Optimizing proton pump inhibitors in Helicobacter pylori treatment:Old and new tricks to improve effectiveness
2019-09-25EnzoIerardiGiuseppeLosurdoRosaFedericaLaFortezzaMariabeatricePrincipiMicheleBaroneAlfredoDiLeo
Enzo Ierardi, Giuseppe Losurdo, Rosa Federica La Fortezza, Mariabeatrice Principi, Michele Barone,Alfredo Di Leo
Abstract The survival and replication cycle of Helicobacter pylori (H. pylori) is strictly dependant on intragastric pH, since H. pylori enters replicative phase at an almost neutral pH (6-7), while at acid pH (3-6) it turns into its coccoid form, which is resistant to antibiotics. On these bases, it is crucial to increase intragastric pH by proton pump inhibitors (PPIs) when an antibiotic-based eradicating therapy needs to be administered. Therefore, several tricks need to be used to optimize eradication rate of different regimens. The administration of the highest dose as possible of PPI, by doubling or increasing the number of pills/day, has shown to be able to improve therapeutic outcome and has often proposed in rescue therapies, even if specific trials have not been performed. A pre-treatment with PPI before starting antibiotics does not seem to be effective, therefore it is discouraged. However, the choice of PPI molecule could have a certain weight,since second-generation substances (esomeprazole, rabeprazole) are likely more effective than those of first generation (omeprazole, lansoprazole). A possible explanation is due to their metabolism, which has been proven to be less dependent on cytochrome P450 (CYP) 2C19 genetic variables. Finally,vonoprazan, a competitive inhibitor of H+/K+-ATPase present on luminal membrane of gastric parietal cells has shown the highest efficacy, due to both its highest acid inhibition power and rapid pharmacologic effect. However current data come only from Eastern Asia, therefore its strong power needs to be confirmed outside this geographic area in Western countries as well as related to the local different antibiotic resistance rates.
Key words:Helicobacter pylori; Proton pump inhibitors; Eradication; Cytochrome P450;Optimization
INTRODUCTION
Helicobacter pylori(H. pylori) is a microaerofilic Gram-negative bacterium that colonizes the human stomach thus causing gastritis, peptic ulcer and malignant diseases (gastric cancer and mucosa associated lymphoid tissue lymphoma)[1].Therefore, a prompt eradication ofH. pylorimay heal non-malignant conditions and stop the development of neoplastic diseases.
Until few decades ago, the human stomach was considered as a “sterile” organ where the acidic pH hampered the growth of any germ. However, the discovery ofH.pylorihas disavowed this axiom[2]. Urease is the most important enzyme of this bacterium, since it allowsH. pyloristomach colonization by degrading urea into carbon dioxide and ammonia, an alkaline molecule able to counteract acidic environment below the layer of neutral mucins that cover the gastric wall[3]. Thus, the survival and growth ofH. pyloriis strictly dependant on urease effect on intragastric pH at this site. Indeed, it has been observed that only bacteria in replicative vegetative phase are susceptible to antibiotics[4-7].H. pylorienters replicative phase at an almost neutral pH (6-7), while at acid pH (3-6) it turns into the coccoid form that is resistant to antibiotics[4-9]. On these bases, it is crucial to increase intragastric pH by proton pump inhibitors (PPIs) when an antibiotic-based eradicating therapy needs to be administered, since an inadequate acid suppression may keep some bacteria in non replicative forms, not susceptible to antibiotics. On the other hand, this mechanism explains some cases of treatment failure, not linked to bacterial genotypic resistance[8].A further relevant property of PPIs is the ability to reduce intragastric bacterial load,thus making more likely the success of antibiotics. This relevant aspect is supported by the observation that patients with very high bacterial load are the most resistant ones to therapeutic approaches[10,11]. Finally, an additional demonstration in favor of PPI role inH. pyloritreatment was suggested by Figuraet al[12], who showed that lansoprazole reduces minimal inhibitory concentration of culturedH. pyloristrains through a possible direct inhibitory effect on bacterial replication. The evidence of the pivotal usefulness of a correct use of PPIs in the eradication ofH. pyloriled us to perform a narrative review aimed to explore some poorly considered aspects of PPI management in this context.
THE OPTIMAL DOSE
Current Maastricht V guidelines[13]recommend high PPI dose, which is equivalent to omeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg or rabeprazole 20 mg, all twice daily, while low doses are discouraged since they are ineffective, as clearly highlighted in 2015 Italian guidelines[14]. Therefore, a very high dose is defined by both doubling usual amount (e.g., lansoprazole 60 mg bid) or multiple administration(e.g., lansoprazole 30 mg tid or qid). At this regard, there is no unanimous consent for the definition of the very high dose of esomeprazole, since some Authors suggest 20 mg bid and others 40 mg bid[15,16]. In the OPTRICON trial[15], esomeprazole 40 mg bid was given with a 14-d triple therapy in 402 naïve patients, with an eradication rate at intention to treat of 81.3%. Unfortunately, in this trial the alternative arm was represented by concomitant therapy, therefore it was inadequate to give useful information about the PPI dose effectiveness. On the other hand, De Francescoet al[16]showed that esomeprazole 40 mg bid with 14-d triple therapy showed eradication rate of 81.9% compared to 73.9% of esomeprazole 20 mg bid. Interestingly, in a Thai study,lansoprazole 60 mg bid within a 10-d triple therapy showed a similar effectiveness of 10-d sequential therapy with lansoprazole at standard dose (80%vs85%)[17]. At this regard, very high PPI doses have been frequently proposed in the context of dual therapy: A meta-analysis[18]demonstrated that such regimen, when used in rescue line, was equivalent to those recommended by guidelines (pooled eradication rate of 81.3%vs81.5%, risk ratio = 1). In Taiwan, an optimized dual therapy with esomeprazole 40 mg tid plus amoxicillin 750 mg qid showed an effectiveness of the 91.7%, higher, even if not significantly (P= 0.21), than that of concomitant regimen,which displayed an outcome of the 86.7%[19]. Additionally, the same high-dose PPIamoxicillin dual therapy achieved a very satisfactory eradication rate (96.1%) in China[20]. These recent evidences suggest that increasing PPI doses may have the power to reach an effectiveness similar to that obtained by adding further antibiotics[21]. Nevertheless, the real “test bed” of very high PPI dose is represented by the comparison with standard dose within the same eradication regimen. A metaanalysis[22]of seven studies, enrolling articles in which 7-d triple therapy was associated to either standard or very high dose, showed that the last one had a significantly higher eradication rate (82% versus 74%, with a risk ratio = 1.09,P=0.03). Other similar head-to-head trials are summarized in Table 1[16,23-28]. As shown, in most cases the dose escalation allowed to gain a small increment in eradication rate without changes in adverse events[18]. Only one study, performed with dexlansoprazole, a new molecule, did not achieve a satisfactory eradication rate(53.8%) for dual therapy, but in this case only 13 patients were enrolled[29]. Finally, the utility of dose doubling has been underlined in a study by Ormeciet al[30]demonstrating that, in extensive metabolizers, triple therapy with standard dose of rabeprazole or pantoprazole failed in 12/75 and 13/81 patients respectively.Conversely, the re-administration of the same regimen with very high PPI dose in patients who failed therapy surprisingly allowed eradication in 10/12 for rabeprazole and 10/13 for pantoprazole. It is, therefore presumable, that in these cases treatment failure was not due to antibiotic resistance, but to an inadequate acid suppression.
PRE-TREATMENT WITH PPI
There is conflicting evidence whether a pretreatment with PPI may affect the efficacy ofH. pylorieradicating regimens. Based on the assumption that PPIs slowly achieve the steady state and the optimal blood concentrations, it has been hypothesized that a pretreatment with these drugs before starting antibiotics may improve therapy effectiveness. Janssenet al. have reported that three-day pretreatment with a PPI(lansoprazole 30 mg twice daily) before quadruple eradication therapy paradoxically decreased eradication rate (66%vs84%)[31]. The authors theorized that PPIs might induce coccoid-persistent forms ofH. pylori, less vulnerable to the antibiotics. Other studies have reported that pretreatment did not affect treatment outcome[32-34]. Inoueet al. have reported that there was no statistically significant difference in the eradication rate with the regimen of lansoprazole, amoxicillin and clarithromycin between patients with and without pretreatment (lansoprazole 30 mg, once daily)[32]. A similar result was observed in a retrospective study by Tokoroet al[33]. The same Author investigated as well the influence of a pretreatment with histamine 2 receptor antagonist onH. pylorieradication; results indicated that bacterium eradication was not changed by this approach[34].
HAVE ALL PPI THE SAME EFFECTIVENESS?
The effectiveness of a PPI is related to the dose, formulation, relative power,frequency of administration and genetic differences in the activity of cytochrome P450(CYP) enzymes of the subjects assuming the drug. A meta-analysis by McNichollet al[35]compared first-generation (omeprazole, lansoprazole and pantoprazole) with second-generation PPIs (rabeprazole and esomeprazole). Thirty-five studies and 5998 patients were analyzed; the main finding of the meta-analysis was that the secondgeneration PPIs appeared to have a small superiority in eradication rate, because of their higher acid inhibition power. The eradication rates for esomeprazole was higher than for first-generation PPIs: 82.3%vs77.6%; odd ratio (OR) = 1.32 [95% confidence interval (CI): 1.01-1.73]; also rabeprazole showed better results than first-generationPPIs: 80.5%vs76.2%; OR = 1.21 (95%CI: 1.02-1.42). The new generation PPIs,esomeprazole and rabeprazole, had similar eradication rates: 78.7%vs76.7%; OR =0.90 (95%CI: 0.70-1.17).
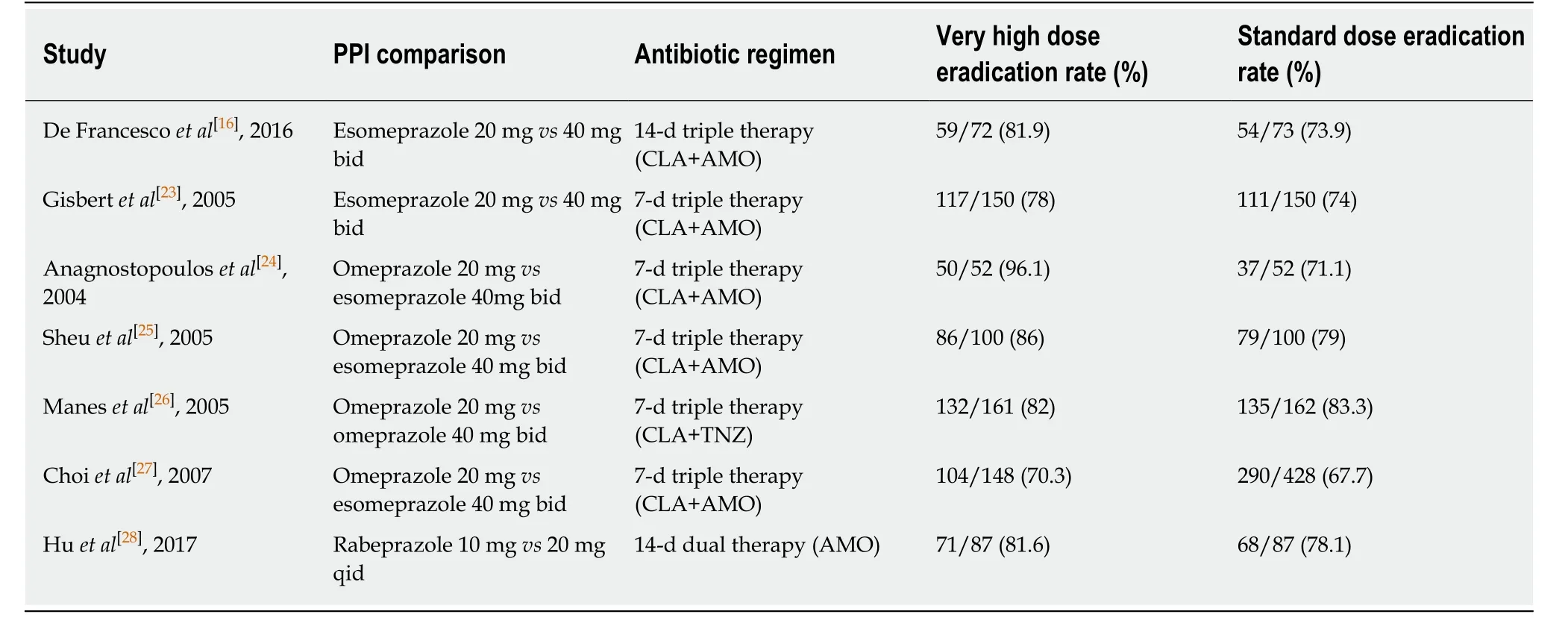
Table 1 Main studies exploring very high dose vs standard proton pump inhibitors dose in Helicobacter pylori eradication regimens
Liver metabolism of PPIs is a relevant parameter since PPIs are prodrugs and,therefore, they are rapidly metabolized. The main enzymes involved in the metabolism of PPIs are CYP2C19 and CYP3A4. There are genetic differences in the activity of CYP2C19. Patients producing the largest amount of this enzyme are called“homozygous extensive metabolizer” (HomEM); “Heterozygous extensive metabolizer” (HetEM) are carriers of one wild-type and one mutation-type allele.Finally “poor metabolizer” (PM) patients have two “loss of function” variant alleles[36].The pharmacokinetics properties of PPIs have significant difference between PM and HomEM. Indeed, in PM patients, a tendency towards a better eradication rate was found when treatment contained first-generation PPIs, whereas EM patients obtained higher eradication rates with therapy regimens based on new-generation PPIs[37].Possible explanations may be that the CYP2C19 has no significant effect on the rabeprazole-based or esomeprazole-based triple therapies. Indeed, rabeprazole is metabolized through a non-enzymatic pathway, with scanty involvement of CYP2C19[38]and esomeprazole has only a minimal first pass metabolism characterized by poor hydroxylation via CYP2C19[39].
A meta-analysis by Padolet al[40]showed a significant difference between HetEM and HomEMs (OR = 1.90, 95%CI: 1.38-2.60,P< 0.0001) in favor of HetEM. A higherH.pylorieradication rates, in dual and triple omeprazole therapies, was recorded in PM over both HomEM (OR = 4.03, 95%CI: 1.97-8.28,P= 0.0001), and HetEM (OR = 2.24,95%CI: 1.09-4.61,P= 0.03). Dual and triple rabeprazole and triple lansoprazole therapies were not significantly different between PM and HomEM (OR = 1.04,95%CI: 0.44-2.46,P= 0.25)[40]. An additional meta-analysis of sixteen randomized clinical trials by Tanget al[41]. investigated the differences in eradication rate between HomEMs, HetEMs and PMs and confirmed the trend of a better eradication rate for PMs over HomEMs and HetEMs. The sub-analysis of individual PPIs showed a significant low eradication rate in both HomEMs versus HetEMs and HomEMsvsPMs with either omeprazole (OR 0.329; 95%CI: 0.195-0.553 and OR 0.232; 95%CI:0.105-0.515, respectively) or lansoprazole (OR 0.692; 95%CI: 0.485-0.988 and OR 0.441;95%CI: 0.252-0.771, respectively), while no significant difference was seen between HetEMs and PMs[41].
Additionally, some novel formulations of PPIs, such as modified releasedexlansoprazole, and instant release-omeprazole or tenatoprazole, which may increase the control of intragastric pH especially at night, are under investigation.However, they have not been tested yet forH. pyloridespite they represent an excellent starting point for future investigations[42].
NOVEL DRUGS: A FOCUS ON VONOPRAZAN
Vonoprazan (VPZ) is a molecule that has been recently introduced in Japan for the treatment of acid-related diseases, and it is currently available only in Japanese market[43]. VPZ is a competitive inhibitor of H+/K+-ATPase present on luminal membrane of gastric parietal cells. The binding power is much more powerful than conventional PPIs,i.e., its biological activity is 300 times greater than lansoprazole[44,45].VPZ has two further pharmacological benefits over other PPIs: It does not require pharmacological activation by gastric acid to inhibit acid secretion, and has a longer half-life, due to its slow dissociation kinetics from proton pump[46]. Another peculiarity consists in the rapid onset of the effect: While PPIs usually require more than 75-100 h to achieve the maximal gastric acid inhibitory effect[47,48], VPZ induces a fast, powerful and long-lasting gastric acid inhibition[49]. Therefore, in conclusion, VPZ seems to be better than conventional PPIs for five reasons: (1) It is more potent in acid secretion blockade; (2) It has a fast onset of action; (3) It is less prone to metabolization variables due to cytochrome polymorphisms; (4) Greater safety; and (5) Better tolerability[50].
VPZ is given at a dose of 20 mg bid in eradication regimens. A meta-analysis of ten studies[51], enrolling 10644 patients overall, demonstrated that triple therapy with VPZ in first line eradicatedH. pyloriin the 87.9%, while the eradication rate with traditional PPIs was 72.8% (risk ratio = 1.19,P< 0.001). Most of such studies were retrospective[52], however even randomized controlled trials, which are summarized in Table 2[53-55], confirmed that VPZ was better than conventional PPIs forH. pyloritreatment in any case. However, the most interesting result comes from studies performed on clarithromycin resistant strains. A meta-analysis of 5 studies[56]showed that the eradication rate in clarithromycin susceptible patients did not differ from that of other PPIs (95.4%vs92.8%), while VPZ was clearly superior in clarithromycin resistant strains (82%vs40%,P< 0.001). Authors explained this finding with the following reasons: (1) VPZ prevents nocturnal acid breakthrough, which is linked to therapy failure; and (2) VPZ is not influenced by CYP2C19 polymorphisms. Finally, it seems that the effectiveness of this drug could be explained by its best suppression of acid secretion. Nevertheless, these enthusiastic results require to be confirmed by forthcoming studies outside Japan[50].
CONCLUSION
Evidence in literature clearly shows that a strong inhibition of gastric acid secretion is crucial forH. pylorieradication. Therefore, several tricks have to be used to optimize eradication rate of different regimens. The administration of the highest dose as possible seems to be able to improve therapeutic outcome even if specific trials are necessary. Pre-treatment does not seem to be effective, therefore it is discouraged.However, the choice of PPI molecule could have a certain weight, since secondgeneration substances are likely more effective because their metabolism is less dependent on CYP2C19 genetic variables. VPZ has shown the highest efficacy, but current data come from Eastern Asia, therefore its strong power needs to be confirmed outside this geographic area in Western countries. In the era of antibiotic resistance, the strategies to improve eradication rate are decreasing[57]. Until novel antibiotics will be discovered, a wise choice of antibiotic combination, may be guided by a tailored approach based on the knowledge of antibiotic susceptibility (preferably with non-invasive modality)[58-61]. Nevertheless, approaches which bypass the problem of antibiotic resistance, such as PPI optimization, vaccine, probiotics or phytochemistry, could be an useful ace up your sleeve[62-64]. In particular, the creation of novel and more effective and powerful PPIs could be an additional weapon againstH.pylori. Finally, the marketing of VPZ in Western countries is expected to confirm its stunning results in the far East countries.
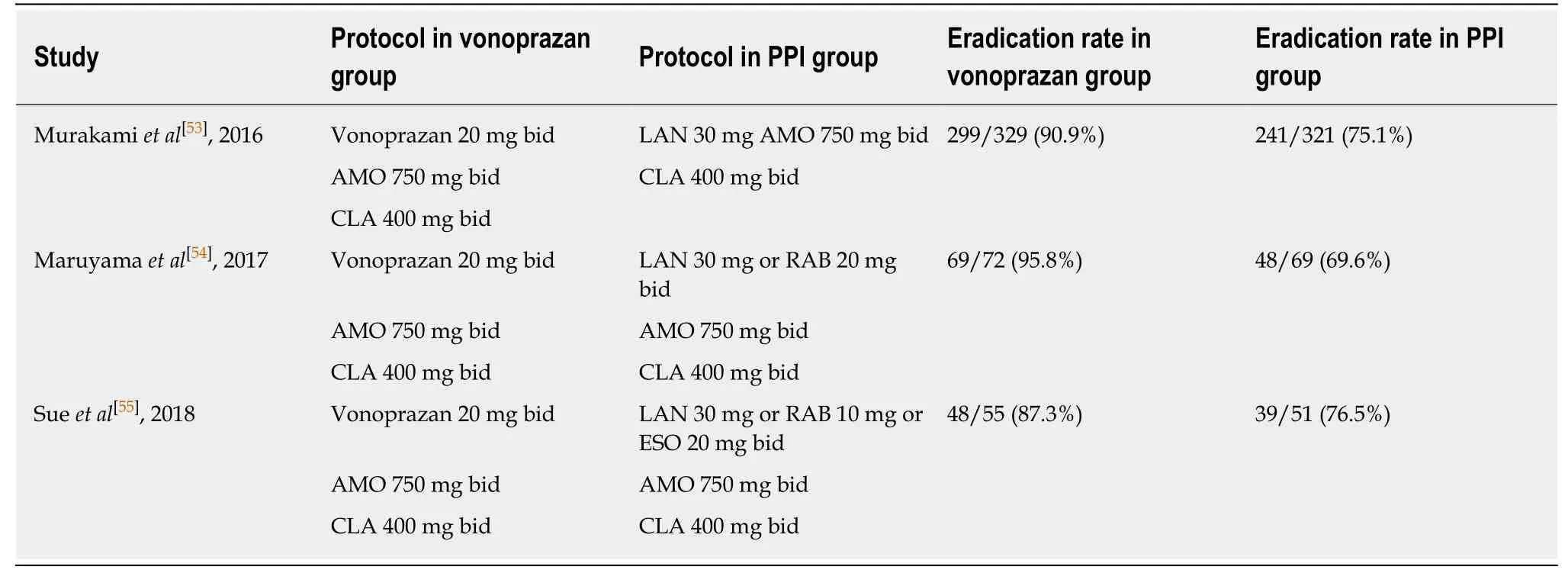
Table 2 Randomized controlled trials comparing vonoprazan vs proton pump inhibitors for Helicobacter pylori eradication regimens
杂志排行
World Journal of Gastroenterology的其它文章
- Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors
- Autoantibodies: Potential clinical applications in early detection of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma
- Role of endoscopic ultrasound in the screening and follow-up of high-risk individuals for familial pancreatic cancer
- Hepatic senescence, the good and the bad
- Regulatory effect of a Chinese herbal medicine formula on nonalcoholic fatty liver disease
- Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways
