Role of endoscopic ultrasound in the screening and follow-up of high-risk individuals for familial pancreatic cancer
2019-09-25DianeLorenzoVincianeReboursFrriqueMaireMaximePalazzoJeanMichelGonzalezMariePierreVulliermeAlainAubertPascalHammelPhilippevyLouisdeMestier
Diane Lorenzo, Vinciane Rebours, Frédérique Maire, Maxime Palazzo, Jean-Michel Gonzalez,Marie-Pierre Vullierme, Alain Aubert, Pascal Hammel, Philippe Lévy, Louis de Mestier
Abstract Managing familial pancreatic cancer (FPC) is challenging for gastroenterologists,surgeons and oncologists. High-risk individuals (HRI) for pancreatic cancer (PC)(FPC or with germline mutations) are a heterogeneous group of subjects with a theoretical lifetime cumulative risk of PC over 5%. Screening is mainly based on annual magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS). The goal of screening is to identify early-stage operable cancers or high-risk precancerous lesions (pancreatic intraepithelial neoplasia or intraductal papillary mucinous neoplasms with high-grade dysplasia). In the literature, target lesions are identified in 2%-5% of HRI who undergo screening. EUS appears to provide better identification of small solid lesions (0%-46% of HRI) and chronicpancreatitis-like parenchymal changes (14%-77% of HRI), while MRI is probably the best modality to identify small cystic lesions (13%-49% of HRI). There are no specific studies in HRI on the use of contrast-enhanced harmonic EUS. EUS can also be used to obtain tissue samples. Nevertheless, there is still limited evidence on the accuracy of imaging procedures used for screening or agreement on which patients to treat. The cost-effectiveness of screening is also unclear. Certain new EUS-related techniques, such as searching for DNA abnormalities or protein markers in pancreatic fluid, appear to be promising.
Key words: Endoscopic ultrasound; Familial pancreatic cancer; Fine-needle aspiration;Intraductal papillary mucinous neoplasm; Pancreatic cancer; Pancreatic intraepithelial neoplasia; Pancreatic cancer screening guidelines
INTRODUCTION
In the past few decades, the incidence of pancreatic cancer (PC) has continuously increased, while its prognosis remains poor, with a 5-year survival rate < 10% for all stages analysed together. PC is expected to become the second leading cause of cancer-related death in the United States in 2030[1]. Early-stage surgical resection is the only potentially curative treatment that increases survival. However, complete surgical resection can only be performed in a minority of patients, since 80% of patients have metastatic or locoregionally advanced disease at diagnosis[1-3].
Five to 10% of PCs are considered to be inherited[4,5]. While pathogenic germline mutations in specific genes have been associated with an increased risk of PC (from 4% to 40%), a causal germline mutation is identified in fewer than 20% of these families[6-10]. Several gene abnormalities and related hereditary syndromes have been associated with an increased risk of PC:BRCA1andBRCA2(hereditary breast ovarian cancer syndrome),PALB2and the genes involved in the Fanconi pathway,CDKN2A(familial atypical multiple mole melanoma: FAMMM), the genes involved in Lynch syndrome (mainlyMLH1,MSH2,MSH6, andPMS2),PRSS1(hereditary pancreatitis),STK11(Peutz-Jeghers syndrome),TP53(Li-Fraumeni syndrome),APC(familial adenomatous polyposis), andATM(ataxia telangiectasia)[4,9,11-16]. In the remaining 85%of families with no identified germline mutation, familial pancreatic cancer (FPC) is defined by the occurrence of PC in ≥ 2 first-degree relatives or in ≥ 3 relatives whatever the degree of relationship on the same side of the family[4,17].
Pancreatic screening is recommended in high-risk individuals (HRI) to identify early (pre)malignant pancreatic lesions and propose surgical resection with curative intent. The purpose of this screening is to reduce mortality related to PC. While numerous large and retrospective studies have reported on the short-term outcomes of pancreatic screening in HRI, follow-up was generally limited, and hence the magnitude of benefit of pancreatic screening in terms of curative potential remains currently unknown[11,12,17-19]. Additionally, although screening is usually based on yearly magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS), the role of the latter has not been clearly defined. Our goal was to comprehensively review current data on pancreatic screening and follow-up of HRI, with an emphasis on the role of EUS.
OBJECTIVES AND CHALLENGES OF PANCREATIC SCREENING IN HIGH-RISK INDIVIDUALS
The goal of screening in HRI (history of FPC or pathogenic germline mutations +/-family history of PC) is to reduce PC-related mortality by identifying morphological abnormalities that suggest the development of PC at an early and potentially curative stage[11,17-19]. In a meta-analysis from our group including all HRI treated by surgery reported in the literature, patients without invasive PC who underwent resection of premalignant lesions had no postoperative recurrence compared to those with invasive PC, and their 3-year overall survival was 100%vs34.6%, respectively (P<0.001)[11]. The lifetime risk of PC in HRI (with germline mutations or FPC) is estimated to be between 1% and 50% depending on the underlying predisposition and the number of affected relatives[12,17,20]. Table 1 presents the risk by mutations and their frequency in inherited cancers. Thus, HRI are theoretically good candidates for pancreatic screening.
The first challenge of PC screening is the effective identification of good candidates,specifically, individuals with a theoretical risk threshold, arbitrarily set at 5%, of developing PC in their lifetime. Signorettiet al[18,21]have shown that the identification of (pre)malignant lesions varies depending on the genetic subgroup (3% in familial PC, 5% in FAMM syndrome, 6.3% in hereditary breast/ovarian cancer, and 12.2% in Peutz-Jeghers syndrome), while it was 42% in patients with hereditary pancreatitis who have the PRSS1 mutation. Corralet al[19]estimated that 135 patients needed to be screened to successfully identify 1 patient with a target lesion (high-risk lesion or PC)(95%CI: 88-303). This low rate was highly questionable, however, due to the very short follow-up period (3.3 years on average) reported in the studies[22]. Indeed, it contrasts with the delay that was estimated for a premalignant lesion to transform into invasive cancer (11 years) and does not enable the drawing of conclusions regarding the global yield of pancreatic screening in HRI.
Relevant imaging pancreatic abnormalities are identified at imaging in approximately 50% of HRI, but this figure is difficult to interpret as there have been too few correlations of these imaging abnormalities with pathological examination due to the limited number of operated patients[11,18,23]. Another challenge of pancreatic screening is to identify and use the most appropriate screening techniques. Ideally, this would be the least invasive and reproducible technique that identifies the greatest number of premalignant lesions and that is the most acceptable for the patient.
The ultimate goal of this approach is to propose surgical resection of premalignant lesions [such as pancreatic intraepithelial neoplasia (PanIN) or intraductal papillary mucinous neoplasms (IPMN) with high-grade dysplasia (HGD)] or even early-stage invasive PC, which are found in approximately 3%-5% of HRI[11,19]. Finally, identifying lesions at high risk of (pre)malignancy and operating neither too early (low-grade dysplasia) nor too late (advanced PC) is challenging[17]. We recently found that an indication for prophylactic pancreatectomy was appropriate (based on identification of HGD or invasive PC) in 42.2% of surgically treated HRI[11]. The factors predicting surgical appropriateness were age > 50 years, presence of a germline mutation and the presence of high-risk radiological pancreatic abnormalities (the presence of“worrisome features”, “high-risk stigmata of malignancy”, or a solid pancreatic mass)[11].
WHAT IS THE BEST SCREENING MODALITY FOR HIGHRISK INDIVIDUALS?
In the past two decades, management of HRI has evolved and varies from one country to another. Screening should be performed in multidisciplinary teams in referral centres, which have more experience and expertise in screening methods (i.e.,EUS and MRI) and in the treatment of invasive PC[17]. A recent meta-analysis estimated that the annual prevalence of high-risk lesions (early invasive PC, IPMN, or PanIN with HGD) detected in HRI was 3.3%, corresponding to 5/1000 person-years during follow-up and an individual probability of 0.5% per year[18].
The screening of HRI is mainly based on pancreatic morphological imaging[computed tomography (CT) scan, MRI and EUS][11,12,18,19]. For a long time, many studies have suggested that EUS might provide better detection of small solid lesions,while MRI can identify small cystic lesions[24-27]. In the study by Cantoet al[25]in 216 HRI, EUS, MRI and CT scan detected pancreatic abnormalities (cysts, solid lesions or chronic pancreatitis) in 42.6%, 33.3% and 11% of patients, respectively. This corresponded to a sensitivity of 93% for EUS for the detection of solid lesions smaller than 2 cm compared to 53% and 67% for CT scan and MRI, respectively[25]. Harincketal[27]performed a prospective comparison of EUS and MRI for the detection of clinically relevant pancreatic lesions at initial screening of 139 HRI. In this study, EUS and/or MRI detected pancreatic lesions in 6% of HRI: 2 solid tumours < 10 mm were only detected by EUS (1 invasive PC and 1 PanIN with low-grade dysplasia), and 25%of cysts were only detected by MRI[27]. Nevertheless, as all patients were not operated on, this study does not enable the evaluation of whether the lesions detected were all of pathological relevance. Table 2 reports the main characteristics of HRI screening techniques and imaging results in 16 published studies. Of note, MRI and CT scan protocols were not clearly described in most studies (e.g., matrix size, contrast enhancement, MRI sequences), and the results of EUS are well known to be operatordependent as well as classical radiological procedures[28,29]. Indeed, Topazianet al[28]report a low interobserver agreement for the interpretation of pancreatic EUS in HRI(Kappa < 0.4 except for cysts). This is probably due to the lack of specific training for EUS, the lack of a standardized collection chart and a specific learning curve.Although all of the abovementioned studies included operated patients, the methods of detection of the pancreatic abnormalities that determined the surgical procedure were not described in detail. Thus, while the precise value of EUS compared to the other modalities is probably high (it may find more (pre)malignant lesions), this is difficult to determine in the absence of a large study correlating anatomopathological specimens to EUS findings.
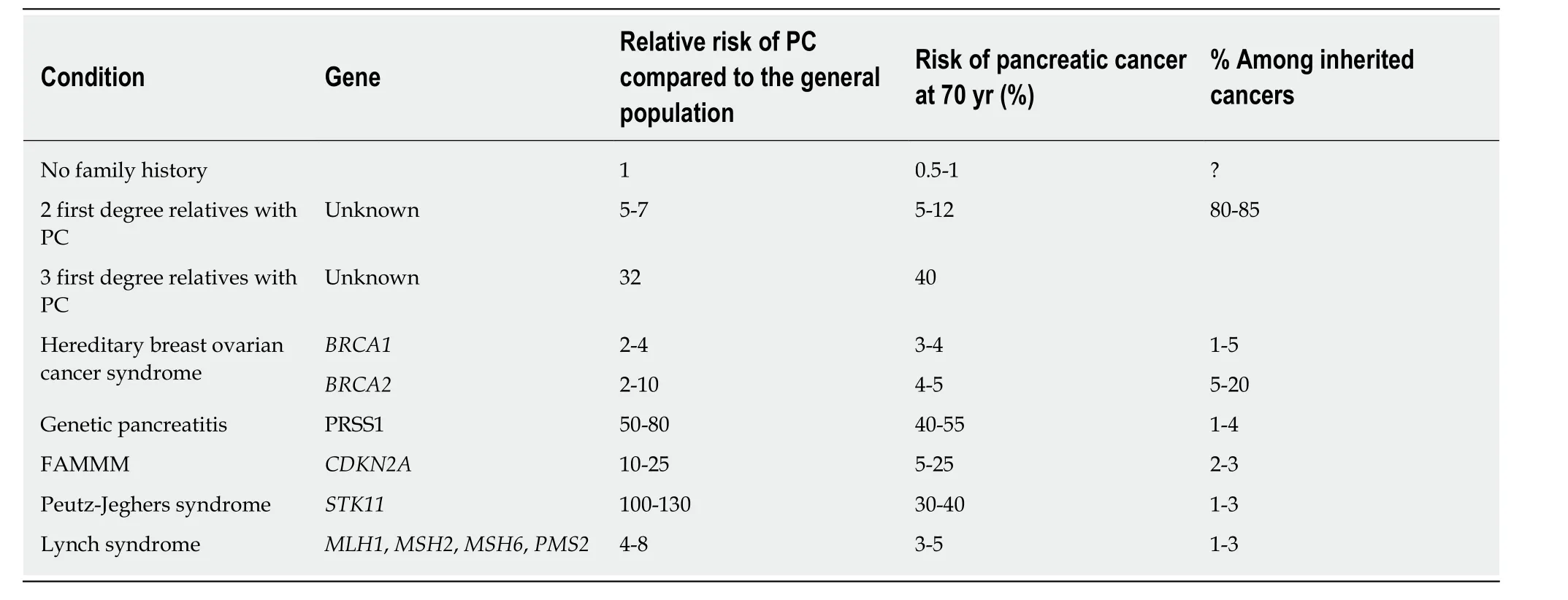
Table 1 Risk of pancreatic cancer based on genetic mutations
There are no approved biomarkers for the screening of PC in HRI. Only one study has reported the results of serum CA19-9 measurement for pancreatic screening in these patients. Twenty-seven out of 546 included patients (4.9%) had elevated CA19-9,and 5 (18.5%) of these had pancreatic lesions (1 PC, 2 IPMN, 1 PanIN, 1 neuroendocrine tumour). Nevertheless, the number of pancreatic lesions in the group with normal CA 19-9 levels was not reported[16]. CA19-9 is not recommended because of its low sensitivity and specificity[11,17-19,30].
The recent CAPS (International Cancer of the Pancreas Screening ) consensus has suggested that annual MRI [including magnetic resonance cholangiopancreatography (MRCP)] and EUS are the best imaging modalities for the detection of significant PC precursor lesions[6]. In summary, and as recommended by the recent statement by the American Society of Clinical Oncology, pancreatic screening and follow-up in HRI should be based on MRI and EUS as complementary tests for the detection of pancreatic lesions[17].
WHEN AND HOW OFTEN SHOULD SCREENING BE PERFORMED?
While pancreatic screening usually begins when HRI are 40 years old, or 10 years before the youngest index case[23], this has recently been challenged because pancreatic screening rarely reveals relevant lesions before the age of 50[4]. We also recently reported that the risk of HGD or invasive cancer was 3 times higher in HRI > 50 years old operated on for pancreatic lesions than in HRI < 50 years old[11].
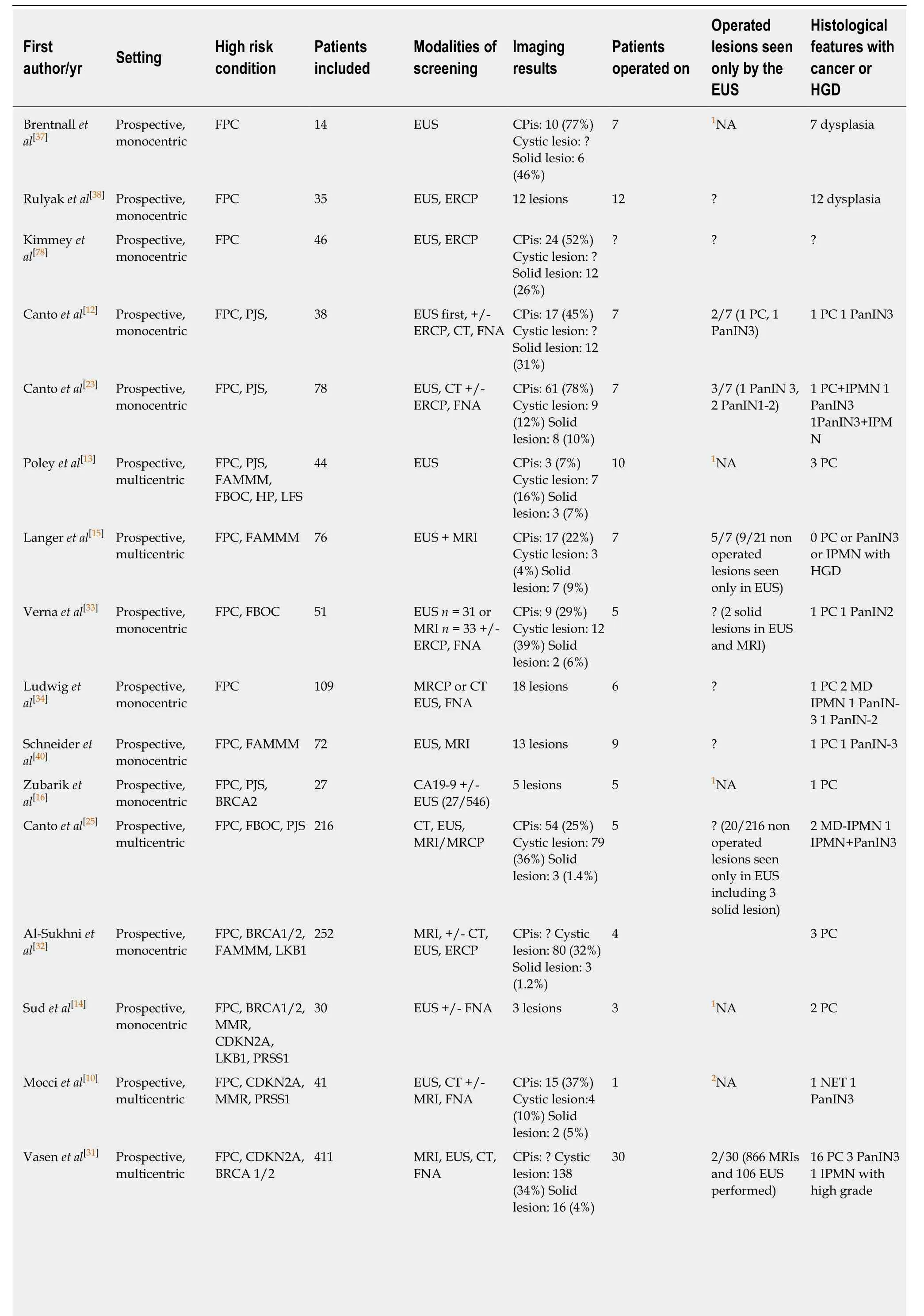
Table 2 Main characteristics of high-risk individuals for pancreatic cancer in the different studies
1NA: Not applicable, because patients had only endoscopic ultrasound (EUS);2NA: Not applicable, because patients had an EUS only if magnetic resonance imaging was abnormal. Three patients developed pancreatic cancer during the screening. CPis: Chronic-pancreatitis-like parenchymal changes; CT: Computed tomography; EUS: Endoscopic ultrasound; FNA: Fine needle aspiration; FPC: Familial pancreatic cancer; IPMN: Intraductal papillary mucinous neoplasm; MRI: Magnetic resonance imaging; PanIN: Pancreatic intraneoplasia; PC: Pancreatic cancer; ERCP: Endoscopic retrograde cholangiopancreaticography; HGD: High-grade dysplasia.
It is important to note that the majority of relevant pancreatic lesions (> 50% of cysts and solid tumours) are identified at the first screening rather than during followup[23,31,32]. The frequency of subsequent follow-up examinations varies depending on different studies and recommendations[4,30-34]. Several multicenter studies have compared different surveillance protocols (e.g., annual MRI and EUS or annual MRI with EUS every 3 years) and did not find any difference in terms of number of diagnosed lesions[4,31]. However, these studies compared the total number of diagnosed lesions, whereas only the number of (pre)malignant lesions would have had clinical relevance. Nevertheless, and unless demonstrated otherwise, it seems reasonable to perform one MRI and one EUS examination per year in HRI[11,19,31,32].
Life-long pancreatic follow-up is recommended in HRI, at least as long as patients remain fit for surgery, without severe comorbidities. In fact, there are no strong data to confirm when follow-up should be stopped[17,23]. Furthermore, the prognosis in another group at risk of PC (patients with IPMN) was poorer in patients who stopped surveillance after 5 years of surveillance, which advocates for prolonged surveillance[35].
PLACE OF ENDOSCOPIC ULTRASOUND FOR PANCREATIC SCREENING OF HIGH-RISK INDIVIDUALS
Pancreatic lesions found in endoscopic ultrasound
EUS pancreatic screening may identify pancreatic abnormalities in 19% to 79% of HRI(13-16, 25, 31, 34, 35, 37-40) (Table 2). These pancreatic “abnormalities” include cystic lesions (13%-49%) (Figures 1 and 2), chronic pancreatitis-like parenchymal changes(14%-77%) (Figure 3) and solid lesions (0%-46%) (Figure 4), which are always considered suspicious[4,10,18,23,26,27,39,40]. Of note, most studies that reported a low rate of EUS-detected abnormalities did not consider chronic pancreatitis-like parenchymal changes.
Pancreatic cystic lesions identified in HRI are mainly IPMN, which are more frequent in HRI (13%-20%) than in the general population (1%-5%)[18,26,41,42]. Whether IPMN in HRI have a different course than sporadic ones is unknown, and hence their monitoring could be similar by analogy with the general population. First, the initial characterization of IPMN should search for potential high-risk stigmata (obstructive jaundice associated with cystic lesions of the head of the pancreas, enhancing mural nodules > 5 mm, main pancreatic duct > 10 mm) and worrisome features (pancreatitis,cyst > 3 cm, enhancing mural nodule < 5 mm, thickened/enhancing cyst walls, main duct size 5-9 mm, abrupt change in calibre of pancreatic duct with distal pancreatic atrophy, lymphadenopathy, increased CA19-9 serum levels, and cyst growth rate > 5 mm per 2 years)[43,44]. These features are highly important for deciding on surgery because their presence is associated with an increased probability of finding invasive PC or HGD on the resected specimen[11]. EUS has been shown to be the best technique for the early detection of malignancy in patients with IPMN[45]. Nevertheless, the entire pancreatic parenchyma is at risk of PC, which can occur away from the cysts[46].Thus, surveillance should not focus on pancreatic cysts alone. However, all cystic lesions found in HRI are not IPMN (but may be other benign cysts such as serous cystadenomas)[15,30,31]. Nevertheless, in the absence of specific worrisome criteria for non-IPMN cystic pancreatic lesions, it seems appropriate to use the abovementioned worrisome features for all cystic lesions, while it may lead to operating on benign cysts without potential pejorative evolution[11].
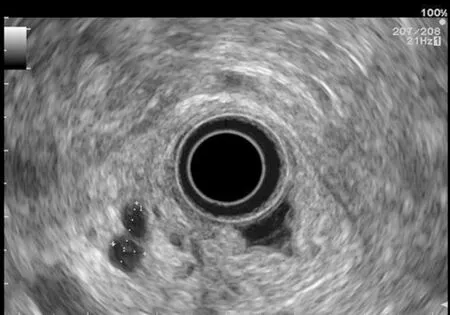
Figure 1 Pancreatic cystic lesion: Intraductal papillary mucinous neoplasm.
Chronic-pancreatitis-like parenchymal changes (CPis) are more common in HRI(up to 67%-80%) than in the general population (15%-17%)[12,23,47-49]. The diagnostic criteria for chronic pancreatitis with EUS (Rosemont Criteria) include major criteria(hyperechoic foci with shadowing and main pancreatic duct calcification, a lobularity with honeycombing) and minor criteria (small cysts, dilated ducts ≥ 3.5 mm, irregular pancreatic ducts, dilated side branches ≥ 1 mm, hyperechoic duct walls, strands, nonshadowing hyperechoic foci, and lobularity with noncontiguous lobules)[50]. Chronicpancreatitis-like parenchymal atrophy and hyperechoic foci may correspond to multifocal PanIN[51-54]. Bruneet al[51]reported a significant correlation between CPis on EUS and percentage of PanIN lesions on surgical specimens. The supposed explanation is that multifocal PanIN lesions produce obstructive lobular atrophy,which is probably the source of CPis[53]. Features of chronic pancreatitis during EUSbased surveillance of HRI are easily seen with good interobserver agreement[48]. One study showed that CPis generally have no or little progression over time, although the follow-up period was limited (3 years)[48]. A fatty pancreas has also been reported to be a risk factor for PC and should be noted on the EUS report[55].
Finally, EUS can identify solid pancreatic lesions in up to 20% of HRI during follow-up. These solid tumours are generally PC but may also be PanIN with HGD or neuroendocrine tumours[10,11,15,26,31,32,40]. In published studies, 14/53 (26%) of operated significant lesions (for which MRI and EUS data were available) were only seen on EUS (Table 1)[12,15,23,27,31]. However, as previously reported, 7/26 HRI operated on for a“pancreatic mass” (27%) had lesions with no/low malignant potential on the pathological specimen[11]. Nevertheless, it is difficult to consider not operating on an HRI with a “pancreatic mass” identified during EUS screening[11]. As discussed below,lesion sampling may be of special interest in this setting.
Endoscopic ultrasound pancreatic screening techniques in high-risk individuals
A study by Shinet al[29]suggested that compared to radial EUS, linear-array EUS improves the detection of pancreatic lesions in HRI, probably due to better visualization of the pancreatic tail. This same study reported a "second-pass effect"with additional lesions detected during a second EUS examination[29].
The use of contrast-enhanced harmonic EUS (CH-EUS) could improve its diagnostic performance. Indeed, it can identify solid neoplastic components in pancreatic cysts/IPMN as hyperenhanced lesions (Figure 5)[56]. The pooled sensitivity and specificity of CH-EUS for the diagnosis of PC are very high (91% and 87%,respectively) in the general population with solid pancreatic neoplasms[56,57].Nevertheless, there is no specific study on the use of CH-EUS for pancreatic screening in HRI.
EUS elastography could also be useful for the characterization of solid pancreatic lesions in this population[58]. Iglesias-Garciaet al[59]reported a sensitivity, a specificity,and positive and negative predictive values of 100%, 85.5%, 90.7%, and 100%,respectively, for the diagnosis of sporadic PC.
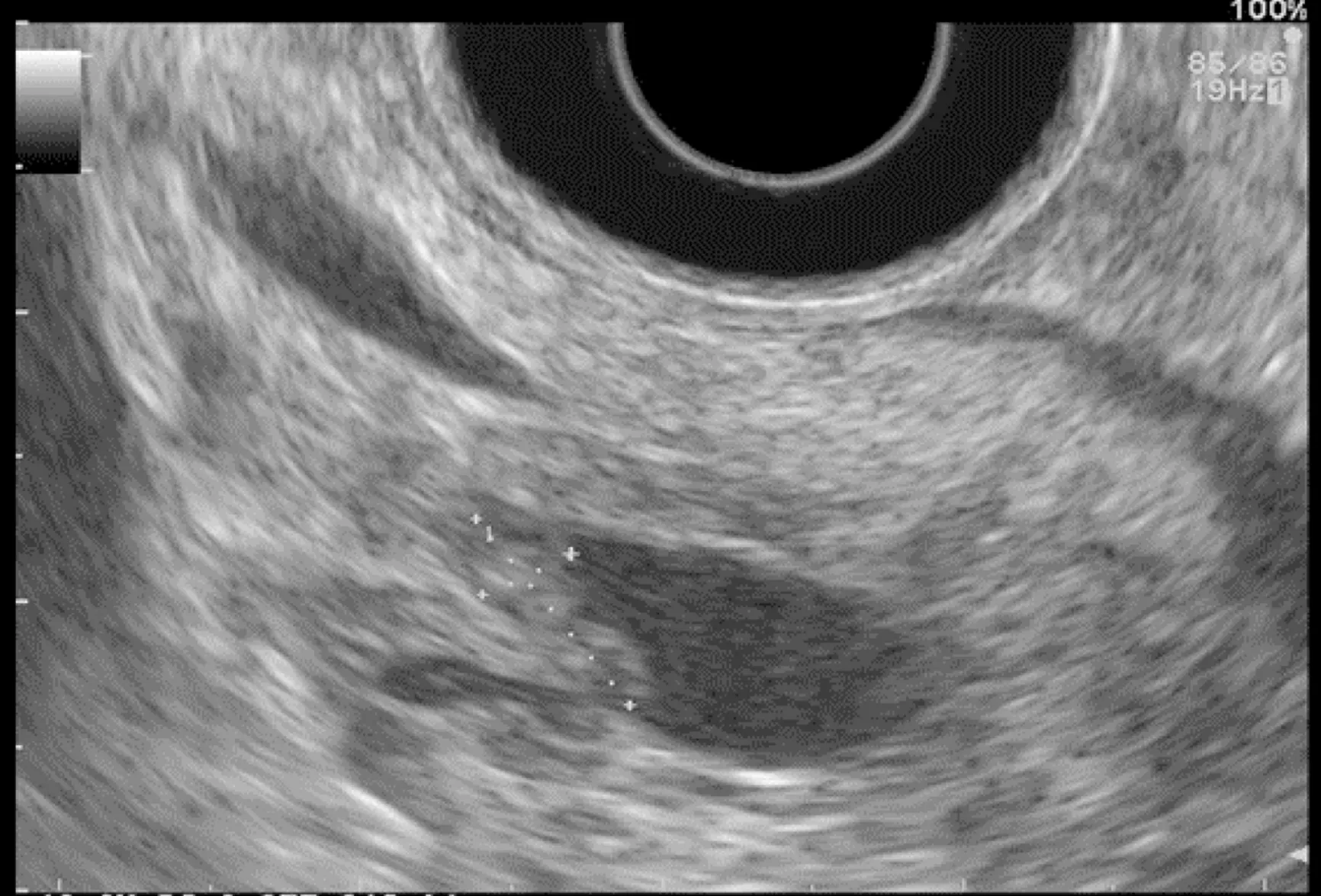
Figure 2 Intraductal papillary mucinous neoplasm with mural nodule.
The results of certain new techniques for the detection of (pre)malignant pancreatic lesions are especially promising in HRI, including the molecular imaging of cathepsin Ein vivousing a confocal laser endomicroscopy miniprobe (overexpression of cathepsin E in PanIN) and the detection of DNA abnormalities or protein markers in pancreatic cyst fluid collected by fine needle aspiration (FNA) (mutations in genes such asTP53) (Figure 6)[57,60-63]. The role of these combined strategies (EUS with other new technological/biological techniques) as well as their impact on survival and costeffectiveness must, however, still be defined[64]. Finally, another lead could also be the identification of molecular abnormalities (p53 mutations,KRASmutations, DNA methylation markers) associated with the presence of PC and precursors of PC within pancreatic fluid collected by endoscopic retrograde cholangiopancreatography[65-69].The role of these exams in FPC screening strategies must still be defined[68].
Endoscopic ultrasound-guided fine needle aspiration and fine needle biopsy
Another advantage of EUS is that FNA or fine needle biopsy (FNB) can be performed in solid pancreatic masses to obtain tissue samples for histopathological characterization with a low risk of complications[11,14,23,33,70]. The complication rate of EUSFNA is approximately 2%[70]. The sensitivity (84.3%), specificity (97%), and accuracy(84%) of EUS-FNA for solid pancreatic masses are high[71]. However, the negative predictive value is low (64%)[26,71], indicating that a negative FNA does not exclude the presence of a (pre)malignant lesion. Thus, in the presence of a solid pancreatic lesion with negative histological samples, FNA (or FNB) must be repeated in HRI, and prophylactic pancreatectomy should be discussed. The use of cutting needles for EUSFNB should improve pancreatic histological samples[72]. In addition, EUS-FNA is associated with a non-negligible rate of false positives (2% in the literature, up to 33%in HRI, especially in patients with CPis). Thus, the diagnostic value of FNA may be limited in HRI screening[11].
IMPROVING THE DETECTION OF HIGH-GRADE PANIN IN HIGH-RISK INDIVIDUALS
The theoretical risk of hereditary transmission of PC-predisposing genetic abnormalities is up to 50%. Hence, in the setting of FPC without a known predisposing gene mutation, approximately 50% of HRI undergo screening theoretically without predisposing genetic abnormalities. Thus, the detection of any cystic lesions or CPis is important in FPC-related HRI without identified mutations because it probably indicates that this subject carries the unidentified mutation and is hence at risk of PC.
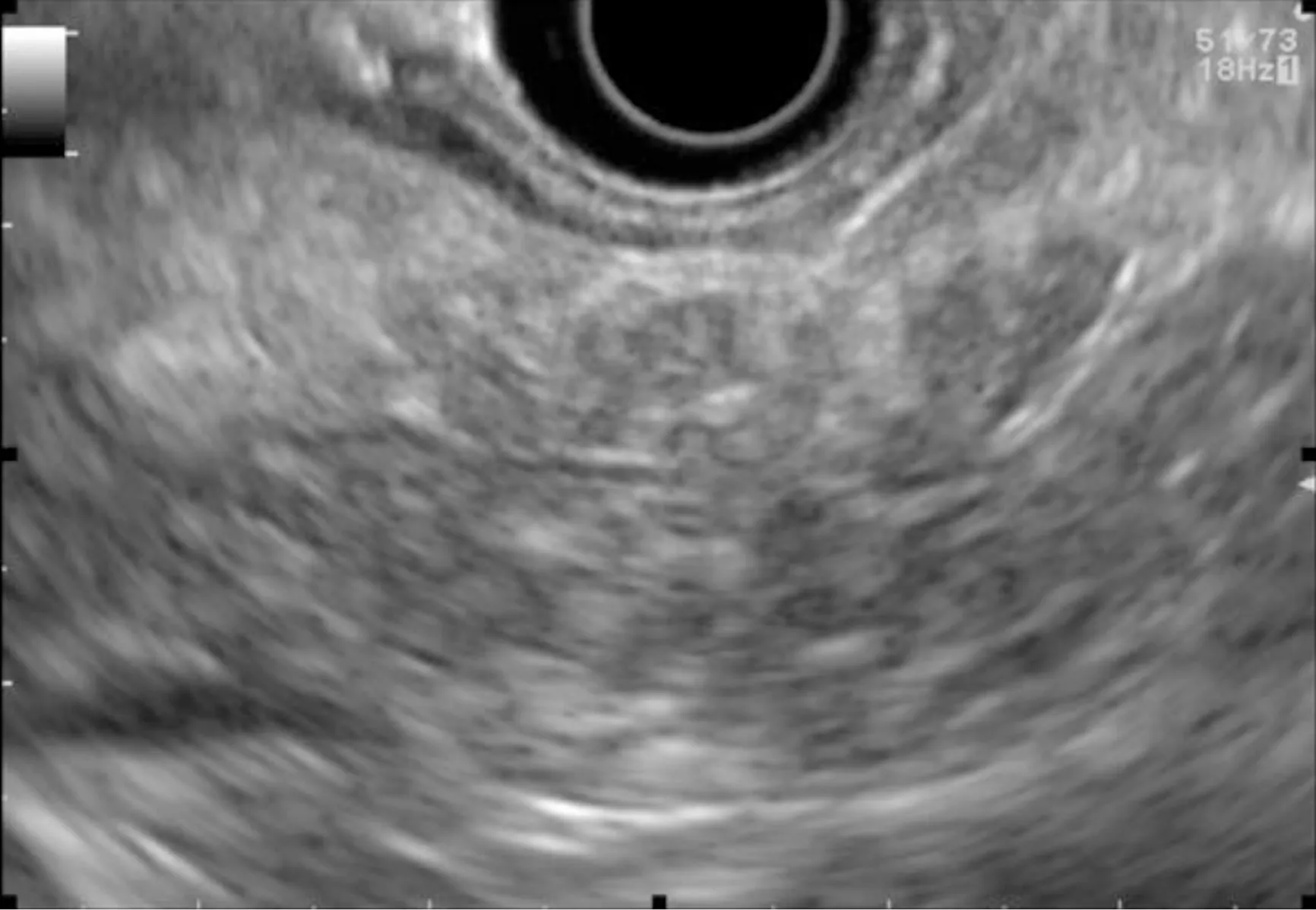
Figure 3 Chronic-pancreatitis-like parenchymal changes.
Most PC arise from PanIN lesions, with a delay of approximately ten years[22]. The challenge of screening is being able to propose surgery at the stage of high-grade PanINs. Some aspects of high-grade PanINs have been described with MRI and EUS,but these lesions lack specificity[23,51,73]. Our group and others previously reported that CPis (microcysts and/or hyperechoic foci of fibrosis) visualized at EUS were associated with PanIN (in up to 83% of cases)[51,54,74]. In an American study, the odds ratio for the association between intermediate-grade PanIN and hyperechoic foci without shadowing in the pancreas head was 8.5 (P= 0.05)[74]. This aspect would concern approximately 70% of HRI[12,23]. However, the assessment of such abnormalities might suffer from a lack of recognition, and the correlation between EUS aspect and pathology should be assessed in a large series of HRI[28,60].
Several studies have reported an increased risk of PC in patients with pancreatic cysts, whatever the etiology[73-75]. In some cases, and especially in HRI, MRI with MRCP and diffusion-weighted MR sequences can help identify very small cysts that do not communicate with the pancreatic ducts[73,74]. In a recent MRI study that included 100 patients, our group recently showed that the identification of noncommunicating pancreatic microcysts had a 52.3% sensitivity, a 77.1% specificity, and a 61% accuracy for the diagnosis of PanIN[73]. In the same study, the association of global atrophy and non-communicating microcysts increased the predictive risk of PanIN. Interobserver agreement for the presence of microcysts was excellent with MRI (kappa = 0.92)[73].
PSYCHOLOGICAL IMPACT OF PANCREATIC SCREENING
The psychological burden in HRI of PC is significant because of the patient’s level of risk and his/her experience with close relatives suffering from this severe disease[15].Patients must be informed that the aim of screening is to identify premalignant lesions or early invasive PC to propose a pancreatectomy with prophylactic and/or curative intent. Additionally, they must be informed of the potential benefits (avoiding the development of PC, being treated at a curable stage, or at least diagnosing PC at the earliest stage possible and prolonging survival) and risks (related to general anaesthesia and/or EUS and especially FNA, unnecessary pancreatic surgery with a risk of complications and diabetes) of pancreatic screening[17]. Moreover, because the sensitivity of the different imaging examinations is not 100%, patients must be informed of the risk of missing (pre)malignant lesions and developing advanced PC during follow-up intervals.
Several studies have investigated the psychological burden of pancreatic screening and repeated examinations. Overall, pancreatic screening may have a positive psychological impact on HRI[76]. Koningset al[48]reported a low psychological burden due to the examinations themselves, which were considered uncomfortable only in 10% of cases. Of note, although it is an invasive procedure, EUS was generally not considered to be more uncomfortable than MRI[48,76].
COST-EFFECTIVENESS OF PANCREATIC SCREENING IN
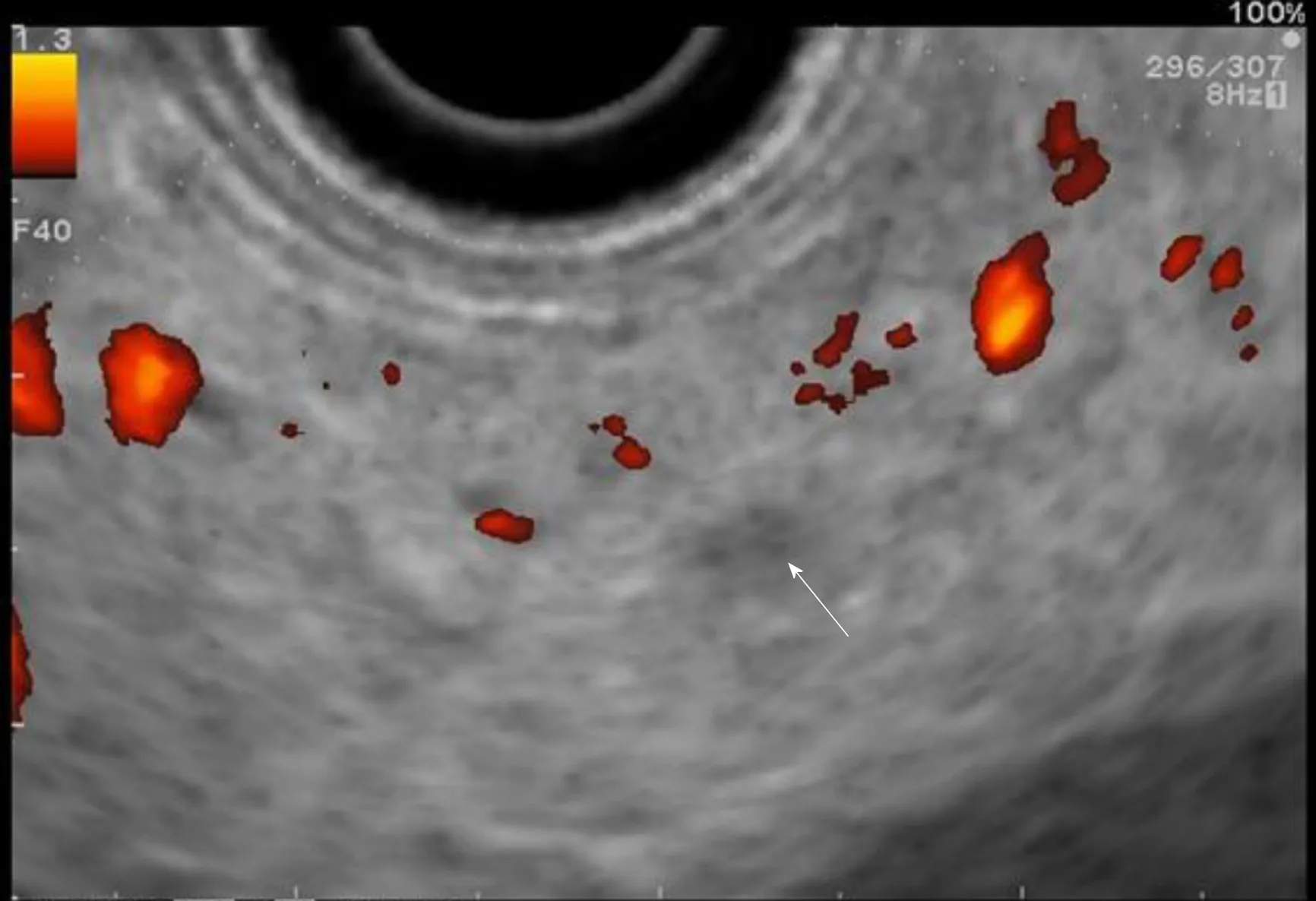
Figure 4 Small hypoechoic nodule.
HIGH-RISK INDIVIDUALS
It is difficult to assess the cost-effectiveness of pancreatic screening in HRI because of the different screening practices in different countries and the results of screening in different studies[10,11,17,18,23,24,40]. A screening test can generally be considered acceptable if there is a positive benefit/cost ratio. Rulyaket al[38]compared one-time EUS-based screening to no screening in a hypothetical cohort of 100 HRI. They showed that screening increased life expectancy (38 years) in a cost-effective manner ($ 16885 per life-year saved). Latchfordet al[77]created a model that showed that seven PCs would theoretically be detected in 250 patients who would undergo yearly EUS between the age of 40 and 55 years old. The cost would be $ 164.285 dollars per PC detected and $372708 per life saved. Overall, it is not clear whether EUS-based screening can be considered cost-effective[60].
CONCLUSION
PC is inherited in 5%-10% of cases. HRI of PC can benefit from pancreatic screening mainly based on annual MRI and EUS. Successful screening targets are early invasive PC and IPMN or PanIN with HGD, which may be treated surgically with curative intent. These lesions are identified in 2% to 5% of all HRI undergoing screening. EUS appears to be the best examination to identify small solid lesions and is complementary to MRI, whose performance may be higher for identifying small cystic lesions. Pancreatic abnormalities found by EUS are cystic lesions (13%-49% of HRI), solid lesions (0%-46%) and chronic pancreatitis-like parenchymal changes (>50%). Of note, the latter frequently correspond to PanIN lesions. Finally, CH-EUS,EUS-elastography and other new techniques (including needle-based confocal laser endomicroscopy miniprobe and the detection of DNA abnormalities or protein markers by FNA) are being developed, but further studies are needed to evaluate their role in the management of HRI. There is still limited evidence on the accuracy,acceptability and cost of screening as it is currently recommended. HRI undergoing screening should continue to be included in large cohorts, such as the CAPS consortium (http://caps-registry.com), which is a major opportunity to improve PC screening.
Areas of currently unmet needs within the scope of hereditary PC include the following: (1) To constitute large cohorts of HRI undergoing long-term prospective follow-up; (2) To study the correlation between the pancreatic abnormalities identified at imaging (MRI and EUS) and the lesions identified at the pathological examination of surgically resected specimens; (3) To develop specific EUS and MRI training modules to improve the recognition of pancreatic lesions in HRI and interobserver agreement; (4) To develop new techniques such as biomarkers or new nuclear imaging techniques; and (5) To study more comprehensively the consequences of screening in terms of cost-effectiveness, psychological burden, and long-term surgical consequences in operated HRI.
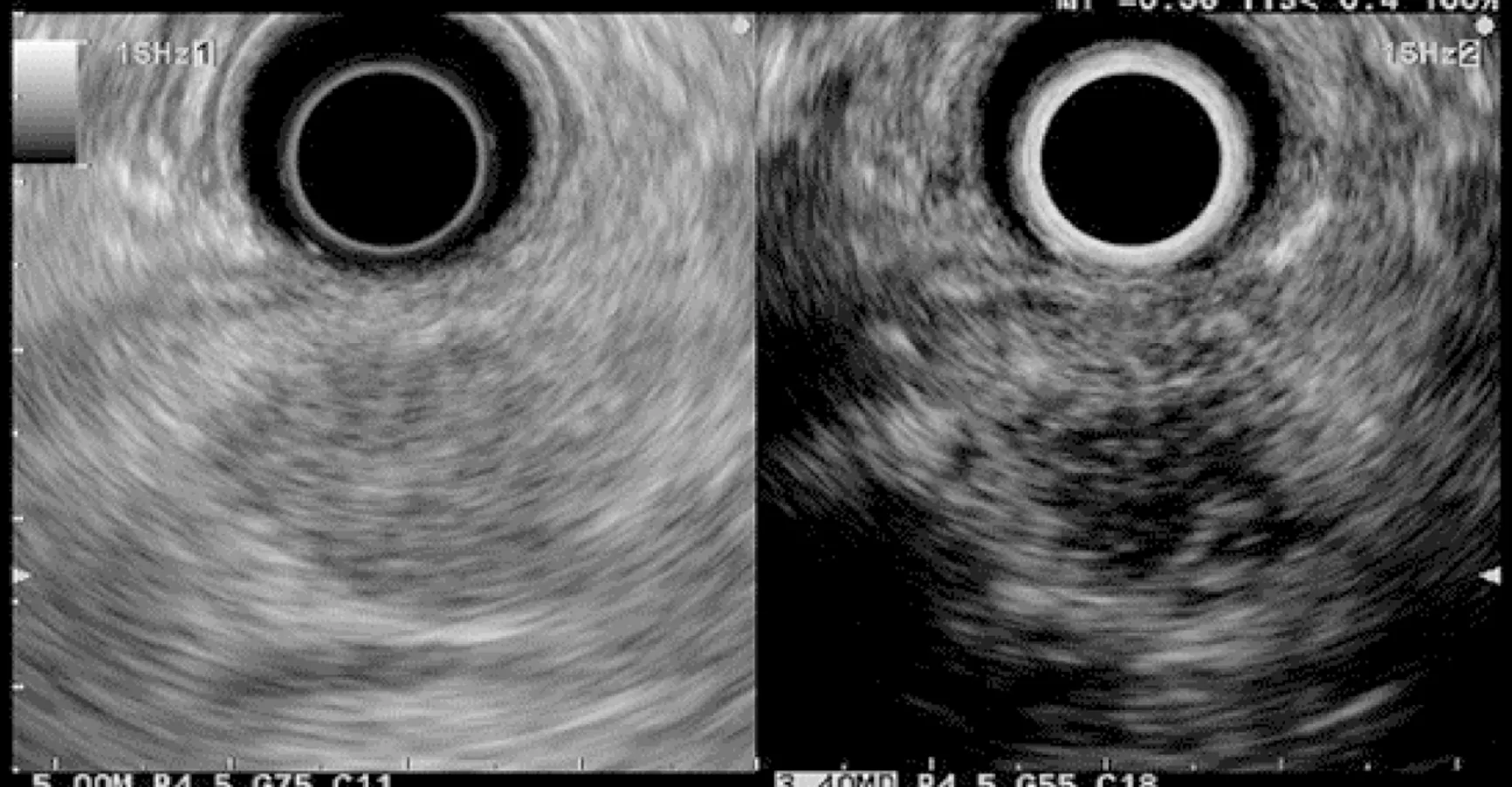
Figure 5 Contrast-enhanced harmonic in endoscopic ultrasound: Hypoechoic suspect nodule.
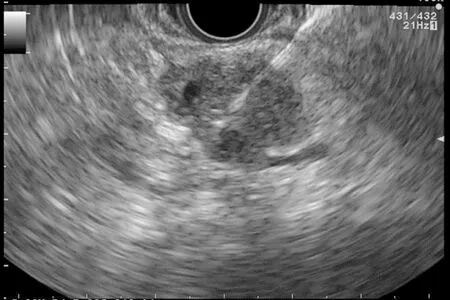
Figure 6 Fine needle aspiration of a solid pancreatic lesion.
杂志排行
World Journal of Gastroenterology的其它文章
- Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors
- Autoantibodies: Potential clinical applications in early detection of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma
- Hepatic senescence, the good and the bad
- Optimizing proton pump inhibitors in Helicobacter pylori treatment:Old and new tricks to improve effectiveness
- Regulatory effect of a Chinese herbal medicine formula on nonalcoholic fatty liver disease
- Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways
