席夫碱类混价七核锰金属配合物的合成、表征及磁性
2019-09-09杨立国耿翠环余智超鑫王张永辉牛永生李大成
杨立国 王 芳 耿翠环 余智超 王 鑫王 凯 张永辉 牛永生 李大成
(1安阳工学院化学与环境工程学院,安阳 455000)
(2聊城大学化学化工学院,聊城 252000)
0 Introduction
Polynuclear manganese complexes are attracting considerable attention in several different fields ranging from bioinorganic chemistry to solid-state physics[1].Low-nuclearity species have been extensively studied as models for the water oxidizing complex in photosystem Ⅱ[2],whereas nanometer-size clusters with high-spin ground states are currently being investigated as single-molecule magnets[3].From a synthetic point of view,chemists have learned to exploit hydrolytic reactions,which naturally lead to insoluble metal oxides and oxohydroxides as final products.By proper use of suitable organic ligands,mainly carboxylates[4],polyamines[5],and polyols[6],the “growth” of the metal cores can be controlled in such a way to obtained finite-size clusters.Some ligands have been used to synthesize the manganese complexes,such as triethanolamine[7],salicylaldoxime[8],Alkoxide ligands[9],dipyridyl ketone[10].Due to the effect of ligand fields and different coordination modes,the complexes will excite different magnetic properties.To our best of knowledge,the Schiff-base ligand,3-ethoxy-6-(((2-hydroxyethyl)imino)methyl)phenol(H2L),has not been used to synthesized the manganese complexes.
To further study the interaction between the ligand and the property,two new manganese complexes,[Na2MnⅡMnⅢ6O2(L)6(N3)4(CH3COO)2]·4DMF (1)and[Na2MnⅡMnⅢ6O2(L)6(SCN)4(CH3COO)2]·2DMF(2),have been synthesized by treating H2L with Mn(OAc)2·4H2O and characterized by elemental analysis,IR spectra and X-ray single crystal diffraction.The magnetic behavior of 1 and 2 indicates an antiferro-magnetic interaction between the Mn2+and Mn3+ions.
1 Experimental
1.1 Chemicals and general instruments
3-ethoxy-6-(((2-hydroxyethyl)imino)methyl phenol was prepared according to the published procedures[11].All other reagents and solvents were used as received.
Thermogravimetric analysis (TGA)experiments were carried out on a NETZSCH STA 449F3 thermal analyzer from 40 to 800℃under N2at a heating rate of 10 ℃·min-1.Powder X-ray diffraction(PXRD)determinations was performed on an X-ray diffractometer(D/max 2500 PC,Rigaku)with Cu Kα radiation(λ=0.154 06 nm).The crushed single crystalline powder samples were scanned at 40 kV (generator voltage)and 40 mA (tube current)from 5°to 50°with a step of 0.1°·s-1.Elemental analyses were performed on an Elementar Vario MICRO CUBE elemental analyzer.The magnetic measurements were performed on SQUID-VSM.
1.2 Synthesis of[Na2MnⅡMnⅢ6O2(L)6(N3)4(CH3COO)2]·4DMF(1)
Mn(OAc)2·4H2O(171.5 mg,0.7 mmol),NaN3(13.0 mg,0.2 mmol)and Schiff base ligand(H2L)(125.4 mg,0.6 mmol)were reacted in DMF(10 mL)in the presence of tetramethyl ammonium hydroxide.The solution was stirred for 5 hours at room temperature and filtered.The filtrate was left undisturbed to allow slow evaporation of the solvent.Black single crystals suitable for X-ray diffraction were obtained after a week.Yield:73.1 mg,32% (based on Mn).IR(KBr,cm-1):3 636(br),2 362(s),1 678(s),1 602(m),1 575(s),1 526(s),1 440(vs),1 381(s),1 357(w),1 310(m),1 234(m),1 201(m),1 116(vw),1 034(vw),971(vw),831(w),786(vw),735(w),650(w),612(w).Anal.Calcd.for C82H112Mn7N22Na2O28(%):C,43.07;H,4.90;N,13.48.Found(%):C,43.12;H,4.95;N,13.42.
1.3 Synthesis of[Na2MnⅡMnⅢ6O2(L)6(SCN)4(CH3COO)2]·2DMF(2)
The synthesis of 2 was same as that of 1 except that NaSCN (16.2 mg,0.2 mmol)was used instead of NaN3.Yield:65.9 mg,28% (based on Mn).IR(KBr,cm-1):3 652(br),2 989(s),1 602(s),1 578(s),1 524(vs),1 440(s),1 384(m),1 312(m),1 299(m),1 183(vw),1 116(vw),1 029(w),978(vw),831(w),755(w),739(w),659(w),605(w).Anal.Calcd.for C84H110Mn7N14Na2O30S4(%):C,42.81;H,4.67;N,8.32.Found(%):C,42.78;H,4.65;N,8.26.
1.4 X-ray crystallography
Data were collected on a Bruker SMART APEXⅡdiffractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm)at room temperature by using an ω-2θ scan mode.The size of the crystal 1 and 2 are 0.18 mm×0.16 mm×0.10 mm and 0.28 mm×0.15 mm×0.12 mm,respectively.The raw data frames were integrated with SAINT+,and the corrections were applied for Lorentz and polarization effects.There was no evidence of crystal decay during data collection in all cases.Absorption correction was applied by using the multi-scan program SADABS[12].The structure was solved by direct methods,and all non-hydrogen atoms were refined anisotropically by least-squares methods on F2using the SHELXLS program[13].The aromatic H atoms were restrained to ride 0.093 nm from the relevant C atom,with Uiso(H)=1.2Ueq(C),while the methyl H atoms were treated as rigid groups,pivoted about the attached C atom,with C-H bond lengths fixed at 0.096 nm and Uiso(H)=1.5Ueq(C).The crystallographic data and structural refinement parameters are provide in Table 1.Selected bond lengths and angles of complexes 1 and 2 are listed in Table 2.
CCDC:1849331,1;1849333,2.
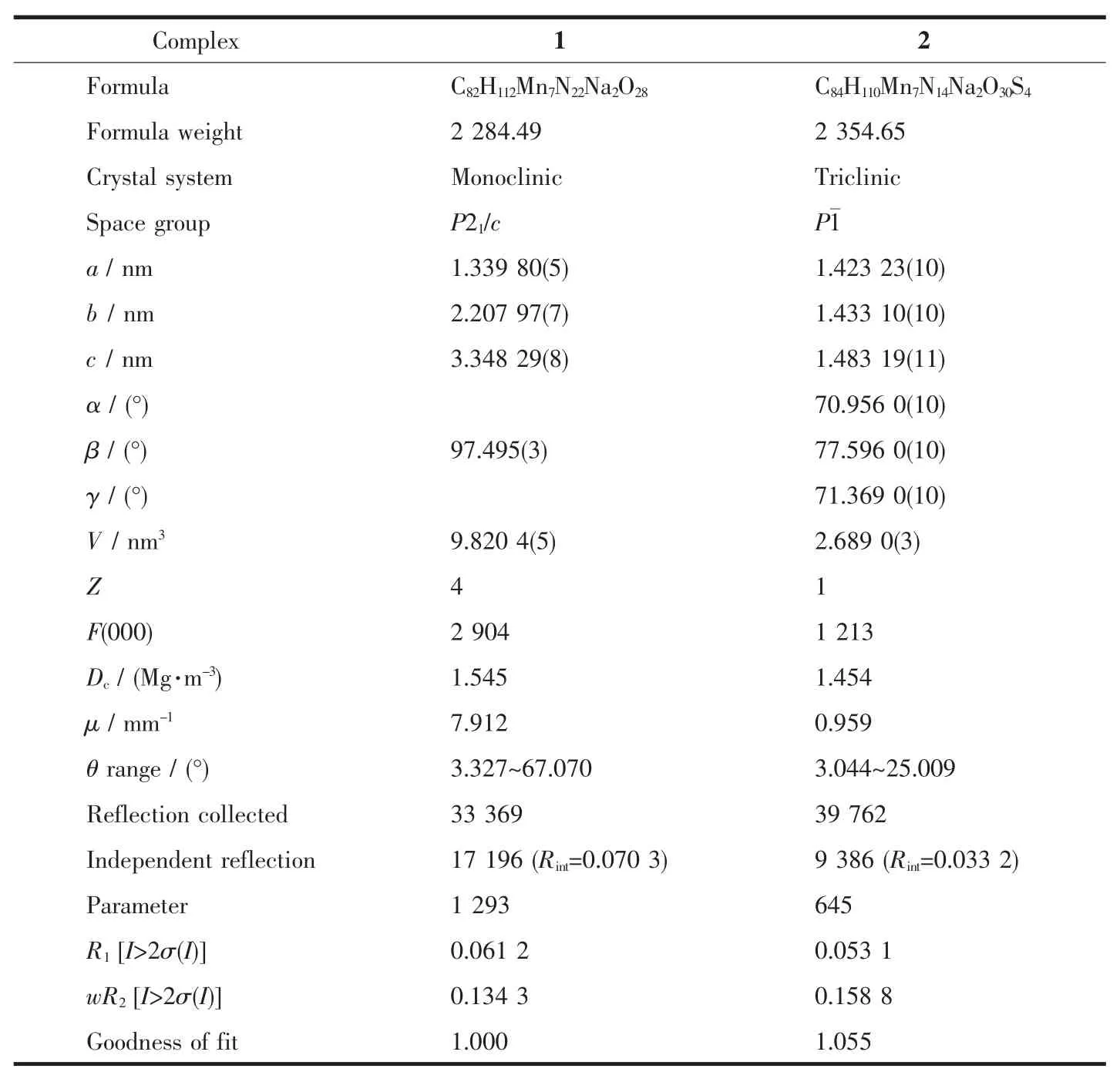
Table 1 Crystallographic data for complexes 1 and 2
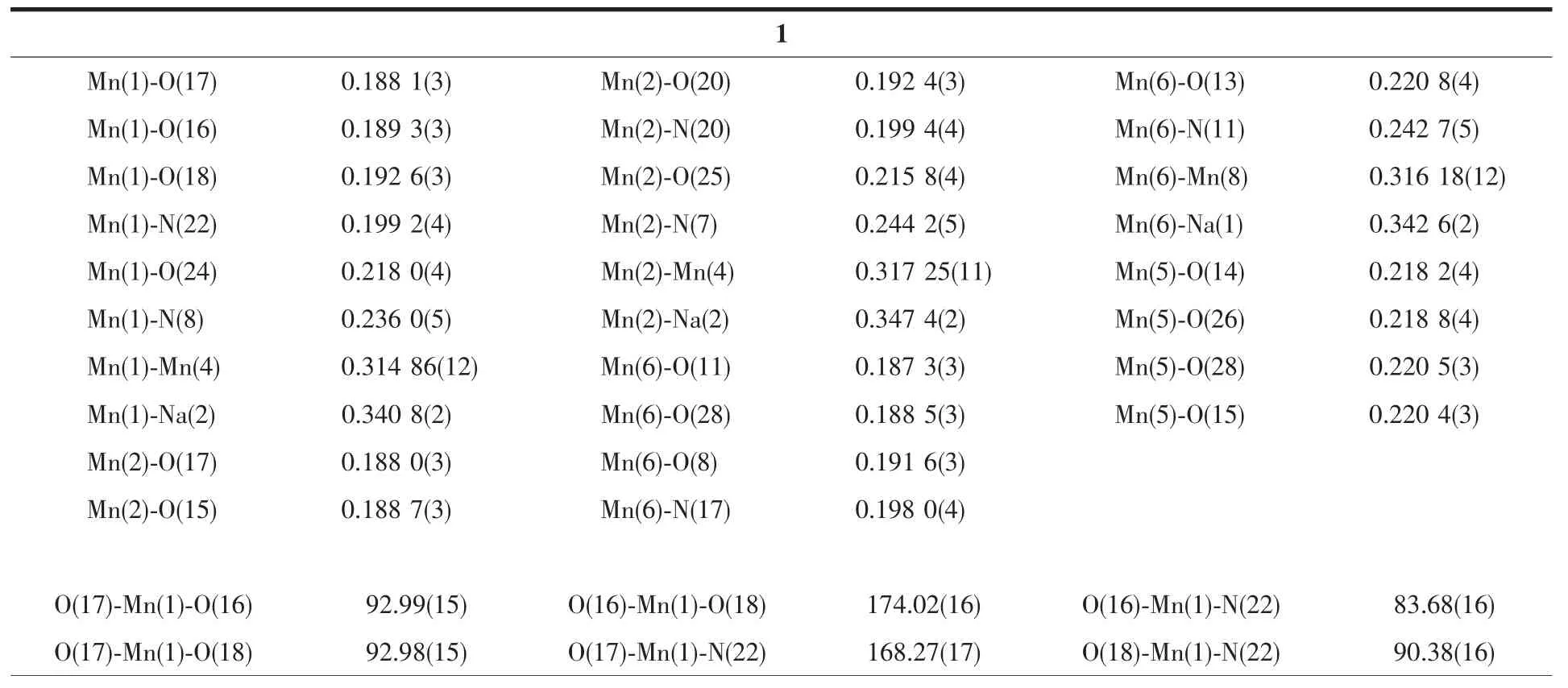
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2

Continued Table 2
2 Results and discussion
2.1 Structure description
Complex 1 crystallizes in the monoclinic system,while 2 crystallizes in the triclinic system with the similar structure.Herein,the molecular structure of complex 1 is described in detail as a representative example.As shown in Fig.1,complex 1 consists of seven Mn ions,two Na+ions,six L2-ions,four N3-anions and four DMF solvent molecules.The two peripheral [MnⅢ3O]7+triangles are each bonded to a central Mn2+ion.This ion is linked to each triangle via the O atom of the ligand.The Mn ions in each triangle are linked to each other via one μ4-O2-ion,one η1∶η1∶μ-acetates,and the two N3-groups via the N atoms to form a[MnⅢ6MnⅡ(μ4-O2-)2(μ-OOCCH3)2(μ-N3)4]10+core.The Na+ion links to the Mn via three O atoms of three L2-ligands.The central μ4-O atom deviates 0.222 nm from the mean plane formed by three Mn ions,which is similar with the reported structure[7a].The angle of Mn-N(N3)-Mn is 83.20°and 85.65°,respectively.The average distance between Na+ion and μ4-O is 0.267 7 nm.The average angle of Mn-O-Mn is 106.76°.The structure of 2 is similar with 1,except that the SCN group replaces the N3group.The central μ4-O deviates 0.018 3 nm from the mean plane formed by three Mn ions,which is similar with 1.The angle of Mn-N(NCS)-Mn is 80.73°and 80.06°,respectively.The distance between Na+ion and μ4-O is 0.270 0 nm.The average angle of Mn-O-Mn is 108.13°.
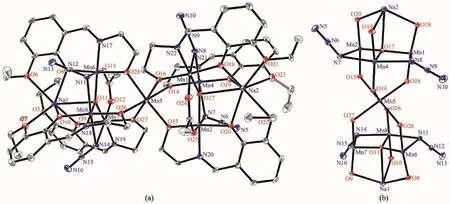
Fig.1 Molecular structure(a)and coordination environment(b)of complex 1 with 40%probability displacement ellipsoids
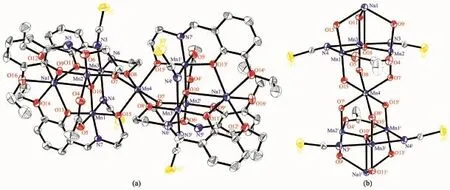
Fig.2 Molecular structure(a)and coordination environment(b)of complex 2 with 40%probability displacement ellipsoids
2.2 X-ray powder diffraction analyses
Complexes 1 and 2 were also characterized by powder X-ray diffraction(PXRD)at room temperature.The patterns calculated from the single-crystal X-ray data of 1 and 2 were in good agreement with the observed ones in almost identical peak positions(Fig.3).The difference in reflection intensities between the simulated and experimental patterns was due to the powder size and variation in preferred orientation for the powder samples during collection of the experimental PXRD data.
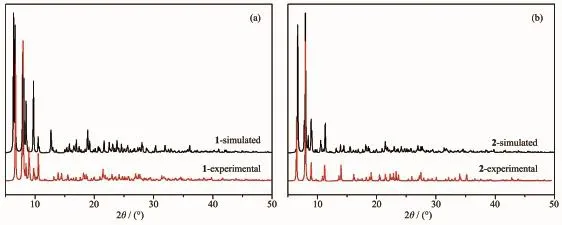
Fig.3 XRD patterns of complex 1(a)and 2(b)
2.3 Thermal stability
Thermal stability is an important aspect for the application of coordination compound.Thermogravimetric analysis(TGA)experiments were carried out to determine the thermal stabilities of 1 and 2(Fig.4).For complex 1,there are many solvent molecules in the structure,and TG curves showed the first consecutive step of weight loss that was observed in a range of 40~250 ℃,corresponding to the release of solvent molecules.Then,the continuously weight loss corresponds to the loss of ligands (in the range of 180~750 ℃).Finally,the weight loss ended untilheating to 750℃and the total weight loss was about 68%.For 2,TG curves was similar with complex 1,the weight loss ended until heating to 750℃and the total weight loss was about 67%.
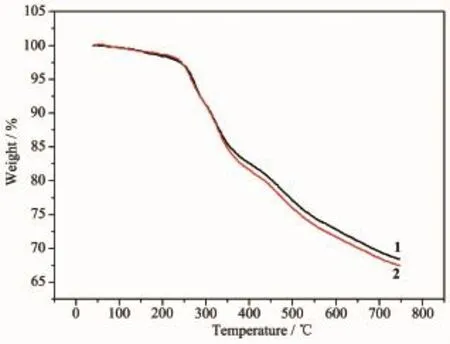
Fig.4 TGA curves of complexes 1 and 2
2.4 Magnetic studies
The variable-temperature dc magnetic susceptibility measurement for complexes 1 and 2 were performed in the temperature range of 2~300 K under an applied magnetic field of 1 000 Oe.The collected data are plotted as χMT vs T in Fig.5 and Fig.6.The χMT value at room temperature of complex 1 was 26.13 cm3·mol-1·K,which is higher than the theoretically predicted value(22.37 cm3·mol-1·K)for six Mn3+and one Mn2+ions[14].With the temperature decreasing,the χMT product of 1 decreased until 8.5 K,and then increased rapidly to 9.89 cm3·K·mol-1at 2 K,suggesting the presence of intramolecular ferromagnetic coupling between the Mn3+and Mn2+for 1 below 8.5 K[15].The temperature dependence of the reciprocal susceptibility χM-1values at high temperature were fitted by the Curie-Weiss law,χM=C/(T-θ),with C=21.50 cm3·mol-1·K and θ=-59.09 K.The negative θ values confirm antiferromagnetic coupling among metal centers of 1.For complex 2,the χMT product at 300 K are 16.14 cm3·mol-1·K,which is lower than the theoretically predicted value for six Mn3+and one Mn2+ions.As the temperature decreasing, χMT values gradually decreased,then underwent a rapid decrease until to 2.09 cm3·mol-1·K at 2 K,indicating the presence of intramolecular antiferromagnetic interactions between the Mn3+and Mn2+ions.The temperature dependence of the reciprocal susceptibility χM-1values above 50 K were fitted by the Curie-Weiss law with the obtained constant C=21.90 cm3·mol-1·K and θ=-103.71 K.The negative value of θ confirms an antiferromagnetic coupling among metal centers of 2.
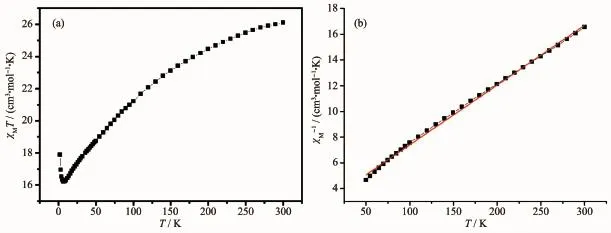
Fig.5 Temperature dependence of χM T at 1 000 Oe(a)and 1/χM vs T plot(b)for 1
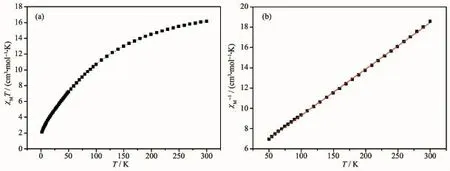
Fig.6 Temperature dependence of χM T at 1 000 Oe(a)and 1/χM vs T plot(b)for 2
To explore the dynamic magnetic property of the complexes,the temperature dependence of the alternating-current(ac)magnetic susceptibility for 1 and 2 under a zero direct-current(dc)magnetic field oscillating at 1~320 Hz was recorded in Fig.7 and Fig.8.As can be seen,1 and 2 didn′t exhibit the frequency-dependent character in out-of-phase(χ″)signals and in-phase(χ′)signals.The result shows that the complexes 1 and 2 are not single molecule magnet(SMM).
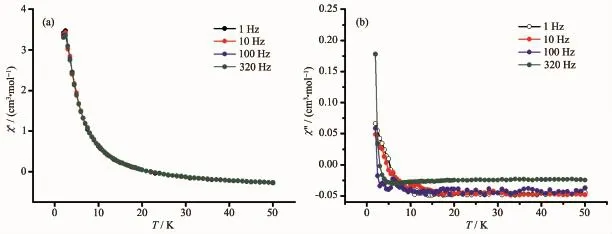
Fig.7 Plots for temperature dependence of in-phase(χ′)(a)and out-of-phase(χ″)(b)ac susceptibility for 1 under zero applied dc magnetic field
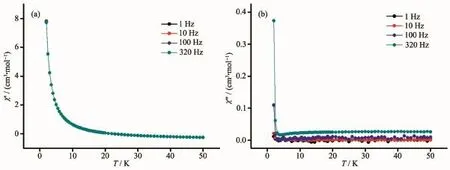
Fig.8 Plots for temperature dependence of in-phase(χ′)(a)and out-of-phase(χ″)(b)ac susceptibility for 2 under zero applied dc magnetic field
3 Conclusions
In summary,two new manganese complexes with Schiff-base ligands were synthesized and characterized by elemental analysis,IR spectra,thermogravimetric analyses and single crystal X-ray diffraction analysis.The investigation of magnetic studies confirms the antiferromagnetic interactions between the Mn2+and Mn3+ions for complexes 1 and 2.Furthermore,the dynamic magnetic property of the complexes 1 and 2 was studied,and it is found that the complexes 1 and 2 are not SMM.
