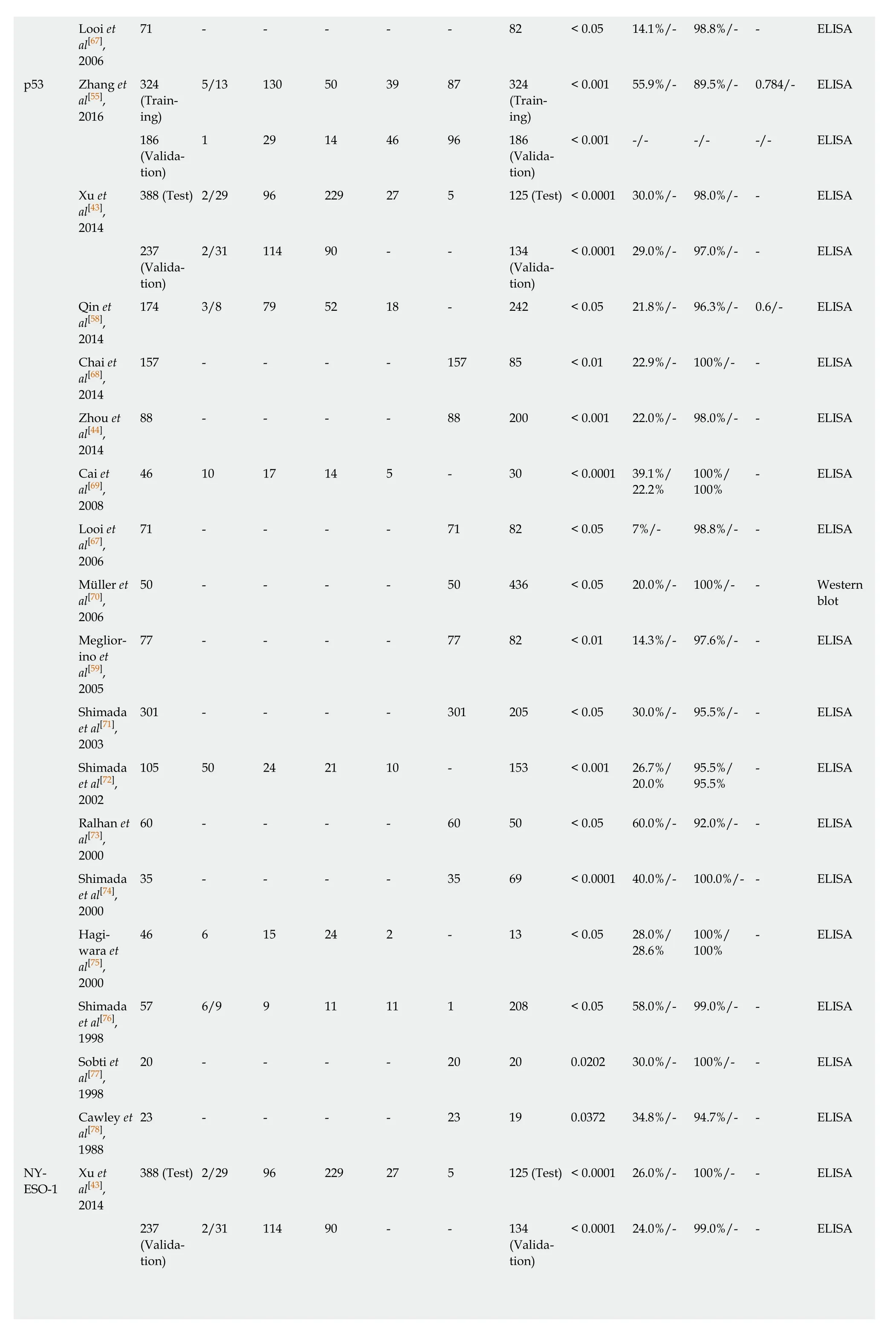
Looi et71 - - - - - 82 < 0.05 14.1%/- 98.8%/- - ELISA al[67],2006 p53 Zhang et3245/13 130 50 39 87 324< 0.001 55.9%/- 89.5%/- 0.784/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.001 -/- -/- -/- ELISA(Valida-(Validation)tion)Xu et388 (Test) 2/29 96 229 27 5 125 (Test) < 0.0001 30.0%/- 98.0%/- - ELISA al[43],2014 2372/31 114 90 - - 134< 0.0001 29.0%/- 97.0%/- - ELISA(Valida-(Validation)tion)Qin et174 3/8 79 52 18 - 242 < 0.05 21.8%/- 96.3%/- 0.6/- ELISA al[58],2014 Chai et157 - - - - 157 85 < 0.01 22.9%/- 100%/- - ELISA al[68],2014 Zhou et88 - - - - 88 200 < 0.001 22.0%/- 98.0%/- - ELISA al[44],2014 Cai et46 10 17 14 5 - 30 < 0.0001 39.1%/100%/- ELISA al[69],22.2%100%2008 Looi et71 - - - - 71 82 < 0.05 7%/- 98.8%/- - ELISA al[67],2006 Müller et50 - - - - 50 436 < 0.05 20.0%/- 100%/- - Western al[70],blot 2006 Meglior-77 - - - - 77 82 < 0.01 14.3%/- 97.6%/- - ELISA ino et al[59],2005 Shimada301 - - - - 301 205 < 0.05 30.0%/- 95.5%/- - ELISA et al[71],2003 Shimada105 50 24 21 10 - 153 < 0.001 26.7%/95.5%/- ELISA et al[72],20.0%95.5%2002 Ralhan et60 - - - - 60 50 < 0.05 60.0%/- 92.0%/- - ELISA al[73],2000 Shimada35 - - - - 35 69 < 0.0001 40.0%/- 100.0%/- - ELISA et al[74],2000 Hagi-46 6 15 24 2 - 13 < 0.05 28.0%/100%/- ELISA wara et28.6%100%al[75],2000 Shimada57 6/9 9 11 11 1 208 < 0.05 58.0%/- 99.0%/- - ELISA et al[76],1998 Sobti et20 - - - - 20 20 0.0202 30.0%/- 100%/- - ELISA al[77],1998 Cawley et23 - - - - 23 19 0.0372 34.8%/- 94.7%/- - ELISA al[78],1988 NY-Xu et388 (Test) 2/29 96 229 27 5 125 (Test) < 0.0001 26.0%/- 100%/- - ELISA ESO-1al[43],2014 2372/31 114 90 - - 134< 0.0001 24.0%/- 99.0%/- - ELISA(Valida-(Validation)tion)
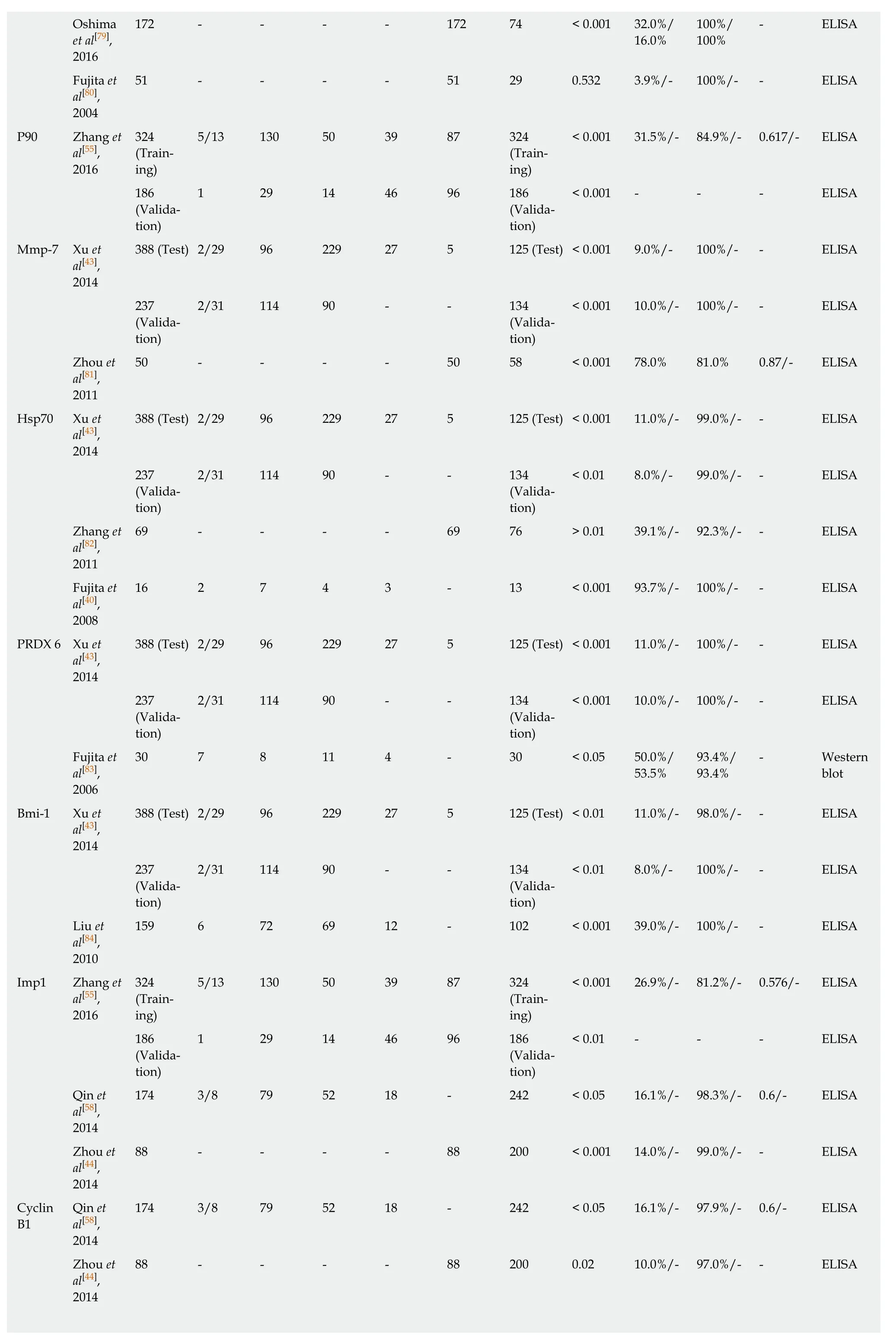
Oshima172 - - - - 172 74 < 0.001 32.0%/100%/- ELISA et al[79],16.0%100%2016 Fujita et51 - - - - 51 29 0.532 3.9%/- 100%/- - ELISA al[80],2004 P90 Zhang et3245/13 130 50 39 87 324< 0.001 31.5%/- 84.9%/- 0.617/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.001 - - - ELISA(Valida-(Validation)tion)Mmp-7 Xu et388 (Test) 2/29 96 229 27 5 125 (Test) < 0.001 9.0%/- 100%/- - ELISA al[43],2014 2372/31 114 90 - - 134< 0.001 10.0%/- 100%/- - ELISA(Valida-(Validation)tion)Zhou et50 - - - - 50 58 < 0.001 78.0% 81.0% 0.87/- ELISA al[81],2011 Hsp70 Xu et388 (Test) 2/29 96 229 27 5 125 (Test) < 0.001 11.0%/- 99.0%/- - ELISA al[43],2014 2372/31 114 90 - - 134< 0.01 8.0%/- 99.0%/- - ELISA(Valida-(Validation)tion)Zhang et69 - - - - 69 76 > 0.01 39.1%/- 92.3%/- - ELISA al[82],2011 Fujita et16 2 7 4 3 - 13 < 0.001 93.7%/- 100%/- - ELISA al[40],2008 PRDX 6 Xu et388 (Test) 2/29 96 229 27 5 125 (Test) < 0.001 11.0%/- 100%/- - ELISA al[43],2014 2372/31 114 90 - - 134< 0.001 10.0%/- 100%/- - ELISA(Valida-(Validation)tion)Fujita et30 7 8 11 4 - 30 < 0.05 50.0%/93.4%/- Western al[83],53.5%93.4%blot 2006 Bmi-1 Xu et388 (Test) 2/29 96 229 27 5 125 (Test) < 0.01 11.0%/- 98.0%/- - ELISA al[43],2014 2372/31 114 90 - - 134< 0.01 8.0%/- 100%/- - ELISA(Valida-(Validation)tion)Liu et159 6 72 69 12 - 102 < 0.001 39.0%/- 100%/- - ELISA al[84],2010 Imp1 Zhang et3245/13 130 50 39 87 324< 0.001 26.9%/- 81.2%/- 0.576/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.01 - - - ELISA(Valida-(Validation)tion)Qin et174 3/8 79 52 18 - 242 < 0.05 16.1%/- 98.3%/- 0.6/- ELISA al[58],2014 Zhou et88 - - - - 88 200 < 0.001 14.0%/- 99.0%/- - ELISA al[44],2014 CyclinQin et174 3/8 79 52 18 - 242 < 0.05 16.1%/- 97.9%/- 0.6/- ELISA B1al[58],2014 Zhou et88 - - - - 88 200 0.02 10.0%/- 97.0%/- - ELISA al[44],2014
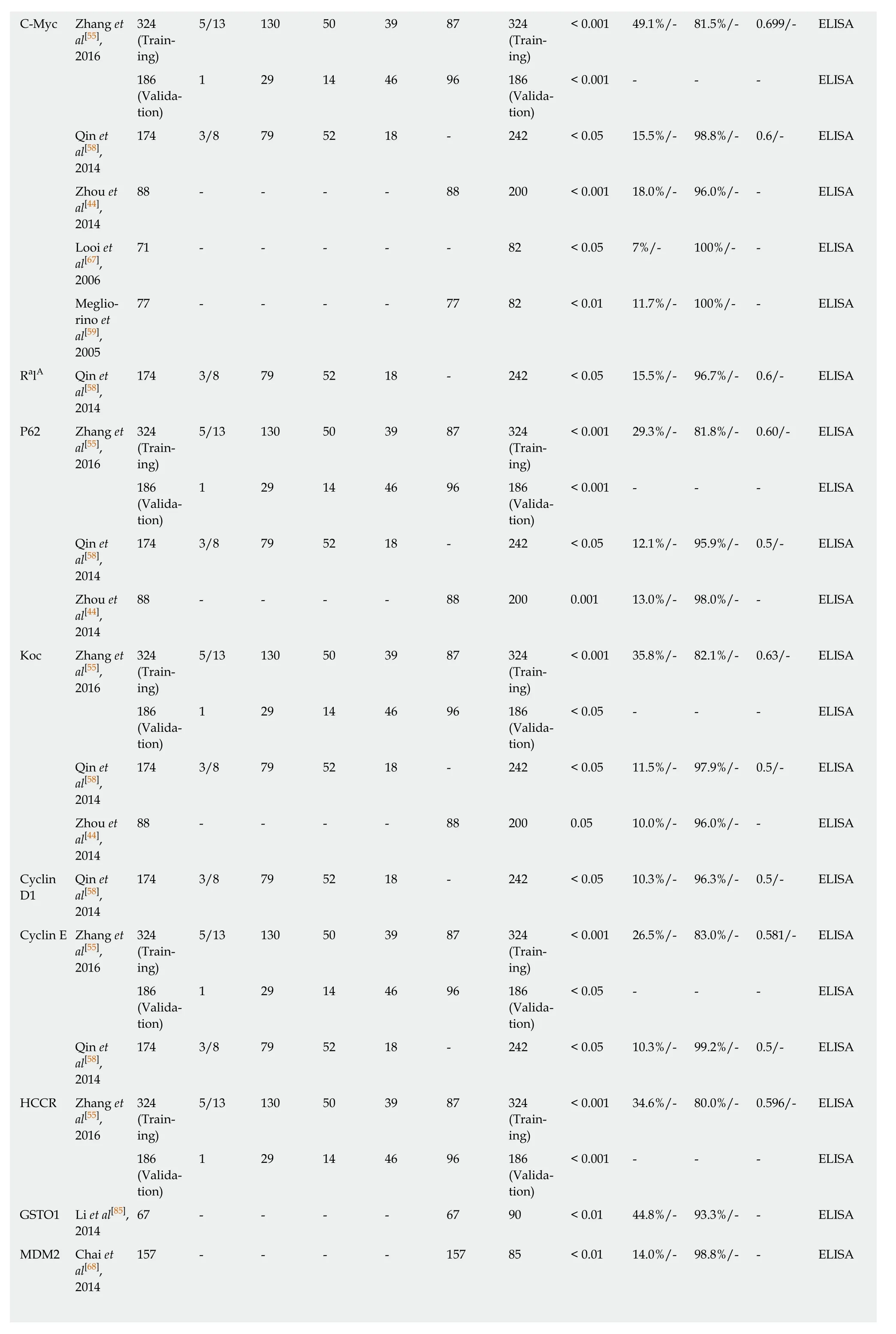
C-Myc Zhang et3245/13 130 50 39 87 324< 0.001 49.1%/- 81.5%/- 0.699/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.001 - - - ELISA(Valida-(Validation)tion)Qin et174 3/8 79 52 18 - 242 < 0.05 15.5%/- 98.8%/- 0.6/- ELISA al[58],2014 Zhou et88 - - - - 88 200 < 0.001 18.0%/- 96.0%/- - ELISA al[44],2014 Looi et71 - - - - - 82 < 0.05 7%/- 100%/- - ELISA al[67],2006 Meglio-77 - - - - 77 82 < 0.01 11.7%/- 100%/- - ELISA rino et al[59],2005 RalA Qin et174 3/8 79 52 18 - 242 < 0.05 15.5%/- 96.7%/- 0.6/- ELISA al[58],2014 P62 Zhang et3245/13 130 50 39 87 324< 0.001 29.3%/- 81.8%/- 0.60/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.001 - - - ELISA(Valida-(Validation)tion)Qin et174 3/8 79 52 18 - 242 < 0.05 12.1%/- 95.9%/- 0.5/- ELISA al[58],2014 Zhou et88 - - - - 88 200 0.001 13.0%/- 98.0%/- - ELISA al[44],2014 Koc Zhang et3245/13 130 50 39 87 324< 0.001 35.8%/- 82.1%/- 0.63/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.05 - - - ELISA(Valida-(Validation)tion)Qin et174 3/8 79 52 18 - 242 < 0.05 11.5%/- 97.9%/- 0.5/- ELISA al[58],2014 Zhou et88 - - - - 88 200 0.05 10.0%/- 96.0%/- - ELISA al[44],2014 CyclinQin et174 3/8 79 52 18 - 242 < 0.05 10.3%/- 96.3%/- 0.5/- ELISA D1al[58],2014 Cyclin E Zhang et3245/13 130 50 39 87 324< 0.001 26.5%/- 83.0%/- 0.581/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.05 - - - ELISA(Valida-(Validation)tion)Qin et174 3/8 79 52 18 - 242 < 0.05 10.3%/- 99.2%/- 0.5/- ELISA al[58],2014 HCCR Zhang et3245/13 130 50 39 87 324< 0.001 34.6%/- 80.0%/- 0.596/- ELISA al[55],(Train-(Train-2016ing)ing)1861 29 14 46 96 186< 0.001 - - - ELISA(Valida-(Validation)tion)GSTO1 Li et al[85],67 - - - - 67 90 < 0.01 44.8%/- 93.3%/- - ELISA 2014 MDM2 Chai et157 - - - - 157 85 < 0.01 14.0%/- 98.8%/- - ELISA al[68],2014
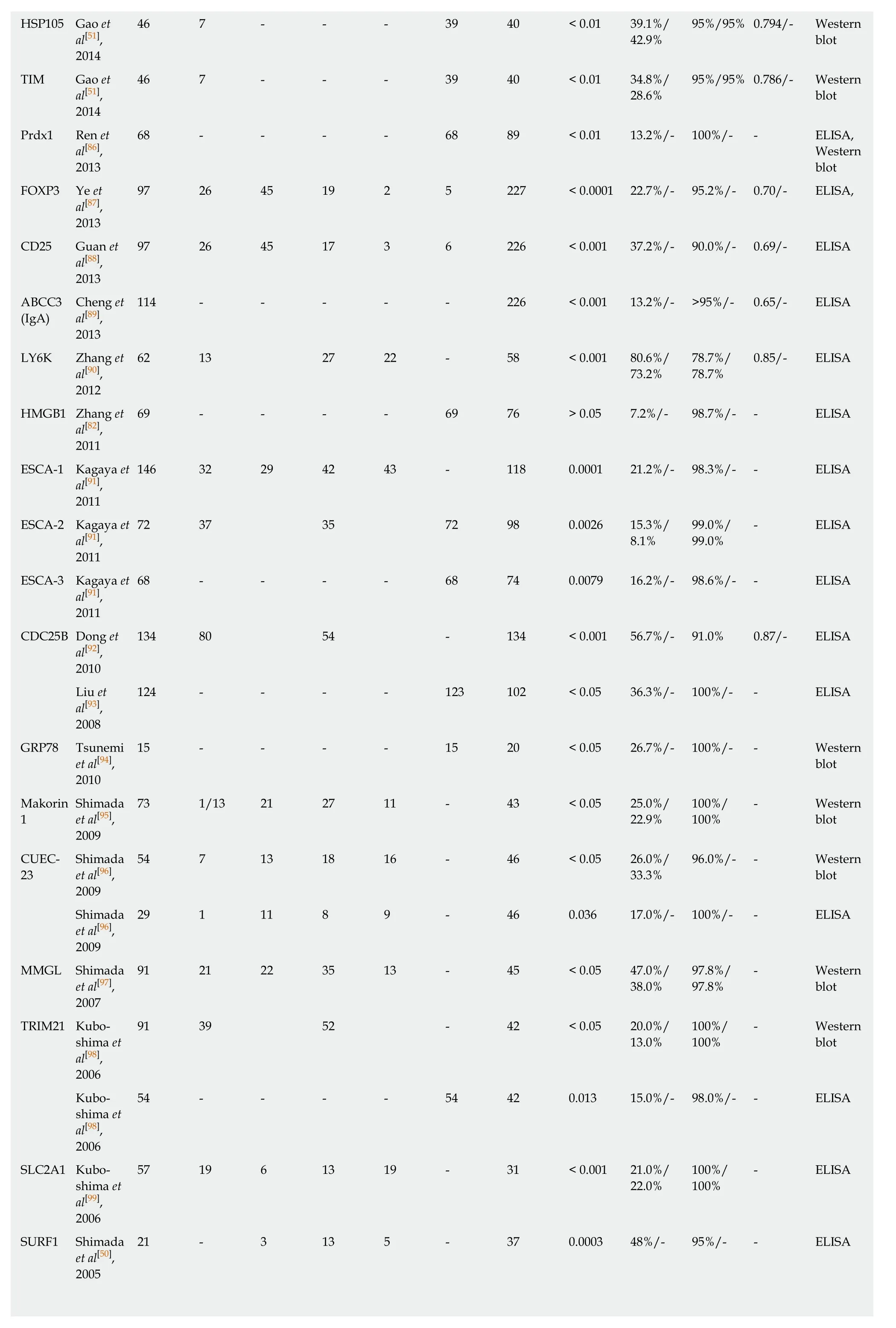
HSP105 Gao et46 7 - - - 39 40 < 0.01 39.1%/95%/95% 0.794/- Western al[51],42.9%blot 2014 TIM Gao et46 7 - - - 39 40 < 0.01 34.8%/95%/95% 0.786/- Western al[51],28.6%blot 2014 Prdx1 Ren et68 - - - - 68 89 < 0.01 13.2%/- 100%/- - ELISA,al[86],Western 2013blot FOXP3 Ye et97 26 45 19 2 5 227 < 0.0001 22.7%/- 95.2%/- 0.70/- ELISA,al[87],2013 CD25 Guan et97 26 45 17 3 6 226 < 0.001 37.2%/- 90.0%/- 0.69/- ELISA al[88],2013 ABCC3Cheng et114 - - - - - 226 < 0.001 13.2%/- >95%/- 0.65/- ELISA(IgA)al[89],2013 LY6K Zhang et62 13 27 22 - 58 < 0.001 80.6%/78.7%/0.85/- ELISA al[90],73.2%78.7%2012 HMGB1 Zhang et69 - - - - 69 76 > 0.05 7.2%/- 98.7%/- - ELISA al[82],2011 ESCA-1 Kagaya et146 32 29 42 43 - 118 0.0001 21.2%/- 98.3%/- - ELISA al[91],2011 ESCA-2 Kagaya et72 37 35 72 98 0.0026 15.3%/99.0%/- ELISA al[91],8.1%99.0%2011 ESCA-3 Kagaya et68 - - - - 68 74 0.0079 16.2%/- 98.6%/- - ELISA al[91],2011 CDC25B Dong et134 80 54 - 134 < 0.001 56.7%/- 91.0% 0.87/- ELISA al[92],2010 Liu et124 - - - - 123 102 < 0.05 36.3%/- 100%/- - ELISA al[93],2008 GRP78 Tsunemi15 - - - - 15 20 < 0.05 26.7%/- 100%/- - Western et al[94],blot 2010 MakorinShimada73 1/13 21 27 11 - 43 < 0.05 25.0%/100%/- Western 1et al[95],22.9%100%blot 2009 CUEC-Shimada54 7 13 18 16 - 46 < 0.05 26.0%/96.0%/- - Western 23et al[96],33.3%blot 2009 Shimada29 1 11 8 9 - 46 0.036 17.0%/- 100%/- - ELISA et al[96],2009 MMGL Shimada91 21 22 35 13 - 45 < 0.05 47.0%/97.8%/- Western et al[97],38.0%97.8%blot 2007 TRIM21 Kubo-91 39 52 - 42 < 0.05 20.0%/100%/- Western shima et13.0%100%blot al[98],2006 Kubo-54 - - - - 54 42 0.013 15.0%/- 98.0%/- - ELISA shima et al[98],2006 SLC2A1 Kubo-57 19 6 13 19 - 31 < 0.001 21.0%/100%/- ELISA shima et22.0%100%al[99],2006 SURF1 Shimada21 - 3 13 5 - 37 0.0003 48%/- 95%/- - ELISA et al[50],2005
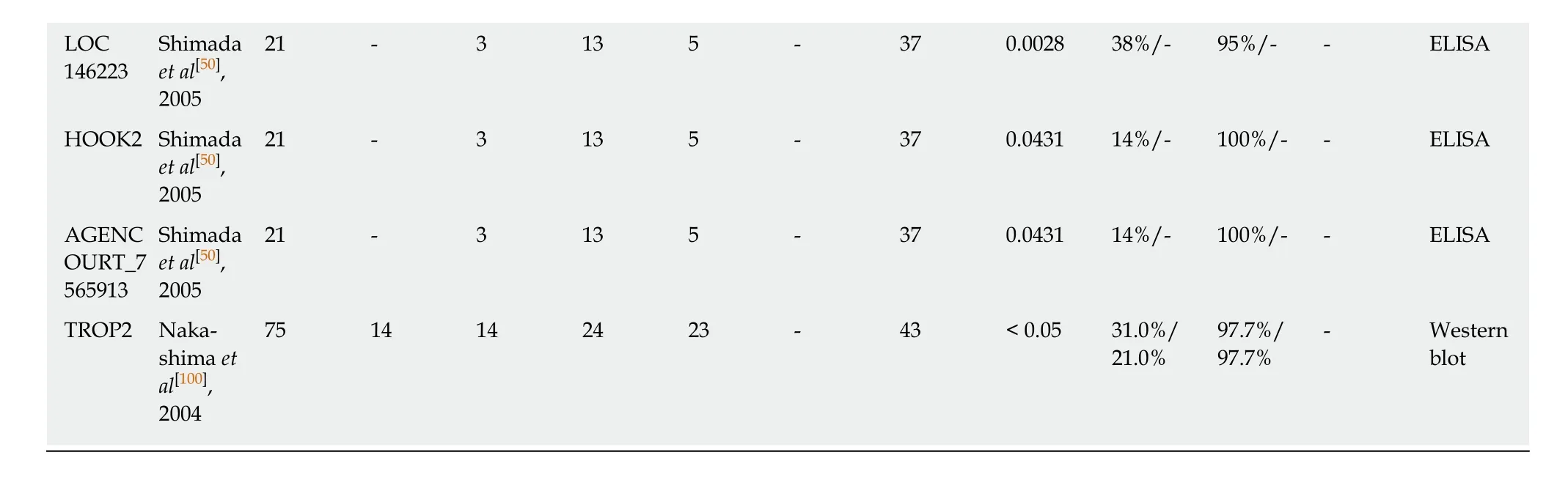
ESCC: Esophageal squamous cell carcinoma; AUC: Area under the curve; L1CAM: L1-cell adhesion molecule; STIP1: Stress induced phosphoprotein 1;DKK-1: Dickkopf 1; Mmp-7: Matrix metallopeptidase 7; Hsp70: Heat shock protein 70; PRDX 6: Peroxiredoxin 6; Bmi-1: BMI1 proto-oncogene, polycomb ring finger; Imp1: Insulin-like growth factor 2 mRNA binding protein 1; C-Myc: MYC proto-oncogene, bHLH transcription factor; Koc: Insulin-like growth factor 2 mRNA binding protein 3; HCCR: LETM1 domain containing 1; GSTO1: Glutathione S-transferase omega 1; MDM2: MDM2 proto-oncogene;HSP105: Heat shock protein family H (Hsp110) member 1; TIM: Rho guanine nucleotide exchange factor 5; Prdx1: Peroxiredoxin 1; FOXP3: Forkhead box P3; CD25: Interleukin 2 receptor subunit alpha; ABCC3: ATP binding cassette subfamily C member 3; LY6K: Lymphocyte antigen 6 family member K;HMGB1, high mobility group box 1; CDC25B: Cell division cycle 25B; GRP78: Heat shock protein family A (Hsp70) member 5; Makorin 1: Makorin ring finger protein 1; MMGL: Myomegalin; TRIM21: Tripartite motif containing 21; SLC2A1: Solute carrier family 2 member 1; SURF1: SURF1 cytochrome c oxidase assembly factor; HOOK2: Hook microtubule tethering protein 2; TROP2: Tumor-associated calcium signal transducer 2.
DIAGNOSTIC PERFORMANCE OF AUTOANTIBODY PANELS IN ESCC
Over the past few years, as single TA autoantibodies do not appear to demonstrate enough diagnostic sensitivity to set up a reliable test for early detection, studies have aimed to identify a suitable panel of TA autoantibodies. These predicaments are presumably due to cancer heterogeneity. In fact, it is unlikely that most patients will respond to the same immunodominant antigens. Even tumors of the same kind are comprised of a diverse mix of biological subtypes; accordingly, cancer patients are more likely to induce an immunoreaction to different sets of TAA, and not all cancers are likely to be detected by autoantibodies against a single antigen. Tables 4 and 5 give an overview of different combinations of multiple autoantibodies as potential blood-based biomarkers for ESCC and EGJA that have been described in the literature by various research groups.
With improvements in technology, several high-throughput methods, such as proteomics platforms, have enabled the uncovering of autoantibodies and the generation of a panel of TAA. These discovery techniques encompass serological analysis of tumor antigens by recombinant cDNA expression cloning[45], serological proteome analysis[46], phage display[47], protein microarrays[48]and multiple affinity protein profiling[49]. Shimadaet al[50]were the first to use the high-throughput method of serological analysis of tumor antigens by recombinant cDNA expression cloning in ESCC. They showed that several TAA that could elicit a humoral immune response that could be detected simultaneously, and the technique enabled the generation of an autoantibody panel that exhibited better diagnostic value (86% sensitivity and 100%specificity) than a single TA autoantibody. Subsequently, a study using serological proteome analysis identified some novel TAA associated with ESCC, and the combination of two TAA (HSP105 and TIM) can give 54.3% sensitivity and 95%specificity in distinguishing ESCC from controls[51]. These studies all show that the combined detection of autoantibodies against several antigens in the panel can greatly increase sensitivity in the diagnosis of ESCC. However, except for the two abovementioned studies, no other relevant literature applying proteomic technology to identify a TAA panel has appeared. This indicates, to some extent, that the identification and development of novel autoantibodies by proteomics platforms for ESCC is limited and behindhand especially compared with other tumor types, such as lung, breast and liver tumors[52-54].
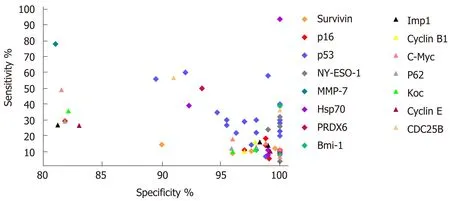
Figure 1 Graphical representation of sensitivity versus specificity for single tumor-associated autoantibody biomarkers in esophageal squamous cell carcinoma reported in more than one study. Mmp-7: Matrix metallopeptidase 7; Hsp70: Heat shock protein 70; PRDX 6: Peroxiredoxin 6; Bmi-1: BMI1 proto-oncogene, polycomb ring finger; Koc: Insulin-like growth factor 2 mRNA binding protein 3; C-Myc: MYC proto-oncogene, bHLH transcription factor; IMP1: Insulin-like growth factor 2 mRNA binding protein 1.
On the other hand, researchers have been more inclined to evaluate the diagnostic performance of combinations of several known TAA. In accord with such thinking,eight studies reported the diagnostic value of different combinations of autoantibodies for ESCC (Table 4). From the list of autoantibodies examined in the panel (Table 4), p53 autoantibodies were the most common choice for inclusion in the biomarker combinations. As is known, p53 as a tumor suppressor gene has been linked to many cancers, including ESCC, and thus would be a rational biomarker to be investigated. Zhanget al[55]assessed a combination of six immunoreactive TAA in ESCC samples and normal controls with independent validation. Then, they sought to identify which biomarkers used in combination were more informative and allowed a similar discrimination between groups. They finally found a restricted panel of four TAA that gave similar sensitivity and specificity in early stage ESCC. Indeed, a similar research strategy had been previously performed by Xuet al[43]who used two independent cohorts to investigate the combination of autoantibodies against p53,NY-ESO-1, MMP-7, Hsp70, Prx VI and Bmi-1. This panel distinguished early stage ESCC from normal controls with a sensitivity/specificity of 45%/95% and 46%/96%,respectively in the test and validation cohorts. Interestingly, the authors also determined a simplified autoantibody panel retaining four out of six biomarkers that exhibited almost the same diagnostic efficacy (Table 4). Although it is reported that a majority of biomarkers with desirable outcomes in a first data set often result in less promising results in additional independent data sets[56], the two above studies with the combinations of known TAA all showed satisfactory diagnostic value in independent validation cohorts. This suggests potential clinical applications for autoantibody combinations to diagnose ESCC. However, we can see from Table 4 that most of the studies reviewed lack validation in an independent population. In practice, the results of biomarkers need to be validated in larger multicenter cohorts and evaluated as a screening test in high-risk populations. However, no study on the evaluation of autoantibodies in ESCC diagnosis has been able to do so. All previously identified autoantibody panels for ESCC should be validated by these procedures to evaluate their true clinical relevance and diagnostic power.
DIAGNOSTIC PERFORMANCE OF AUTOANTIBODY PANELS IN EGJA
As the combined detection of selected autoantibodies as a panel could generally increase diagnostic sensitivity while keeping relatively high specificity in ESCC, two studies have attempted to evaluate the same panels of autoantibodies identified in ESCC for early detection of EGJA and have shown promising results. They demonstrated sensitivities above 50% and specificities above 86% (Table 5). Zhouet al[41]detected autoantibodies to eight TAA, comprised of p53, IMP1, P16, cyclin B1,P62, c-Myc, survivin and Koc and suggested that successive addition of seven TAA(p53, Koc, P62, c-Myc, IMP1, survivin and P16) led to stepwise increases in sensitivity and specificity, ultimately achieving a sensitivity of 64.0% with a specificity of 87.0%.This optimized combination is somewhat different from an optimized panel identified for ESCC (p53, IMP1, P16, cyclin B1, P62 and c-Myc) studied by the same research team. Subsequently, Xuet al[42]showed that autoantibodies against a combination of p53, NY-ESO-1, MMP-7, Hsp70, PRDX6 and Bmi-1, which is the same as the panel used for evaluation of ESCC, could be potentially used for early diagnosis of EGJA.When comparing stage I and II patients to normal controls, the authors showed sensitivities and specificities of 50.0% and 90.5% and 56.0% and 90.0%, respectively, in the training and validation cohorts. It should be noted that a strict panel of p53, NYESO-1 and Bmi-1 to comprise informative biomarkers for EGJA gives similar diagnostic performance. Interestingly, as discussed above, a different restricted combination (p53, NY-ESO-1, PRDX6 and Hsp70) from the same autoantibody panel in early stage ESCC retains high sensitivity and specificity.
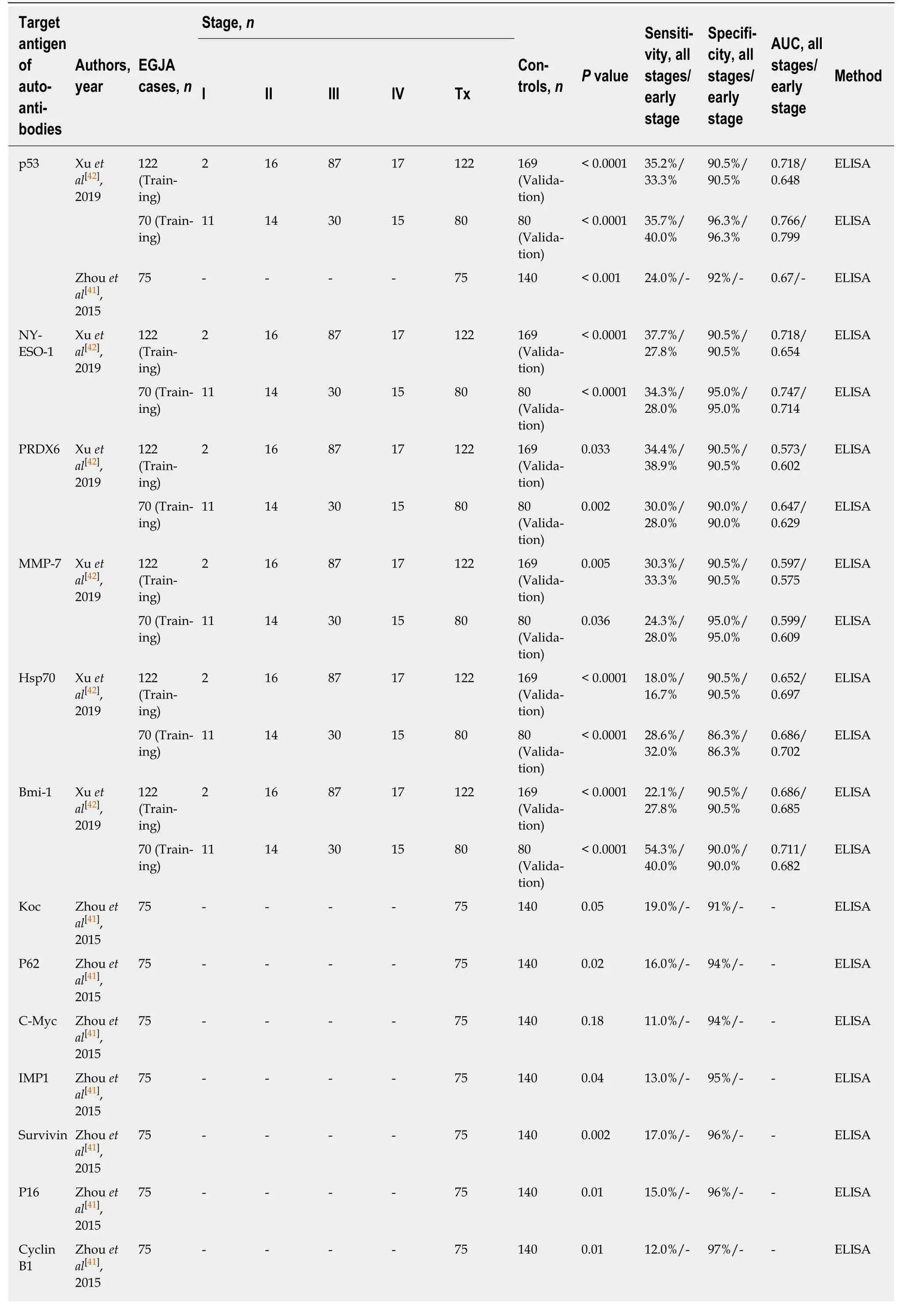
Table 3 Diagnostic performance of single tumor-associated autoantibody biomarkers in esophagogastric junction adenocarcinoma
These studies suggest that the importance of individual autoantibodies in the panel assay varies in different types of cancers. However, we still need to determine which TA autoantibodies applied in combination are more informative and allow a better diagnostic value. In future work, more TA autoantibodies need to be discovered and characterized to identify the best combination for EGJA. Meanwhile, the identified signatures for EGJA should be verified in larger multicenter-appropriated cohorts of early stage patients and controls to test the diagnostic power.
CONCLUSION AND PERSPECTIVES
Endoscopic examination is a current but invasive diagnostic and screening procedure for early detection of ESCC and EGJA. The development and validation of noninvasive biomarkers is of great need for ESCC and EGJA screening. In recent decades,a large number of blood-based cancer biomarkers, such as cell-free circulating tumor DNAs, various non-coding RNAs, proteins and TA autoantibodies, have been identified and indicate the potential for early detection of esophageal cancer. Among these biomarkers, TA autoantibodies are a promising biomarker entity in early cancer detection as they are capable of identifying cancer in high-risk individuals. Moreover,they are highly stable and can be easily detected by routine methods (e.g., ELISA).Recently, a TA autoantibody assay namedEarlyCDT-Lung (against p53, NY-ESO-1,CAGE, GBU4-5, MAGE A4, SOX2 and Hu-D) approved by the FDA has been clinically and analytically validated. An ongoing prospective randomized trial is evaluating the clinical utility of this TA autoantibody panel and its use in a clinical setting of which the results are expected to be announced in the near future. Once this assay is successful for lung cancer, we would predict that tests for all solid tumors,including ESCC and EGJA, will follow.
Biomarker development needs several gradual steps covering preclinical studies,retrospective studies of stored specimens, multicenter validation studies and prospective screening studies. However, in ESCC and EGJA, autoantibody studies on early detection are hampered by several issues. First, the availability of sera from early stage patients seems limited. Only few studies have investigated the diagnostic value of TA autoantibody panels in patients with early stage tumors. Access to large early stage sample cohorts is an essential and necessary issue to examine a test’s value for early stage disease. Moreover, few patients with pre-diagnostic serum samples or high-risk ESCC or EGJA cohorts are available, and up to now no study has reported on the immune response in the form of autoantibodies in these populations. Thus, an investigation of TA autoantibodies for the early detection of ESCC and EGJA will be limited mainly by the availability of human samples. On the other hand, current studies (Tables 4 and 5) investigating autoantibodies show promise but still lack the necessary validation stages. These studies need clinical multicenter validation through use of a broader population to further determine diagnostic value.
It seems that there are different patterns of TA autoantibody frequencies in different types of cancers. Thus, one encountered difficulty is the definition of the panel. This leads to the question of how to choose the optimized combination that works best in terms of sensitivity, specificity and predictive value. At this moment,these is no good guiding principle, but more advanced high-throughput proteome technology might be helpful. On the other hand, it should also be pointed out that TA autoantibodies may not be unique for specific types of cancers. Therefore, TA autoantibody panels identified for ESCC or EGJA are likely to be used as a screening test to discover the existence of cancer, and in general more specific diagnostic tools,such as endoscopy, should be carried out in the event of a positive result.
In conclusion, this review suggests that TA autoantibodies have the potential to serve as diagnostic biomarkers for ESCC and EGJA possibly as part of a general cancer screen. However, present studies in ESCC and EGJA remain at an early stage.It is clear that extensive efforts are needed to uncover promising autoantibody signatures to detect these cancers especially at the early stage. Moreover, it is too early to evaluate the diagnostic value of the autoantibodies reviewed here for clinical use.Standardized assay protocols facilitating the establishment of autoantibodies as highly accurate biomarkers is of great need in ESCC and EGJA. Finally, future studies performed with precise design and collaborative efforts among groups to buildstandardized guidelines to report results will contribute greatly in this research area.
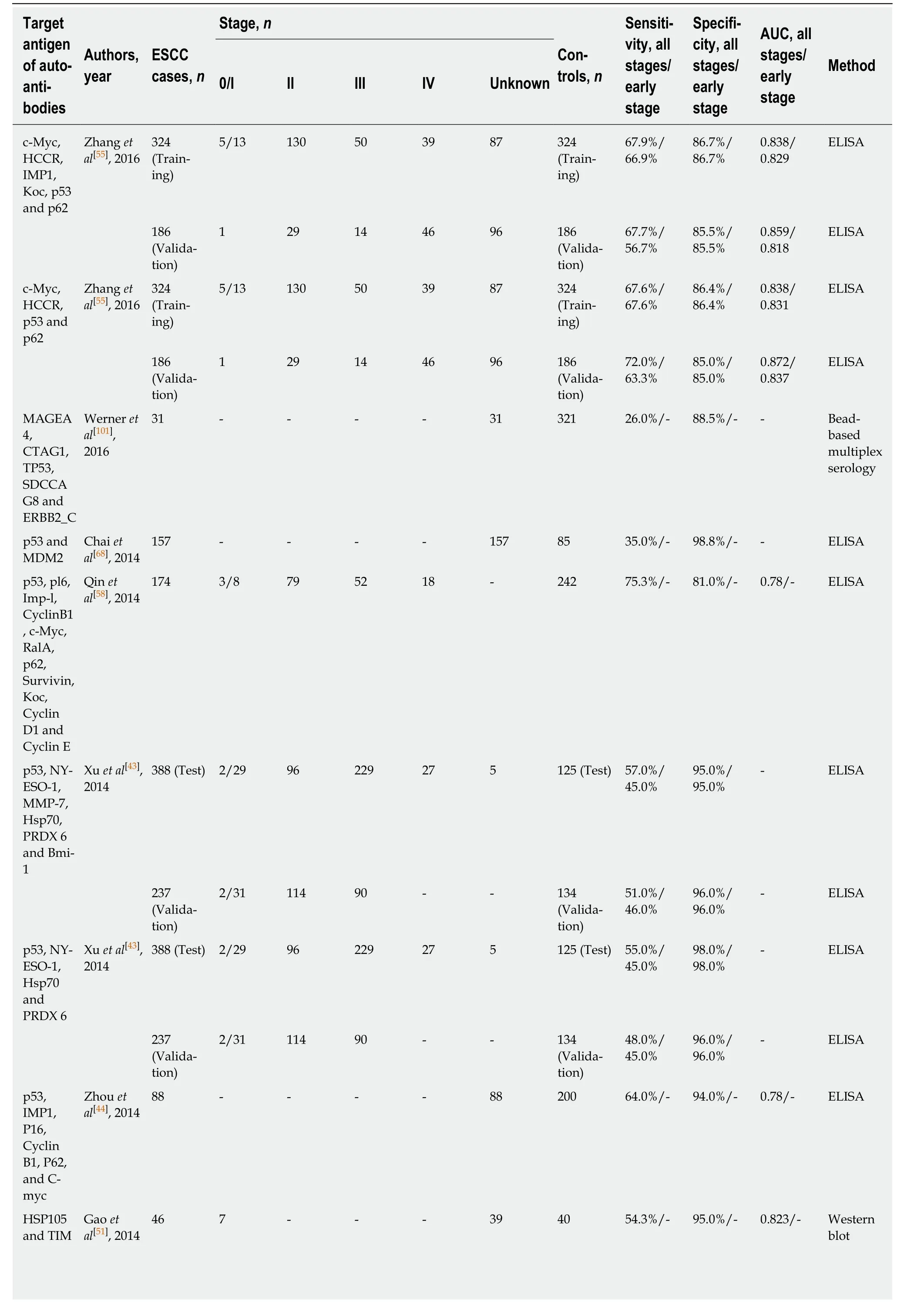
Table 4 Diagnostic performance of tumor-associated autoantibody panel in esophageal squamous cell carcinoma
ESCC: Esophageal squamous cell carcinoma; AUC: Area under the curve; C-Myc: MYC proto-oncogene, bHLH transcription factor; HCCR: LETM1 domain containing 1; IMP1: Insulin-like growth factor 2 mRNA binding protein 1; Koc: Insulin-like growth factor 2 mRNA binding protein 3; MAGEA4: MAGE family member A4; CTAG1: Cancer/testis antigen 1B; SDCCAG8: Serologically defined colon cancer antigen 8; ERBB2: Erb-b2 receptor tyrosine kinase 2;MDM2: MDM2 proto-oncogene; HSP105: Heat shock protein family H (Hsp110) member 1; TIM: Rho guanine nucleotide exchange factor 5; SURF1: SURF1 cytochrome c oxidase assembly factor; HOOK2: Hook microtubule tethering protein 2.
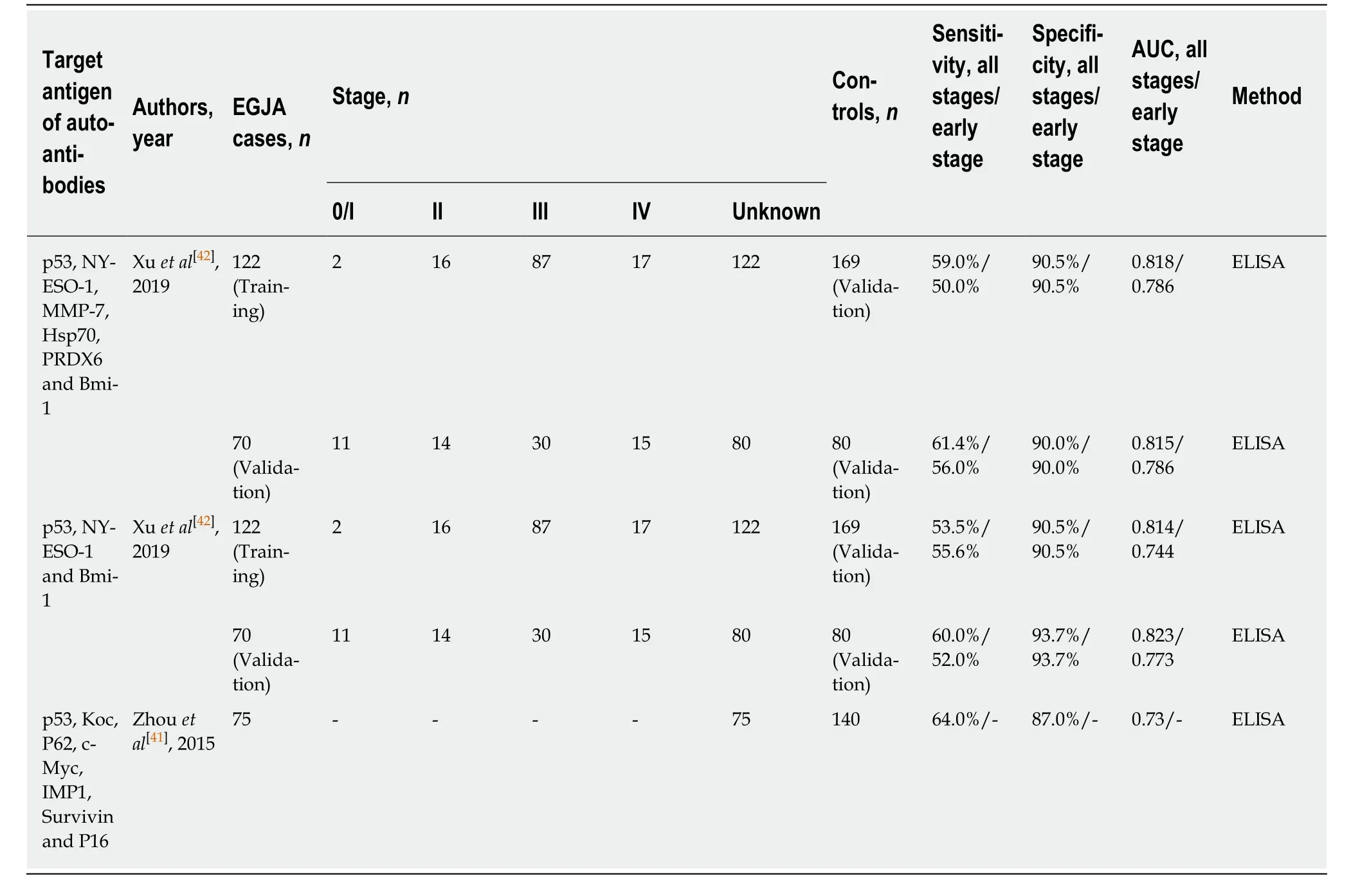
Table 5 Diagnostic performance of tumor-associated autoantibody panel in esophagogastric junction adenocarcinoma
ACKNOWLEDGEMENTS
We thank Professor Stanley Li Lin who re-read this manuscript carefully.
杂志排行
World Journal of Gastroenterology的其它文章
- Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors
- Role of endoscopic ultrasound in the screening and follow-up of high-risk individuals for familial pancreatic cancer
- Hepatic senescence, the good and the bad
- Optimizing proton pump inhibitors in Helicobacter pylori treatment:Old and new tricks to improve effectiveness
- Regulatory effect of a Chinese herbal medicine formula on nonalcoholic fatty liver disease
- Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways
