一种具有Z-Scheme机制的高效可见光驱动的介孔AgI@Fe-MIL-88B-NH 2光催化剂
2019-07-10刘雪霆朱洪程张勇涛晋冠平魏凤玉
刘雪霆 朱洪程 李 缘 王 芳 张勇涛 晋冠平 魏凤玉
(合肥工业大学化学与化工学院,可控化学与材料化工安徽省重点实验室,合肥 230009)
0 Introduction
The increasing environment contamination and energy crisis have become the world′s largest contemporary issues,which urge us to find clean and reproducible energy resources.Due to the advantages of clean,sustainable,abundant and economical characteristics,solar energy from sunlight is widely believed to be the key to addressing the problems of the environment and energy resource[1-7].Photocatalysis as a promising green technology,has been extensively explored for potential applications in environmental remediation[8],CO2fixation[9]and water splitting[10-11].Dyes are generally water-soluble organic colorants,gradually being increased owing to the tremendous increase of industrialization and requirements of human beings for color[11-12].Various techniques for treatment of dyeslike RhB (rhodamine B)containingwaste water have been developed,including chemical precipitation,ion exchange,membrane separation,adsorption and photocatalysis[12-19].
Metal-organic frameworks(MOFs)are crystalline porous materials whose structure is composed of metal-oxide units joined by organic linkers through strong covalent bonds.Compared with the traditional photocatalysts,the superiority of MOFs is based on their desirable topology and high surface area,which is beneficial for fast transport and good accommodation of guest molecules[20-21].However,because of their microporosity (generally,pore size <2 nm),the potential applications of these materials are limited.To overcome this drawback,we used monodispered microsized sulfonated polystyrene(PS)spheresprepared by dispersion polymerization according to Paine et al.[22]as templates to prepare mesoporous Fe-MIL-88B-NH2spherical shell with open pore.This unique Fe-based MOF shows high adsorption capacity for RhB with the particle size of 1.5 μm,moreover,it exhibits an excellent reusability.
By far,silver halide(AgCl,AgBr and AgI)-based photocatalysts have been explored in the photooxidation of organic pollutants,due to their perfect activities[23].However,their stability is still low,and it can be improved by combination of silver halide with other materials.Considering this situation,AgI particles were adhered on the surface and inward of mesoporous Fe-MIL-88B-NH2spherical shells to yield AgI@Fe-MIL-88B-NH2composite photocatalyst via in situ reaction method.The visible-light activity of this composite is obviously higher than that of pure materials irradiated under visible light.Moreover,the stability of AgI in the composite has been enhanced.
Currently,the Z-scheme system photocatalysts have gained great interests owing to their comparatively stronger redox capacity and improved photocatalytic performance relative to the single component or even type-Ⅱheterojunction photocatalyst[24-25].The AgI loading remarkably promoted the photocatalytic activity of Fe-MIL-88B-NH2during the photo-oxidation process that was found to be a Z-scheme heterojunction systembased on theactivespeciestrappingand electron spin resonancel(ESR)measurements.This unique Z-scheme heterojunction system based on mesoporous MOFs spherical shell and silver salt may apply as a platformtoconstructother highefficientphotocatalysts.
1 Experimental
1.1 Materials
Relative molecular mass of poly(N-vinylpyrrolidone)(PVP)is about 40000.Azoisobutyronitrile(AIBN,Aladdin Reagent)initiator was crystallized before being used.Styrene monomer was distilled under reduced pressure.FeCl3·6H2O (Aldrich,97%),CH3COOH(Fisher,99.7%),N,N-dimethyl formamide(DMF),silver nitrate,potassium iodide,2-aminoterephthalic acid(BDC-NH2)and concentrated sulfuric acid are of analytical grade without further purification.All reagents except AIBN,FeCl3·6H2O and CH3COOH used in this study were supplied by Sinopharm Chemical Reagent Co.Ltd.
1.2 Synthesis of monodispersed polystyrene(PS)microspheres
PS fine powder consisting of microspheres was prepared by dispersion polymerization reported by Paine et al[22]. The PVP stabilizer(1.000 g)was dissolved in ethanol(38.2 mL)in a three-necked round bottom flask fitted with a condenser and a magnetic stirrer.The reaction vessel was then heated to 70℃under a nitrogen blanket and purged with nitrogen for 12 h at 70℃.A solution of AIBN (0.150 g)predissolved in styrene monomer(15.000 g)was added to the reaction vessel with vigorous stirring.The styrene polymerization was allowed to proceed for 12 h before cooling to room temperature.The product was purified by repeated centrifugation and washed with ethanol.A white fine powder(PS)was finally obtained after being dried in a vacuum oven at 50℃.
1.3 Preparation of sulfonated PSmicrospheres
After purification and drying,PS fine powder(1.700 g)and concentrated sulfuric acid(98%,60 mL)were introduced to a 100 mL conical flask.After ultrasonic dispersion,the sulfonation was allowed to take place at 40℃under magnetic stirring for 5 h.When cooled to the room temperature,the product was separated by repeated centrifugation(6 000 r·min-1)and washed with a large excess of ethanol.Finally,a yellow fine powder(sulfonated PS)was obtained after being dried.
1.4 Preparation of hollow Fe-MIL-88B-NH 2 microspheres
The size-controlled synthesis of Fe-MIL-88B-NH2nanocrystals was accomplished using a hydrothermal route with Fe(Ⅲ)salt and 2-aminoterephthalic acid as the metal source and organic linker,respectively.In a typical synthesis,0.160 g of sulfonated PS template was mixed with 13.3 mL of deionized water,to which 1.66 mL of FeCl3·6H2O aqueous solution(0.4 mol·L-1,0.66 mmol)was poured.The resulting mixture was stirred for 2 h before 0.3 mL of acetic acid was injected.After stirring for an additional 1 h,0.060 g(0.33 mmol)of BDC-NH2was added.The reaction mixture was stirred for 2 h before transferring into an autoclave for the crystallization.The dark brown solid product was recovered and washed several times with DMF and ethanol by centrifugation to remove the sulfonated PStemplate and excess reactants.
1.5 Preparation of AgI
KI solution (15.0 mL,0.13 mol·L-1)was added into 19.7 mL of AgNO3solution (0.10 mol·L-1).After stirring for 1 h,the precipitate was filtered,rinsed with deionized water and dried at 60 ℃ for 12 h.Finally,yellow AgI was obtained.
1.6 Preparation of AgI@Fe-MIL-88B-NH 2
The AgI@Fe-MIL-88B-NH2photocatalysts was prepared by in situ reaction as follows:0.300 g of Fe-MIL-88B-NH2was mixed with 20 mL of deionized water then with stirring for 2 h.9.8 mL AgNO3(0.1 mol·L-1)and 7.5 mL KI(0.13 mol·L-1)were added dropwise into the resulted mixture with stirring for 4 h,after that the suspension was washed with ethanol and dried at 60 ℃.By this method,the AgI@Fe-MIL-88B-NH2photocatalysts with different mass ratios of Fe-MIL-88B-NH2were synthesized,and the overall process for the fabrication of AgI modified mesoporous Fe-MIL-88B-NH2spherical shell is shown in Scheme 1.The prepared composite is called x∶y AgI@Fe-MIL-88B-NH2,where the mass ratio of AgNO3to Fe-MIL-88B-NH2is x∶y.

Scheme 1 Schematic illustration for the fabrication of AgI@Fe-MIL-88B-NH2 composites
1.7 Photocatalytic experiments
The photocatalytic degradation of model pollutant RhB solution was measured at ambient pressure and 298 K in a set of home-made photochemical reaction equipment.The light source was a PHILIPS 70 W metal halidelamp(the light withλ<420 nmwasfiltered out by a cut off filter).20 mg of photocatalyst was added into 100 mL RhB(initial concentration,C0′=10 mg·L-1) aqueous solution.Before irradiation,the suspension was stirred continuously for 12 h in the dark in order to reach the adsorption-desorption equilibrium between RhB and the photocatalyst.The supernatant liquid was obtained through filtration by 0.22 μm filter,and examined using a Shimadzu UV-240 spectrophotometer.
1.8 Characterization
A SU8020 field emission scanning electron microscope (FESEM)was used to characterize the surface morphologies of Fe-MIL-88B-NH2,AgI and AgI@Fe-MIL-88B-NH2composite,and the operating voltages were 1 kV,15 kV and 5 kV,respectively.Transmission electron microscope (TEM)image of sulfonated PS was obtained from JEM-2100F(Japan),and the operating voltage was 200 kV.The crystal structures and phase states of AgI@Fe-MIL-88B-NH2composites were determined by X-ray diffractometry(XRD)using a Rigaku D/max-2500V X-ray diffractometer with Cu Kα (λ=0.154 nm)radiation at an operating voltage of 40 kV,an operating current of 40 mA with 2θrange of 5°~90°.N2adsorption-desorption was performed on a Tristar II3020M surface area and porosity analyzer at 77 K.The infrared absorption spectra were investigated using a Fourier transform infrared (FT-IR)spectrophotometer (Nicolet 67,America)in a wavenumber range from 400 to 4 000 cm-1.X-ray photo-electron spectroscopy(XPS)measurements were performed on ESCALAB250 spectrometer to identify the elemental compositions and chemical states of AgI@Fe-MIL-88B-NH2.UV-Vis spectra were recorded on a DUV-3700 spectrometer.Photoluminescence emission spectra (PL)were measured on a PL measurement system(Fluorolog Tau-3)with the excitation wavelength of 320 nm.The electron spin resonance(ESR)measurements were carried out on a Bruker model ESR JES-FA200 spectrometer with a microwave power of 1.0 mW and a modulation frequency of 100 kHz.
Photocurrent measurements were performed on a CHI 660B electrochemical work station(Chenhua Instrument,China)in a conventional three electrode configuration with a Pt foil as the counter electrode and an Ag/AgCl (saturated KCl)as the reference electrode(referring to the following reference).A 70W metal halide lamp served as a light source.0.1 mol·L-1Na2SO4aqueous solution was used as the electrolyte.The working electrodes were prepared as follows:10 mg of the as-prepared photocatalyst and 10μL Nafion dispersing reagent were added in 1 mL absolute ethanol and sonicated for 30 min.The slurry was then spread on a 1.0 cm×1.0 cm indium tin oxide(ITO)glass substrate and dried in air.The photoresponse of the samples,as light on and light off,was measured at 0.0 V[2-6].
2 Results and discussion
The morphology and particle sizes of sulfonated PStemplate,AgI,Fe-MIL-88B-NH2and AgI@Fe-MIL-88B-NH2were observed by TEM and SEM,and the results are presented in Fig.1(a)~(d),respectively.Fig.1(a)shows the sulfonated spherical PStemplates,which had uniform size with diameter of about 1.3μm.As the sulfonation reaction occurs inwardly from the PS particles surface,the functional groups on the surface of spheres are-SO3H.AgI samples(Fig.1(b))were mainly composed of irregular particles with diameters between 0.4 to 1.5μm.Fig.1(c)shows Fe-MIL-88BNH2,which had open pores on the spherical shells with diameter of about 1.5μm.SEM image of AgI@Fe-MIL-88B-NH2is showed in Fig.1(d).It can be clearly seen that the AgI particles were adhered on the surface and inward of open pore of Fe-MIL-88BNH2shells,which can lead to more efficient use of the visible light.Meanwhile these characteristics are especially beneficial to the intimate contact between AgI and Fe-MIL-88B-NH2,and can enhance the stability of the resulted composite.As a result,the efficient separation of photogenerated charge-carriers and the corresponding high photocatalytic capacity can be obtained.Although pure AgI samples have large diameters(0.4 to 1.5 μm),their sizes in AgI@Fe-MIL-88B-NH2composite are smaller,because AgNO3and KI react on the surface and inward of open pore of Fe-MIL-88B-NH2shells,and the growth of resulted AgI particles are limited.
The crystallinity of samples was tested by XRD,and the results are presented in Fig.2.The diffraction peaks of Fe-MIL-88B-NH2agreed with those of previously reported pure Fe-MIL-88B-NH2single crystals[26].For AgI,the diffraction peaks are indexed to crystal planes of the hexagonalβ-AgI crystal phase(PDF No.09-0374)[27].After compounding of Fe-MIL-88B-NH2and AgI,the peaks of AgI were enhanced,whereas those of Fe-MIL-88B-NH2became weaker.This means that Fe-MIL-88B-NH2can enhance the structure stability of AgI[6,28-29].
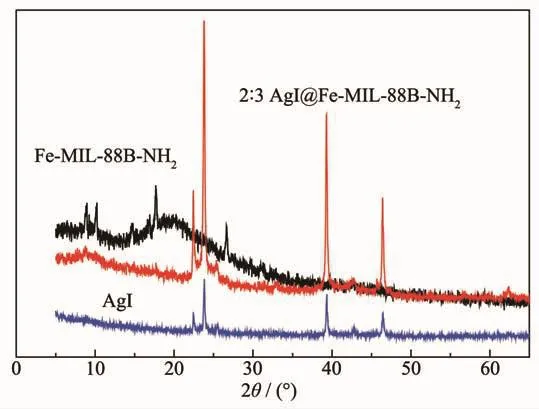
Fig.2 X-ray diffraction patterns of Fe-MIL-88B-NH2,AgI and 2∶3 AgI@Fe-MIL-88B-NH2
To identify the presence of amino groups in the resulting nanocrystals,FT-IR spectra for Fe-MIL-88BNH2and 2∶3 AgI@Fe-MIL-88B-NH2samples were recorded (Fig.3).For Fe-MIL-88B-NH2,the peak at 1 564 cm-1can be ascribed to stretching vibrations of C=C.The peak of asymmetric bending vibration of CH is at 1 458 cm-1.The vibrational bands of the-NH2groups appear at 3490 and 3370 cm-1.FT-IR of 2∶3 AgI@Fe-MIL-88B-NH2was almost the same with that of Fe-MIL-88B-NH2,proving that no obvious change happened for Fe-MIL-88B-NH2during the in situ reaction.No band at around 1 700 cm-1that is characteristic of protonated carboxylic groups was observed in the spectra of Fe-MIL-88B-NH2and 2∶3 AgI@Fe-MIL-88B-NH2in Fig.3,suggesting the absence of protonated carboxylic groups in the nanocrystals[30].This also indicates that almost all protonated carboxylic acids including acetic acid and BDC-NH2are deprotonated.
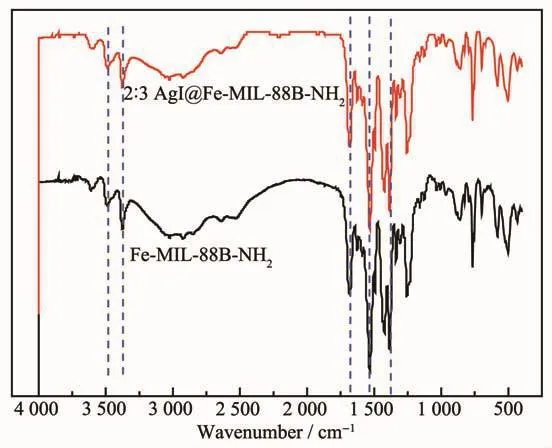
Fig.3 FT-IR spectra of Fe-MIL-88B-NH2 and 2∶3 AgI@Fe-MIL-88B-NH2
The N2adsorption-desorption isotherms of Fe-MIL-88B-NH2and 2∶3 AgI@Fe-MIL-88B-NH2are shown in Fig.4(a).The BET surface area and total pore volume of 2∶3 AgI@Fe-MIL-88B-NH2were calculated to be 13 m2·g-1and 0.018 cm3·g-1,respectively,which were slightly lower than that of Fe-MIL-88B-NH2(14 m2·g-1and 0.020 cm3·g-1).The decrease in the amount of N2adsorption and the pore volume of 2∶3 AgI@Fe-MIL-88B-NH2indicates that the portion of pores in Fe-MIL-88B-NH2may be occupied by the highly dispersed AgI particles.The pore diameter of Fe-MIL-88B-NH2and 2∶3 AgI@Fe-MIL-88B-NH2ranged from 2.5 to 50 nm(Fig.4(b))The N2isotherm of 2∶3 AgI@Fe-MIL-88B-NH2is categorized as typeⅣ.This property implies the presence of mesopores.

Fig.4 (a)N2 adsorption-desorption isotherms of Fe-MIL-88B-NH2 and 2∶3 AgI@Fe-MIL-88B-NH2;(b)BJH pore diameter distribution curve of Fe-MIL-88B-NH2 and 2:3 AgI@Fe-MIL-88B-NH2
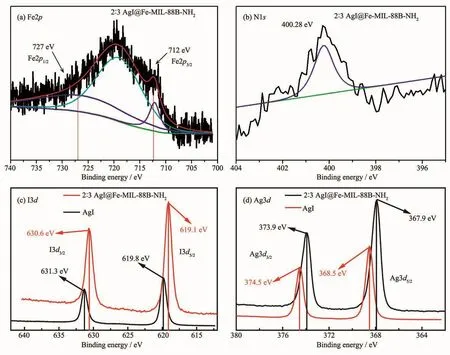
Fig.5 XPSspectra of 2∶3 AgI@Fe-MIL-88B-NH2 and AgI
The chemical composition and surface chemical states of 2∶3 AgI@Fe-MIL-88B-NH2and AgI was analyzed by XPS.As shown in Fig.5.Fe2p peaks of 2∶3 AgI@Fe-MIL-88B-NH2at about 712 and 727 eV are assigned to Fe2p3/2and Fe2p1/2for iron(Ⅲ) oxide,which is consistent with the previously reported iron(Ⅲ)-based MOFs[31](Fig.5(a)).The N1s XPS spectrum located at 400.28 eV,and the binding energy of the N atom in the amine group is slightly higher than that of the ligand 2-aminoterephthalate(399.1 eV)because of the static polarization in the confined MOF cavities[32](Fig.5(b)).Th e I3d5/2and I3d3/2peaks of AgI located at 619.8 and 631.3 eV(Fig.5(c)),respectively,indicating the monovalent oxidation state of iodine[33-34],and the peaks of I for 2∶3 AgI@Fe-MIL-88B-NH2composite shifted to 619.1 and 630.6 eV.The XPSspectra of Ag species in the composite displayed two peaks at approximately 367.9 and 373.9 eV,which correspond to the binding energies of Ag3d5/2and Ag3d3/2of Ag+,respectively[35-36](Fig.5(d)).Peaks assigned to Ag0(369.2 and 375.2 eV)were not observed[37].It can be concluded that there exists an intense interfacial interaction between AgI and Fe-MIL-88B-NH2.
The UV-Vis diffuse absorption spectra of Fe-MIL-88B-NH2,2∶3 AgI@Fe-MIL-88B-NH2and AgI are shown in Fig.6.Compared with AgI,the absorption edge of 2∶3 AgI@Fe-MIL-88B-NH2exhibited a red-shift which showed significantly stronger light absorption than AgI(Fig.6(a)).The band gap energies of assynthesized Fe-MIL-88B-NH2,2∶3 AgI@Fe-MIL-88BNH2composite and AgIcan be estimated by Tauc plot:(ahν)=A(hν-Eg),where a,hνa nd A are absorption coefficient,photon energy and a constant,respectively.The band gap energies can be taken as the intercept of the linear part of Tauc plot at(ahν)2=0 based on Kubelka-Munk theory[38],and they were 2.79 and 2.25 eV for AgI and Fe-MIL-88B-NH2,respectively(Fig.6(b)).The band gap energy of 2∶3 AgI@Fe-MIL-88BNH2was 2.61 eV,which lay between those of AgI and Fe-MIL-88B-NH2.This is due to the synergetic effect AgI and Fe-MIL-88B-NH2in mixed phase structure which facilitates the migration of photo-induced charges and suppresses the charge recombination,thus enhancing the photo-catalytic performance of 2∶3 AgI@Fe-MIL-88B-NH2.Moreover,the relatively narrow band-gap energy observed for 2∶3 AgI@Fe-MIL-88BNH2is likely attributed to the strong interaction of the hybrid structure,which enables more efficient utilization of the solar spectrum.
Based on the conduction band (CB)of Fe-MIL-88B-NH2(0.13 eV)[39]and its energy gap(2.25 eV),the valence band(VB)positions of Fe-MIL-88B-NH2could be calculated to be 2.38 eV,meanwhile the CB and VB of AgI were-0.42 eV and 2.35 eV,respectively[40].CB positions for AgI is more negative than O2/·O2-(-0.33 eV)and O2/·OOH(-0.037 eV),indicating that it is possible for the transfer of photogenerated electrons from CB of AgI to O2to form·O2-.
The photoluminescence (PL)spectrum is often used to study the separation efficiency of the photogenerated electron-hole pairs in semiconductor particles,and has been proved as a powerful technique to study the photocatalytic mechanism[41-42].Fig.7 shows the PL spectra of Fe-MIL-88B-NH2,2∶3 AgI@Fe-MIL-88B-NH2and AgI.When excited at 320 nm,all samples showed PL emissions in a range of 400~550 nm and the intensity of PL signal in a range of 450~500 nm for 2∶3 AgI@Fe-MIL-88B-NH2was lower than pure Fe-MIL-88B-NH2indicating that the composite has a lower recombination rate of electrons and holes.In general,2 ∶3 AgI@Fe-MIL-88B-NH2sample holds the best capability in charge separation.This shows that 2∶3 AgI@Fe-MIL-88B-NH2can surely suppress the recombination process of the photogenerated electron-hole pairs.As displayed in Fig.1(d),the mesoporous spherical shell having open pore with AgI particles adhered on surface and inward of Fe-MIL-88B-NH2may be responsible for this low PL emission,because there exist more contact interfaces between AgI and Fe-MIL-88B-NH2.

Fig.6 UV-Vis spectra(a)and estimated band gap(b)of Fe-MIL-88B-NH2,2∶3 AgI@Fe-MIL-88B-NH2 composite and AgI
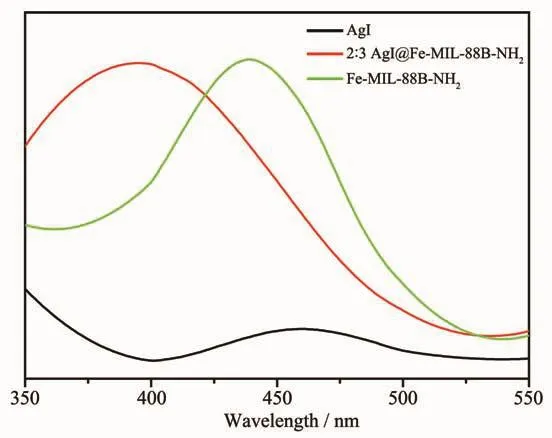
Fig.7 PL emission spectra for Fe-MIL-88B-NH2,2∶3 AgI@Fe-MIL-88B-NH2 and AgI
The photocurrent response test was conducted to further prove the separation efficiency of the photogenerated electron-hole pairs.As indicated in Fig.8,the photocurrent response became stronger(about 80 times larger)for the composite as compared with Fe-MIL-88B-NH2and AgI,which means that the composite has the highest separation efficiency.
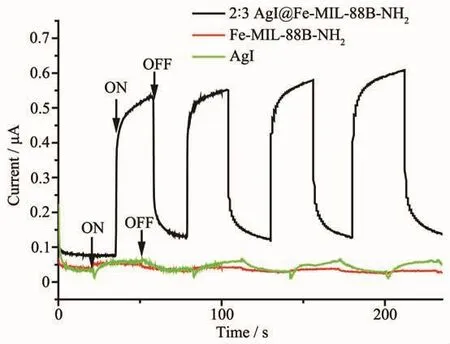
Fig.8 Photocurrent response of Fe-MIL-88B-NH2,2∶3 AgI@Fe-MIL-88B-NH2 and AgI
The AC impedance diagrams of Fe-MIL-88BNH2,2∶3 AgI@Fe-MIL-88B-NH2and AgI(Fig.9)also verify the better separation efficiency of the composite as compared with Fe-MIL-88B-NH2and AgI.
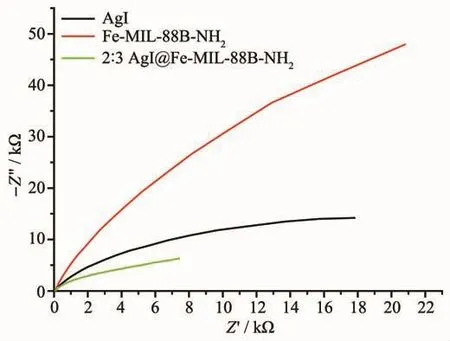
Fig.9 ACimpedance diagrams of Fe-MIL-88B-NH2,2∶3 AgI@Fe-MIL-88B-NH2 and AgI
The adsorption performance of pure materials and compositeswasstudied(Fig.10).Theadsorption capacity qtcan be calculated using the following relationship:

where qt(mg·g-1)is the amount of dye adsorbed onto the adsorbent at time t,and when the adsorption reaches equilibrium,qt=qe;C0and Ct(mg·L-1)denotes the liquid-phase concentrations of dye at initial and time t;V(L)and m(g)are the volume of dye solution and the mass of adsorbent,respectively.As shown in Fig.10,after 8 hours,the adsorption can generally reach equilibrium.Fe-MIL-88B-NH2displayed higher adsorption capacity of RhB in contrast with AgI(Fig.10).This is due to the bigger BET surface area and the mesoporous structure of Fe-MIL-88B-NH2and the electrostatic attraction interaction between RhB and Fe-MIL-88B-NH2[43].It is interesting that,when the content of Fe-MIL-88B-NH2in thecomposite increased,the adsorption capacity for RhB also increased(Fig.10),and when the ratio of AgI to Fe-MIL-88B-NH2increased to 2 ∶3,the adsorption capacity was the biggest.Although the BET surface area of 2∶3 AgI@Fe-MIL-88B-NH2composite(13 m2·g-1)was lower than that of Fe-MIL-88B-NH2(14 m2·g-1),its adsorption capacity for RhB was about 1.6 times higher than that of Fe-MIL-88B-NH2.The reason needs to be further investigated.
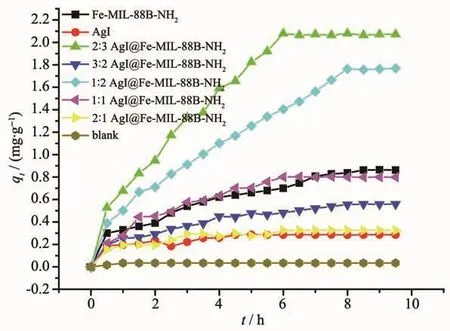
Fig.10 Adsorption capacity of RhB onto AgI@Fe-MIL-88B-NH2 composites,Fe-MIL-88B-NH2 and AgI
The photocatalytic capability of Fe-MIL-88B-NH2,AgI,and AgI@Fe-MIL-88B-NH2with different mass ratios under visible light illumination was evaluated by comparing their photodegradation efficiencies for RhB as presented in Fig.11.During illumination for 3.0 h,theoptimal photocatalytic capability wasachieved when the proportion of AgI@Fe-MIL-88B-NH2reached 2∶3 with the photodegradation efficiency being 97.9%.Before the mass ratio of AgI to Fe-MIL-88B-NH2increased to 2∶3,the photocatalytic capability of AgI@Fe-MIL-88B-NH2composite increased with the increasing of Fe-MIL-88B-NH2content.This is because the mesoporous structure in the Fe-MIL-88B-NH2composite promotes the adsorption capacity,which is also vital for enhancing the photocatalytic capacity.By comparing Fig.10 and Fig.11,one can see that,the variation trends of photocatalytic capacity and adsorption capacity for the composite are the same.Furthermore,photocatalytic capacities of the composite are all higher than those of Fe-MIL-88B-NH2and AgI,although the adsorption capacity of Fe-MIL-88B-NH2is higher than some of the composites.This implies that,besides the adsorption capacity,there exist other factors to affect the photocatalytic capacity.
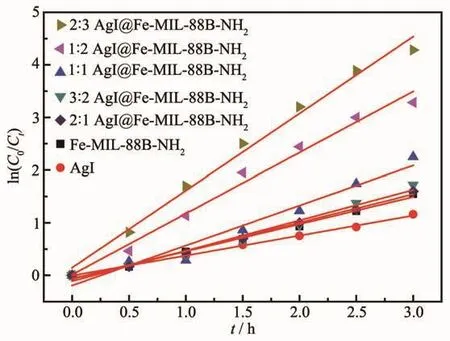
Fig.11 Process of photocatalytic degradation of RhB under visible light irradiation by AgI@Fe-MIL-88B-NH2 composites,Fe-MIL-88B-NH2 and AgI
The kinetic behaviors of the as-prepared samples for photodegradation of RhB were investigated further,and all of them fit well with the pseudo-first order correlation:ln(C0/C)=kt,where C is the concentration of RhB remaining in the solution at irradiation time of t,C0is the initial concentration at t=0,and k is the degradation apparent rate constant.The k values of different samples are shown in Table 1,and decrease as follows:2∶3 AgI@Fe-MIL-88B-NH2>1∶2 AgI@Fe-MIL-88B-NH2>1∶1 AgI@Fe-MIL-88B-NH2>3∶2 AgI@Fe-MIL-88B-NH2>2∶1 AgI@Fe-MIL-88B-NH2>Fe-MIL-88B-NH2>AgI.Obviously,the kinetic constant shows that 2∶3 AgI@Fe-MIL-88B-NH2has the best photocatalytic effect.
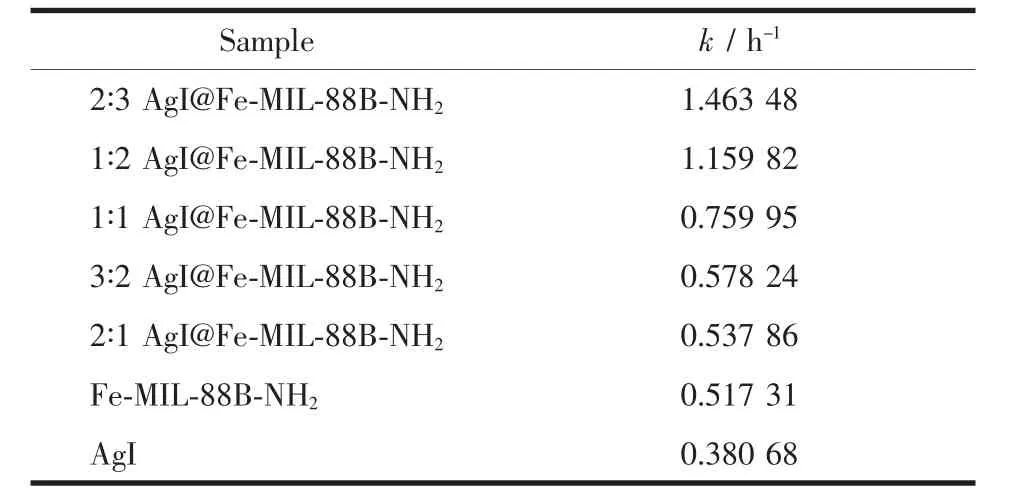
Table 1 Apparent rate constants for RhB degradation
The regeneration of the photocatalyst is one of the important steps for practical applications.To evaluate the photocatalytic stability,2∶3 AgI@Fe-MIL-88B-NH2sample was reused in five successive degradation experiments.As shown in Fig.12(a),the photocatalytic activity of 2∶3 AgI@Fe-MIL-88B-NH2had a little decrease in the first four successive experimental runs,and after the fifth round of circulation,the k value stabilized at about 1.30 min-1,which was 89%of the first cycle.Besides the cycle experiment,the good structural stability of AgI@Fe-MIL-88B-NH2can be also confirmed by the XRD patterns(Fig.12(b)),which indicates that the structure or integrity of photocatalyst changes little after photocatalytic process.
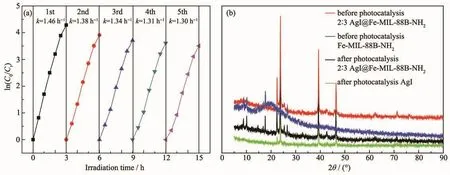
Fig.12 (a)Repeated adsorption and photocatalytic degradation by 2∶3 AgI@Fe-MIL-88B-NH2 under visible light;(b)XRD patterns of 2∶3 AgI@Fe-MIL-88B-NH2 before and after photocatalysis
The trapping experiments of different active species for the degradation of RhB over the AgI@Fe-MIL-88B-NH2composite were carried out to explore the enhanced photocatalytic mechanism,in which tertiary butyl alcohol (t-BuOH),ethylenediamine tetraacetic acid (EDTA-2Na)and benzoquinone(BQ)acted as the scavengers for·OH[44],h+[45]and ·O2-[46],respectively.As shown in Fig.13,the addition of EDTA-2Na in the RhB solution has little effect on the photocatalytic activity of AgI@Fe-MIL-88B-NH2,suggesting that h+does not play a key role for the degradation of RhB.On the contrary,the RhB decomposition process was significantly inhibited by BQ and t-BuOH.On the basis of these results,it can be concluded that·O2-and·OH are the main active oxidation species for AgI@Fe-MIL-88B-NH2in the RhB solution under visible light irradiation.
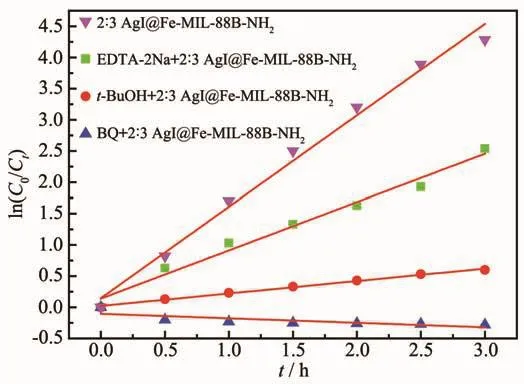
Fig.13 Photocatalytic activities of 2∶3 AgI@Fe-MIL-88BNH2 on the degradation of RhB in presence of different scavengers under visible light irradiation(>420 nm)
The ESR measurements were performed to further confirm the reactive oxygen species evolved in the photocatalysis over AgI@Fe-MIL-88B-NH2nanocomposite with 5,5-dimethyl-1-pyrroline N-oxide(DMPO)in methanol and aqueous solutions.As presented in Fig.14(a),six obvious signals for AgI@Fe-MIL-88B-NH2in methanol were produced,which could be assigned to DMPO-·O2-under light illumination,while no·O2-signal was observed in the dark under identical conditions.The obtained ESR information indicates that O2in solution was reduced to generate·O2-by photogenerated electrons from AgI@Fe-MIL-88B-NH2composite and became the dominant oxygen species during the photocatalytic process.Moreover,the four weak characteristic peaks of DMPO-·OH adducts were also found (Fig.14(b)),which was well consistent with a previous report[47].Thus,the radical trapping experiments and ESR information both verifies the important role of·O2-and·OH in leading to the enormous photocatalytic activity improvement.
Based on the SEM,the N2adsorption-desorption isotherms and the BJH pore diameter distribution curves test,it can be suggested that when AgI particles were adhered on the surface and inward of mesoporous Fe-MIL-88B-NH2spherical shells,more active adsorption sites were offered for AgI@Fe-MIL-88B-NH2,and the interfacial photoreaction was enhanced.On the other hand,the mesoporous spherical shell structure of Fe-MIL-88B-NH2facilitated the propagation of light waves in the photocatalyst,and increased the optical path length in photocatalyst by multiple reflections and scattering of visible light within the interior cavity.Moreover,the unique structure of AgI@Fe-MIL-88B-NH2especially with AgI adhered inward of mesoporous Fe-MIL-88B-NH2spherical shells may protect AgI and contribute to its high stability in the composite.Furthermore,the interface between AgI and Fe-MIL-88B-NH2can promote the separation of photogenerated charge carriers.
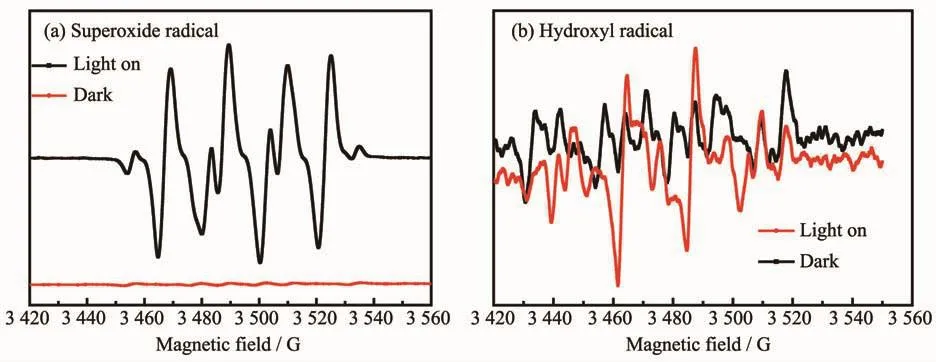
Fig.14 ESR spectra of radical adducts trapped by DMPO spin-trapping in AgI@Fe-MIL-88B-NH2 dispersion in the dark and under visible light irradiation:(a)in methanol dispersion for DMPO-·O2-and(b)in aqueous dispersion for DMPO-·OH
On the basis of the above discussion and the experimental results,apossible “Z-scheme” mechanism for the degradation of RhB by the composite was proposed,as illustrated in Scheme 2.The electrons in the VB of Fe-MIL-88B-NH2can be excited to its CB,leaving holes in the VB.However·O2-radicals cannot be formed because the CB potential of Fe-MIL-88B-NH2(0.13 eV)is more positive than that of the O2/·O2-potential(-0.33 eV vs NHE)[48].Meanwhile,for AgI,the photogenerated electrons transfer to the surface and activate oxygen molecular to yield·O2-under visible light due to the position of CB of AgI(-0.42 eV)[40]is more negative than the potential of O2/·O2-.Both Fe-MIL-88B-NH2and AgIcan photogenerate electron-hole pairs under visible light.The photogenerated electrons in the CB of Fe-MIL-88B-NH2tend to transfer to the VB of AgI and recombine with the photogenerated holes there.Therefore the photogenerated holes left in the VB of Fe-MIL-88B-NH2and the photogenerated electrons accumulated in the CB of AgI are the powerful active species to decompose RhB.Consequently,it can draw a conclusion that the RhB degradation relies on a redox-mediator-free Z-scheme system,which not only enhances the separation efficiency of photogenerated electron and hole pairs,but also improves the photocatalytic performance of RhB degradation.

Scheme 2 Mechanism diagram of the RhB photodegradation by AgI@Fe-MIL-88B-NH2
3 Conclusions
In this study,a novel visible-light-driven mesoporous AgI@Fe-MIL-88B-NH2photocatalyst was successfully prepared by in situ reaction method.Various technical characterizations have been conducted on this composite.AgI modified mesoporous Fe-MIL-88BNH2spherical shell with open pore in the composite shows multiple advantages on the photocatalytic degradation of RhB.A Z-scheme mechanism of the composite was rationalized based on active species trapping and ESR measurements.This unique Z-scheme heterojunction system based on mesoporous MOFs spherical shell and silver salt can act as a platform to construct other high efficient composite photocatalysts in the future research.
