两个由双咪唑基配体构筑的镉配合物的合成、晶体结构及理论计算
2019-07-10李秀梅潘亚茹
李秀梅 潘亚茹 刘 博 周 实
(1通化师范学院化学学院,通化 134002)(2吉林师范大学环境友好材料制备与应用省部共建教育部重点实验室,四平 136000)
0 Introduction
The crystal engineering of metal coordination polymers(MCPs)is one of the most rapidly developing areas of chemical science owing to their diversity of type and physical-chemical properties[1].Obviously,it is the important responsibility for chemists to rationally design and synthesize more MCPs with diverse structures.It is well known that the assembly processes and structures of MCPs are influenced by many factors,such as the coordination preferences of metal ions[2-3],the conformation of bridging ligands[4],solvent systems[5],counteranion[6]and pH value of the solution[7],which can also affect the nature of coordination networks and the framework formation.
In recent years,the direct use of two types of organic ligands has been found to be an effective method for the synthesis of MCPs. Aromatic carboxylate ligands are frequently chosen to construct various dimensions of MCPs because of their rich coordination modes,including monodentate,bridging and chelating.For example,benzoic acid,1,3-benzenedicarboxylate,1,4-benzenedicarboxylate,and 1,2,4,5-benzene tetracarboxylate[8-11]are well used in the construction of MCPs due to their structural rigidity,chemical stability and appropriate connectivity.Besides the carboxylate linkers,bis(imidazole)bridging ligands with different length and flexibly,for example,1,3-bis(imidazol-1-ylmethyl)-benzene,1,4-bis(imidazol-1-ylmethyl)-benzene,1,4-bis(1H-benzimidazolyl)butane are frequently used in the assembly process of MCPs as bridging linker[12-15].
In view of these factors,we herein report the syntheses and characteristics of two new complexes containing 4-nitrobenzoic acid(nba)and 1,3-bis(imidazol-1-ylmethyl)-benzene(mbix)or 1,4-bis(imidazol-1-yl)-butane(bib),namely,[Cd(nba)2(mbix)]2(1)and[Cd((bib)2Br2]n(2).
1 Experimental
1.1 General procedures
All reagents were purchased commercially and used without further purification.Elemental analyses(C,H and N)were measured on a Vario EL(Ⅲ)Elemental Analyzer.IR spectra were recorded in a range of 4 000~400 cm-1on a Nicolet 6700 spectrometer using a KBr pellet.The fluorescence spectra were performed on an F-7000 photospectrometer(Hitachi,High-Tech,Science,Japan).The powder X-ray diffraction (PXRD)studies were performed with a Bruker D8 Discover instrument(Cu Kα radiation,λ=0.154 184 nm,U=40 kV,I=40 mA)over a 2θrange of 5°~50°at room temperature.
1.2 Synthesis
[Cd(nba)2(mbix)]2(1):The pH value of a mixture of Hnba(0.033 g,0.2 mmol),Cd(OAc)2·2H2O(0.2 mmol,0.053 g),mbix(0.048 g,0.2 mmol),and 18 mL H2O was adjust to 7 with 40%NaOH,sealed in a Teflon-lined stainless steel vessel,heated to 150 ℃for 5 days,and followed by slow cooling to room temperature.Yellow block crystals were obtained.Yield:34%.Anal.Calcd.for C56H44Cd2N12O16(%):C,49.24;H,3.25;N,12.31.Found(%):C,48.57;H,2.99;N,11.65.IR(cm-1):3 127w,1 615m,1 574s,1 515m,1 435w,1 406m,1 343s,1 240w,1 107w,939w,834m,724m,657w,518w.
[Cd((bib)2Br2]n(2):The pH value of a mixture of Hnba(0.033 g,0.2 mmol),Cd(OAc)2·2H2O(0.2 mmol,0.053 g),bib (0.038 g,0.2 mmol),and 18 mL H2O was adjust to 8 with 40%NaOH,sealed in a Teflonlined stainless steel vessel,heated to 120 ℃ for 5 days,and followed by slow cooling to room temperature.Brown block crystals were obtained.Unfortunately,Hnba ligand did not participate in the coordination.Bromide ions are derived from reagent(1,4-dibromobutane)for the synthesis of bib ligand.Yield:23%.Anal.Calcd.For C20H28Br2CdN8(%):C,36.80;H,4.32;N,17.17.Found(%):C,36.21;H,4.01;N,16.85.IR(cm-1):3 434w,2 938w,1 616w,1 512s,1 468w,1 444w,1 404w,1 343w,1 272w,1 231s,1 116s,1 105s,1 087s,1 034w,929s,834m,742s,660s,624w,522w.
1.3 Structure determination
Single-crystal X-ray diffraction data for 1 and 2 were recorded on a Bruker D8 QUEST CMOS diffractometer with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at 293 K.The structures were solved with the direct method of SHELXS-97 and refined with full-matrix least-squares techniques using the SHELXL-97 program[16-17].The non-hydrogen atoms of the complexes were refined with anisotropic temperature parameters.The hydrogen atoms attached to carbons were generated geometrically.Crystallographic parameters and the data collection statistics for structures 1 and 2 are given in Table 1.Selected bond lengths and bond angles are listed in Table 2.
CCDC:1890724,1;1890726,2.
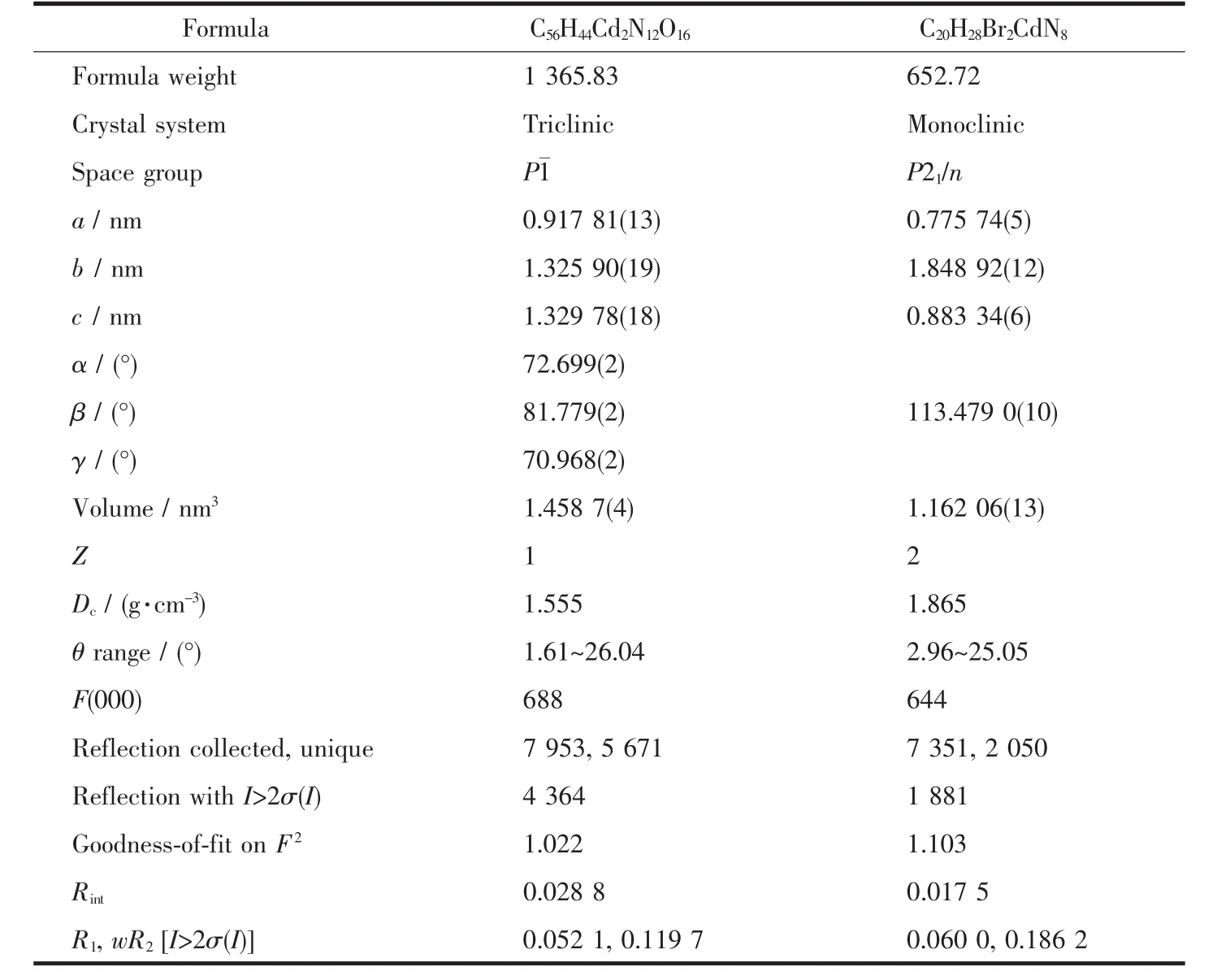
Table 1 Crystal data and structure refinement for 1 and 2

Table 2 Selected bond lengths(nm)and bond angles(°)for 1 and 2
2 Results and discussion
2.1 Description of the structure
Single crystal X-ray analysis revealed that the asymmetric unit of complex 1 is composed of one crystallographically Cd(Ⅱ) ion,one mbix ligand,and two nba ligands.Each Cd(Ⅱ)ion is six-coordinated by two nitrogen atoms(N1 and N4A)from two mbix ligands respectively,and four oxygen atoms from three nba ligands(O1,O2,O5A and O6)respectively in a distorted octahedron coordination sphere.The atoms O1,O2,O5A and O6 constitute the basal plane of the octahedron,and the atoms N1 and N4A are located at the two vertex position.The Cd-O bond distance range from 0.235 3(4)to 0.246 8(3)nm,and the Cd-N distances vary from 0.224 5(4)to 0.224 8(4)nm.The N(O)-Cd-O(N)angles fall in a range of 53.79(12)°~176.91(14)°.
In complex 1,the mbix ligand takes cisconformation bridging mode with a dihedral angle between the two imidazole rings of 25.78°,and the nba ligand coordinates to Cd ions in a bidentate bridging and chelting fashion.As depicted in Fig.1,two Cd(Ⅱ)ions are bridged by four oxygen atoms of nba ligands to give crystallographically dimers with Cd1…Cd1A distance of 0.387 8 nm,and exhibits zerodimensional structure.The two mbix ligands are attached on both sides of dimer.It is noteworthy that there exist π-π interactions among benzene rings of mbix/nba ligands of neighboring dimer.The benzene ring of mbix ligand centroid distance is 0.361 3(3)nm for C19C20C21C22C23C24 and C19′C20′C21′C22′C23′C24′rings(Symmetry codes:3-x,-y,1-z),with the vertical distance to be 0.347 6(2)nm.Benzene ring of nba ligand centroid distance is 0.396 2(5)nm for C2C3C4C5C6C7 and C9C10C11C12C13C14 rings(Symmetry codes:1+x,y,z),with the vertical distance to be 0.362 2(4)nm,indicating the existence ofπ-π effect,so that the structure is more stable.Therefore,an interesting three-dimensional supramolecular network structure is formed byπ-π stacking(Fig.2).
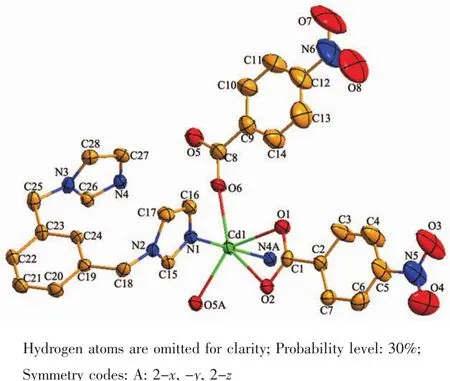
Fig.1 View of coordination environments of Cd(Ⅱ)ions of 1
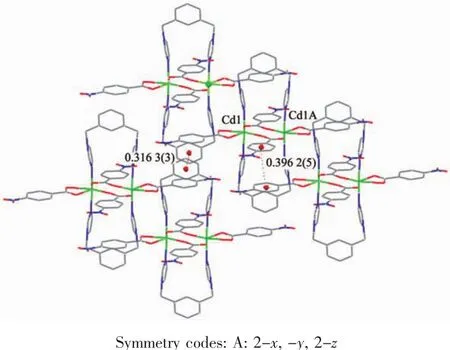
Fig.2 View of 3D supramolecular structure of complex 1 formed by π-π interactions
A single-crystal X-ray diffraction study reveals that complex 2 crystallizes in monoclinic space group P21/n and features a 2D network structure.The coordination environment of Cd(Ⅱ)ion in 2 is shown in Fig.3.The Cd(Ⅱ) ion is six-coordinated by four nitrogen atoms (N1,N1A,N3,N3A)from four bib ligands respectively and two bromine ions (Br1 and Br1A)to furnish a slightly distorted octahedral coordination architecture.The bond distances of Cd-N in complex 2 fall in a range of 0.233 9(6)~0.236 5(7)nm,Cd-Br is 0.276 71(11)nm and the coordination anglesaround the Cd(Ⅱ) ion are in a range of 89.26(17)°~180.00(15)°.
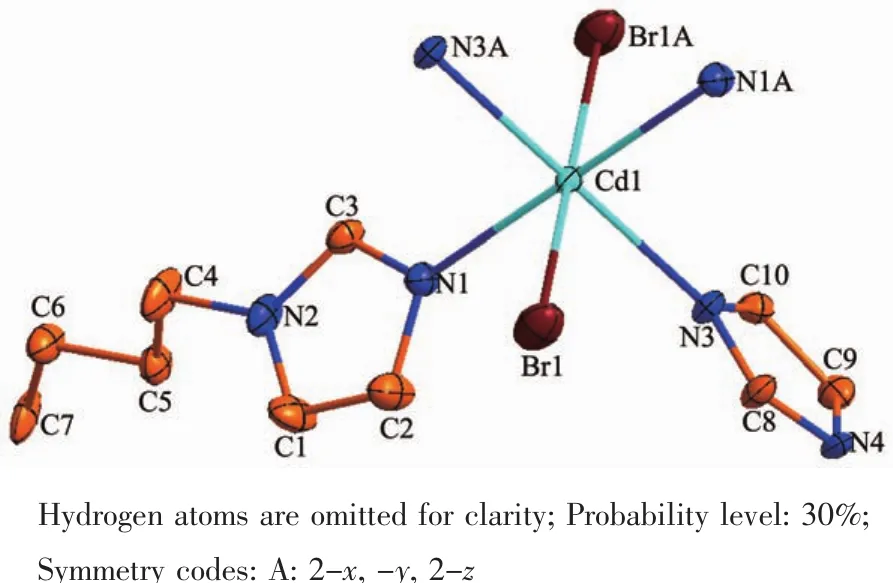
Fig.3 View of coordination environments of Cd(Ⅱ)ion of 2
In the coordination environment,the four nitrogen atoms(N1,N1A,N3,N3A)are located in the basal plane,whereas two bromine ions (Br1,Br1A)occupy the axial positions from the opposite directions.In the crystal structure of complex 2,the bib ligand adopts a cis-conformation bridging mode with a dihedral angle between two imidazole rings of 58.73°and link the Cd(Ⅱ)ions to form a two-dimensional network structurewith(4,4)topology,asshown in Fig.4.
To investigate whether the analyzed crystal structure is truly representative of the bulk materials,X-ray powder diffraction(PXRD)technology has been performed for the complex at room temperature(Fig.5).The main peak positions observed were in good agreement with the simulated ones.Although minor differences can be found in the positions,widths,and intensities of some peaks,the bulk synthesized materials and analyzed crystal can still be considered as homogeneous.The differences may be due to the preferred orientation of the powder samples[18-19].
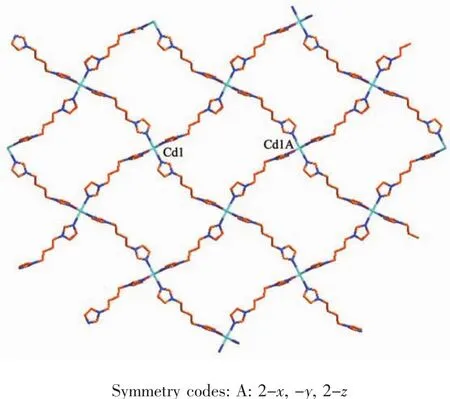
Fig.4 Two dimensional network structure of 2 with(4,4)topology along c-axis

Fig.5 PXRD analysis of complexes 1 and 2
2.2 Photoluminescent properties
Metal-organic coordination polymers,especially d10metal centers,such as Ag(Ⅰ),Au(Ⅰ),Zn(Ⅱ) and Cd(Ⅱ),and conjugated organic linker have been researched because of their fluorescent properties and potential applications as fluorescent-emitting materials,chemical sensors and electroluminescent displays[20].Therefore,in the present work,the photoluminescent properties of Hnba,mbix,bib,complexes 1 and 2 have been investigated in the solid state at room temperature.

Fig.6 Solid-state emission spectrum of 1 and 2 at room temperature
When excited at 207 nm,complex 1 exhibited blue luminescence and showed emission peak at 467 nm,and complex 2 showed green luminescence with emission peak at 553 nm (Fig.6).The free ligands Hnba,mbix and bib showed photoluminescence with the emission maximum at 470,436 and 440 nm,respectively,which can be assigned to intraligand(π→π*)transition[21].Because the Cd(Ⅱ) ion is difficult to oxidize or reduce due to the d10configuration,the emission of this compound is neither MLCT nor LMCT in nature[22-23].Thus,the emission bands of complex 1 and 2 could be assigned to the emission of ligand-toligand charge transfer[24-26].
2.3 Theoretical calculations
All calculations in this work were carried out with the Gaussian03 program[27].The parameters of the molecular structure for calculation were all from the experimental data of the complex.Natural bond orbital (NBO)analysis was performed by density functional theory(DFT)[28]with the PBE0[29-30]hybrid functional and the LANL2DZ basis set[31-32].
The selected natural atomic charges and natural electron configuration for complex 1 is shown in Table 3.It is indicated that the electronic configurations of Cd(Ⅱ) ion,Nand Oatomsare5s0.314d9.995p0.31,2s1.382p4.19~4.20and 2s1.67~1.702p5.02~5.11,respectively.Based on the above results,one can conclude that the Cd(Ⅱ) ion coordination with N and O atoms is mainly on 4d,5s,and 5p orbitals.N atoms form coordination bonds with Cd(Ⅱ)ion using 2s and 2p orbitals.All O atoms supply electrons of 2s and 2p to Cd(Ⅱ) ion,forming the coordination bonds.Therefore,the Cd(Ⅱ) ion obtained some electrons from two N atoms of mbix ligand,four O atoms of nba ligand[32].According to valence-bond theory,the atomic net charge distribution and the NBO bond orders of the complex 1 (Table 3)shows the obvious covalent interaction between the coordinated atoms and Cd(Ⅱ)ion.The differences of the NBO bond orders for Cd-O and Cd-N bonds make their bond lengths become different[32],which is in good agreement with the X-ray crystal structural data of compound 1.
As can be seen from the Fig.7,lowest unoccupied molecular orbital(LUMO)of 1 is mainly composed of nba and mbix ligands,whereas highest occupied molecular orbital(HOMO)mainly consists of nba ligand.So,LLCT may be inferred from some contours of molecular orbital of complex 1.
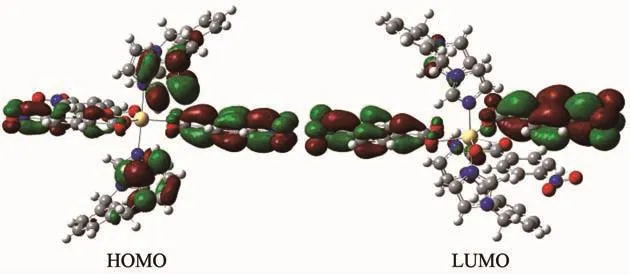
Fig.7 Frontier molecular orbitals of complex 1

Table 3 Natural atomic charges,natural valence electron configurations,Wiberg bond indexes and NBO bond orders for 1 and 2
The selected natural atomic charges and natural electron configuration for complex 2 is displayed in Table 3.It means that the electronic configurations of Cd (Ⅱ) ion,N and Br atoms are 5s0.364d9.995p0.47,2s1.382p4.15~4.28and 4s1.924p5.84,respectively.In view of the above results,one can conclude that the Cd(Ⅱ) ion coordination with N and Br atoms is mainly on 4d,5s,and 5p orbitals.N atoms form coordination bonds with Cd(Ⅱ)ion using 2s and 2p orbitals.All Br atoms supply electrons of 4s and 4p to Cd(Ⅱ)ion and form the coordination bonds.Therefore,the Cd(Ⅱ) ion obtained some electrons from four N atoms of bib ligand,two Br ions[32].Thus,on the base of valencebond theory,the atomic net charge distribution and the NBO bond orders of the complex 2 (Table 3)shows the obvious covalent interaction between the coordinated atoms and Cd(Ⅱ)ion.The differences of the NBO bond orders for Cd-O and Cd-Br bonds make their bond lengths be different[32],which is in good agreement with the X-ray crystal structural data of complex 2.
As can be seen from the Fig.8,lowest unoccupied molecular orbital(LUMO)and highest occupied molecular orbital (HOMO)of 2 are all mainly made up of bib ligand,So,ILCT may be inferred from some contours of molecular orbital of complex 2.
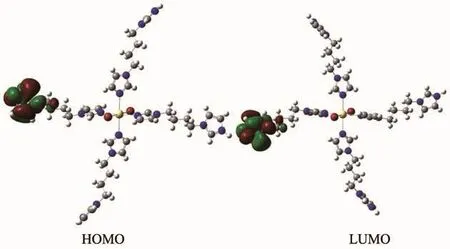
Fig.8 Frontier molecular orbitals of complex 2
