CoMo/Al2O3催化剂柴油加氢脱芳烃集总反应动力学模型
2019-05-21江洪波吕海龙陈文斌李明丰
江洪波, 吕海龙, 陈文斌, 秦 康, 李明丰, 聂 红
(1.华东理工大学 石油加工研究所, 上海 200237; 2.中国石化 石油化工科学研究院, 北京 100083)
Crude oils are becoming heavier and their quality is also changing worse compared with previous years. Thus, there is an increasing trend toward processing more heavy oils all over the world. Heavy crudes typically contain a large number of undesirable aromatic compounds and are more difficult to be converted into clean transportation fuels. Polycyclic aromatics widely present in heavy oils as coke precursors, and aromatics with two or more rings have adverse effects on product quality. For example, high aromatic content in diesel will lead to poor quality and low cetane number. According to the latest diesel national standard of China, polycyclic aromatics in diesel needs to be less than 7%[1]. Therefore, efficient conversion of polycyclic aromatics has an important impact on diesel quality.
Catalytic hydrotreating (HDT) is one of the most important processes in the refining industry from technical, economic, and environmental points of view[2]. To achieve the goal of reducing aromatic content in diesel, understanding impacts of operating parameters is necessary. Modelling and simulation of diesel hydrotreating process can guide operation optimization, provide quantitative fundamentals for best catalyst packing practice in industrial reactor, evaluate catalyst performance through intrinsic relationship of kinetic parameters, and provide guidance for catalyst development.
Most studies on kinetic models for diesel hydrodearomatization (HDA) reaction are based on model components. Huang et al.[3]investigated hydrogenation ofdi-aromatic compound, i.e., naphthalene, in a trickle bed reactor over Pt/Al2O3catalyst. In their work, naphthalene dissolved inn-hexadecane was used to simulate the aromatic compounds in diesel fuels and used the above system to study the kinetics of hydrogenation reaction. Rautanen et al.[4]developed a kinetic model of tetralin hydrogenation in decane on a Ni/Al2O3catalyst. The proposed kinetic model was based on Langmuir-Hinshelwood-type mechanism which could explain their experimental results well. However, their kinetics based on model components could not describe HDA kinetics of diesel. Chowdhury et al.[5]investigated the hydrogenation of aromatics in diesel over NiMo/Al2O3catalyst. Kinetic equations for hydrogenation ofmono-,di- and poly-aromatics were established, but competitive adsorption between aromatics with different rings was not considered.
In this study, kinetics of HDA of diesel oil has been studied on a high-throughput reactor with CoMo/Al2O3as catalyst. A lumped kinetic model for diesel HDA was proposed, and its kinetic parameters were estimated through experimental data regression at various pressures, hydrogen/oil volume ratios, temperatures and liquid hourly space velocities (LHSV). Experimental data and model calculation results are consistent with each other in all experimental conditions.
1 Experimental
To estimate kinetic parameters of hydrodearomatization reaction of diesel, different reaction conditions were examined. Reaction temperature,hydrogen partial pressure, liquid hourly space velocity and hydrogen/oil volume ratio were in the range of 573.15-633.15 K, 4.4-6.4 MPa, 0.75-3 h-1and 200-800, respectively. Effects of external diffusion and internal diffusion were also investigated in experiments.
1.1 Raw materials and catalyst
Properties of the diesel oil used in the experiment are provided in Table 1.

Table 1 Properties of feed oil used in the experiment
CoMo/Al2O3catalyst, purchased from SINOPEC Catalyst CO. LTD, was used in this study. Its specific surface area and pore volume are 227.6 m2/g and 0.31 cm3/g, respectively.
1.2 Reaction conditions and experimental procedure
Hydrogenation activity of CoMo/Al2O3catalyst was tested on the high throughput workflow HDS system (imported from Avantium, Netherlands). This system is equipped with several parallel reactors, which can perform hydrogenation reaction at the same condition (pressure, temperature, flow of the feed, hydrogen/oil volume ratio). The deviations of controlling parameters for these reactors were less than 3%.
Catalysts (particle size 250-380 μm) were loaded in four different size reactors and the volume ratio of above four reactors is 1∶2∶3∶4. The catalysts were first sulfided with a 2% (mass fraction) CS2solution in kerosene under 6.4 MPa of hydrogen pressure at 523.15 K (ramp of 25 K/h) for 6 h and then at 593.15 K (ramp of 15 K/h) for another 4 h. After the sulfidation, the catalysts were activated by hydrogenated diesel (S mass fraction of 685 μg/g) for 24 h at 598.15 K. Finally, the diesel feed was switched to perform hydrogenation reactions. Kinetic reaction study was performed under different conditions through changing temperature, pressure, hydrogen/oil volume ratio, and feed flowrate.
For each condition, the time on stream was all maintained 72 h. Compositions of diesel were quantitatively analyzed by near-infrared spectroscopy method on spectrometer Antaris II from Thermo Scientific equipped with InGaAs detector. Each sample was scanned for 128 times and the obtained infrared spectroscopy was compared with the in-house near-infrared spectroscopy calibration model which was established by partial least squares regression method based on more than 100 kinds of diesel samples[6].
2 Diesel hydrodearomatization experimental results
In present study, effects of space time, temperature, pressure, and hydrogen/oil volume ratio on the reaction were systematically investigated. Some experimental data under different reaction conditions were given in Fig.1.
Fig.1(a) shows that as space time (τ) increases, mass fractions ofdi- andtri-aromatics decrease. However, the mass fraction ofmono-aromatics increases at first and descends later. This implies that the reaction rate ofdi-aromatics to producemono-aromatics is faster than that ofmono-aromatics to produce naphthenic hydrocarbon.
Mass fractions of reaction products at different temperatures are shown in Fig.1(b). As reaction temperature increases, mass fractions ofdi- andtri-aromatics decrease. This means that, under the operation conditions, higher temperature is favor to hydrogenation ofdi- andtri-aromatics even though the hydrogenation of aromatics is exothermic. Mass fraction ofmono-aromatics increases with temperature before 613.15 K and then decreases as reaction temperature further goes up.
As reaction pressure increases, mass fractions ofdi- andtri-aromatics decrease. However, mass fraction ofmono-aromatics increases with the pressure in the beginning and then slightly decreases when pressure is further up as shown in Fig.1(c). As shown in Fig.1(d), it has been observed that hydrogen/oil ratio has little impact on the experimental results.
3 Kinetic modelling of aromatics hydrogenation
Hydrogenation of aromatics has been extensively studied by different scholars[7-9], and most scholars believe that hydrogenation of aromatics is reversible under normal hydrogenation conditions. Aromatic rings of polycyclic hydrocarbons are hydrogenated step by step, and hydrogenation of each ring is also reversible thermodynamically. Hydrogenation of the first aromatic ring will be easier for those polyaromatics with more aromatic rings.
3.1 Model assumption
Based on the analysis of reaction system and high-throughput hydrogenation reactor, the lumped kinetic model was developed with the following assumptions:
(1) The reactor operates isothermally under isobaric and steady-state conditions. No catalyst deactivation happens during hydrogenation reaction.
(2) Reactor behaves like a plug-flow reactor
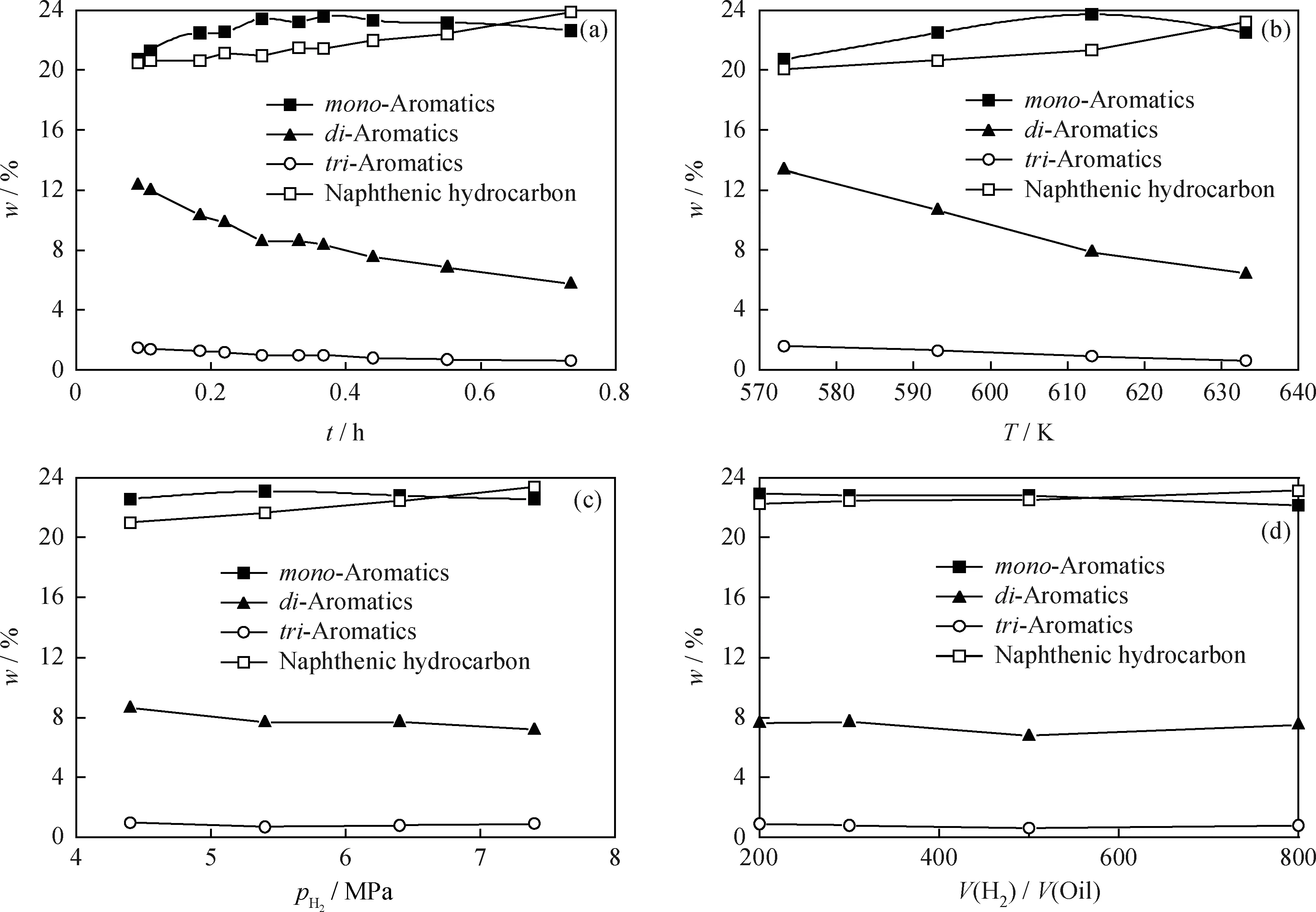
Fig.1 Experimental results of diesel hydrodearomatization on CoMo/Al2O3 under different reaction conditions(a) pH2=6.4 MPa, T=613.15 K, V(H2)/V(Oil)=300; (b) pH2=6.4 MPa, V(H2)/V(Oil)=300, LHSV=2 h-1; (c) T=613.15 K, V(H2)/V(Oil)=300, LHSV=2 h-1; (d) pH2=6.4 MPa, T=613.15 K, LHSV=2 h-1
with pseudo-homogeneous reaction assumption, and evaporation of diesel during reaction is neglected.
(3) Naphthenics and paraffinics in diesel are categorized into two lumps (N and P), respectively. Aromatics with different aromatic rings are categorized into three lumps, namelymono-,di-, andtri-aromatics (A1, A2and A3).
(4) Each of aromatic lumps undergoes hydrogenation reactions is reversible under hydrogenation conditions. However, hydrogenation of naphthenics to paraffinics is irreversible. The reaction network between lumps can be described as follows:
wherekb(b=A0-A3, -A1- -A3) is rate constant. Reactions are first order with respect to the concentration of each lump and hydrogen is in excess during hydrogenation reaction.
(5) Based on the analysis of experimental data for external diffusion study, the effect of external diffusion can be neglected under the experiment conditions. The size of catalyst used in the kinetic experiment is 250-380 μm, which is usually used in micro-reactor test to avoid the internal diffusion[10]. Experimental data for internal diffusion study also suggest that the effect of internal diffusion can be neglected while the size of catalyst ranges from 150 to 1000 μm. Therefore, hydrodearomatization reactions on the catalyst surface can be considered as rate controlling steps.
(6) Langmuir-Hinshelwood-Hougen-Watson (LHHW) equation is used to account for the competitive adsorption of all aromatic hydrocarbons. It is also assumed that the adsorption constants for aromatics with the same number of aromatic rings are the same.
3.2 Kinetic equation
Reaction rate of HDA in diesel oil is mainly affected by temperature, pressure, space time, and hydrogen/oil ratio. The effect of temperature on reaction rate constant can be expressed by Arrhenius equation. The effects of reaction pressure and hydrogen/oil volume ratio on reaction rate constant are expressed in the form of power exponent. Referencing to the reaction networks, the competitive adsorption theory of LHHW was used to obtain kinetic equations. The rate expression can be expressed as follows:

(1)

(2)

(3)
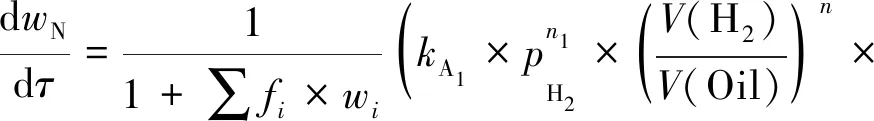
(4)
in which
1+∑fi×wi=1+fA1wA1+
fA2wA2+fA3wA3+fNwN
(5)
Informula (1)-(5),wiis the mass fraction of lumpi(i=A1, A2, A3or N, the same below);nj(j=0, 1, 2, 3) are the influence factors of hydrogen partial pressure;kbis the rate constant, MPa-nj·h-1;pH2is the hydrogen pressure, MPa;V(H2)/V(Oil) is hydrogen/oil volume ratio;fiis adsorption equilibrium constants of lumpi;τis space time, h;nis the influence factor of hydrogen/oil volume ratio.
4 Results and discussion
The parameters of HDA system were estimated with Powell optimization method[11]. The calculated reaction rate constants were shown in Table 2, and the adsorption constant of individual lumps was shown in Table 3. Table 4 gives the influence factors of the pressure and the hydrogen/oil volume ratio.

Table 2 Reaction rate constants of aromaticshydrogenation model
pH2=6.4 MPa;T=613.15 K;V(H2)/V(Oil)=300; The data ofnj,nandfican be seen in Table 3 and Table 4.

Table 3 Adsorption constants of individual lumps
T=613.15 K

Table 4 Influence factors of pressure andhydrogen/oil volume ratio

Table 5 Deviation between experimental data andaromatics hydrogenation modeled values
As indicated in Table 5, deviation errors of all products are within acceptable range. As an example. the comparison between experimental data and model fitted values under some specific conditions is illustrated in Fig.2.

Fig.2 Comparison between experimental data andaromatics hydrogenation model fitted valuespH2=6.4 MPa; T=613.15 K; V(H2)/V(Oil)=300
As shown in Table 2, the forward and backward reaction rates of aromatics hydrogenation are both obvious, which means the reversible assumption of aromatics hydrogenation reactions is acceptable. Forward reactions are faster than the corresponding backward reactions. Under the same reaction conditions, reaction ofdi-aromatics tomono-aromatics is faster than that ofmono-aromatics to naphthenic hydrocarbon. This can explain why the content ofmono-aromatics increases then decreases as the space time increases (Seen Fig.2). A similar trend of experimental observation was also reported by Stanislaus et al.[9].
In this work, competitive adsorption on the catalyst surface is assumed following Langmuir-Hinshelwood mechanism. It can be seen from Table 3 that the adsorption capability of different compounds in descending order istri-aromatics,di-aromatics,mono-aromatics, and naphthenics. Adsorption parameters increase with the increasing aromatic ring number of compounds, which is consistent with the report by Korre et al.[12].
From Table 4, it can be noticed that the influence factors of pressure are larger than 0.5, which means that hydrogenation reaction rate of aromatics increases as reaction pressure increases. However, the influence factor of hydrogen/oil volume ratio is only 0.16. This suggests hydrogen/oil volume ratio has little effect on the HDA reaction of diesel under the experimental conditions.
The influence of temperature on reactions was investigated by changing the reaction temperature. The effect of temperature on reactions follows Arrhenius equation:
(6)
Wherekb0is the pre-exponential factor, MPa-nj/h;Eabis the activation energy of the reaction, kJ/mol;Tis the reaction temperature, K.
The effect of temperature on adsorption is also can be described as Arrhenius equation.
(7)
Wherefi0is the pre-exponential factor;Efiis the influence factor of adsorption, kJ/mol.
In general, high temperature favors desorption of molecules on the surface of catalyst. Thus the influence factors of temperature on adsorption constant are negative as illustrated in Table 6.

Table 6 Activation energy (Eab) of reaction andtemperature influence factors of adsorption (Efi)for aromatics hydrogenation model
5 Validation of aromatics hydrogenation model
As shown in Fig.3, the established kinetic model was used to predict the reaction product distribution and compared with those from experiments. Reaction conditions of the validation experiments are given in Table 7. It can be seen from Table 7, that both model predicted and experimental data are very close with each other and distribute evenly along the diagonal line. This means that the agreement between the experimental and simulated results is very good.

Table 7 Reaction conditions of validation experimentsfor aromatics hydrogenation model

Fig.3 Comparison between experimental and simulatedresults of aromatics hydrogenation modelExperiment conditions were listed in Table 7.exp—Experimental data; cal—Calculated data
6 Conclusion
(1) Based on the reaction mechanism and kinetic experiments from a high-throughput reactor, a lumped kinetic model with considering the influence of competitive adsorption was established for the hydrodearomatization reaction of diesel. The proposed model can well describe the reaction behavior of aromatics in the hydrotreating system. Further validation confirmed the reliability of the model prediction.
(2) It has been confirmed that hydrogenation of aromatics is reversible under the reaction conditions of diesel hydrogenation. The optimized model parameters suggest that hydrogenation of the first aromatic ring is easier for those polyaromatics with more aromatic rings, and the impact of reaction pressure on hydrodearomatization is higher than that of hydrogen/oil volume ratio.
