Ectopic Expression of a Fungal AbGDH Gene from Alternaria brassicicola Improves Nitrogen Use Efficiency in Rice
2019-05-16CHENMingdongLIUCongLIUDerongTANGQilongLINJianzhongLIUXuanming
CHEN Ming-dong,LIU Cong,LIU De-rong,TANG Qi-long,LIN Jian-zhong,LIU Xuan-ming
(Hunan Province Key Laboratory of Plant Functional Genomics and Developmental Regulation,College of Biology,Hunan University,Changsha 410082,Hunan,China)
Abstract:Nitrogen is one of the most important elements in agriculture industry.NADP(H)-dependent glutamate dehydrogenase(GDH)has a lower affinity for ammonium in higher plants,whose nitrogen is thus mainly assimilated by the glutamine synthetase(GS)/glutamate synthase(GOGAT)pathway.But,in lower organ isms such as fungi,GDHs play an important role in nitrogen assimilation due to their higher affinity for am-monium.In this study,a glutamate dehydrogenase gene AbGDH from Alternaria brassicicola was cloned and successfully over-expressed in rice(Oryza sativa L.cv.Kitaake).Enzyme activity assay in vitro demonstrated that the Kmvalues of AbGDH for NH4+,2-oxoglutarate and glutamate were 2.144±0.141 mmol/L,2.690±0.233 mmol/L and 96.772±0.542 mmol/L,respectively.Meanwhile,the enzyme activity assay in vivo revealed that,compared with the nontransgenic plants,the AbGDH over-expressing plants showed a higher aminating activity and a lower Kmvalue for NH4+.Additionally,the hydroponic experiments revealed that,compared with control plants,the transgenic seedlings exhibited a significant increase in plant height and dry weight at lower nitrogen conditions.These results suggested that ectopic expression of AbGDH was inclined to convert 2-oxoglutarate to glutamate,which subsequently improved the ammonium assimilation and enhanced the nitrogen use efficiency particularly under low nitrogen fertility.
Key words:glutamate dehydrogenase(GDH);nitrogen assimilation;rice;Alternaria brassicicola
Rice(Oryza sativa L.)is one of the most important food crops in the world,especially in Asian countries.Nitrogen,a necessary nutrient,is an important growth limiting factor for plant development and productivity.The doubling of global agricultural food production over the past forty years has been associated with a seven-fold increase in the use of nitrogen fertilizers[1].However,more than half of the nitrogen could not be assimilated by crops and has been leached inevitably into the underground water system,ultimately diffusing into the atmosphere and causing severe environmental problems such as eutrophication and damage of marine ecosystems[2].Thus,it is crucial that the nitrogen applied to crops is effectively used by the plants.
Recently,an increasing knowledge of regulatory mechanisms for nitrogen assimilation in rice,the model plant of cereals,has been obtained.It is vital for improving nitrogen use efficiency and for reducing the excessive input of fertilizers,while maintaining an acceptable yield[3~4].Most plants absorb inorganic nitrogen in the form of ammonium(NH4+)or nitrate(NO3-),but the main form of nitrogen available for rice in irrigated paddy fields is NH4+.There are two pathways involved in the assimilation of ammonium in higher plants.One is glu tamine synthetase(GS)/glutamate synthase(GOGAT)pathway,and the other is glutamate dehydrogenase(GDH)pathway[5].In rice,assimilation of ammonium into amino acids is performed mainly through the GS/GOGAT pathway including two enzymes:glutamine synthetase(GS,EC 6.3.1.2)and glutamate syn thase(ferredoxin[Fd]-GOGAT,EC 1.4.7.1;NADHGOGAT,EC 1.4.1.14)[6].GS catalyzes the ATP-dependent condensation reaction of ammonium with glutamate to glutamine,which is the first reaction of ammonium assimilation[7].GOGAT catalyzes the transfer of amino group of glutamine to 2-oxoglutarate(2-OG)to form two glutamate molecules[8].The role of amino acid transport was first assigned for NADHGOGAT in rice because it was found in the vascular tissues[9].Fd-GOGAT was presumably complementary to NADH-GOGAT during development because NADH-GOGAT occurred at higher levels in young and nonexpanded leaves and decreased with leaf age[10].
Since the discovery of GS/GOGAT pathway in higher plants in the 1970s,the physiological role of GDH has been a subject of controversy.GDHs exist widely in the biosphere from bacteria to higher plants and catalyze the reversible reaction for the reductive amination of 2-OG to produce glutamate in the presence of the cofactor NAD(P)H[11].In higher plants,there are two types of GDH isoenzymes[12]:NAD(H)dependent[NAD(H)-GDH,EC 1.4.1.2]and NADP(H)dependent[NADP(H)-GDH,EC 1.4.1.4].Compared with GS,GDHs exhibit high Kmvalues(10~80 mmol/L)for ammonium and catalyze a reversible reaction in ammonium assimilation[13~15],which indicates that GDHs have lower affinities for ammo-nium and are capable of assimilating ammonium in to glutamate only at a high NH4+concentration.However,in contrast to higher plants,GDHs from lower organisms show differences in their amino acid sequences,biochemical characteristics and assimilatory capacities[16~18].Especially,in many microorganisms such as bacteria and fungi,the NADP(H)-GDHs have a lower Kmfor ammonium but a higher Kmfor glutamate[14,18].For example,Kmvalues of SsGDH from Sclerotinia sclerotiorum for ammonium and glutamate are 0.28±0.03 mmol/L and 1 352.62±74.32 mmol/L,respectively[18].Additionally,when provided ammonium as the sole nitrogen source,the mutants of Aspergillus nidulans deficient in NADP(H)-glutamate dehydrogenase(gdhA)grew more poorly[19~20].These previous studies suggest that,compared with those from higher plants,GDHs from microorganisms play important roles in the ammonium assimilation,even at a lower NH4+concentration.
On the other hand,compared with the GS/GOGAT pathway,the GDH does not use ATP,so the ammonia assimilation via GDH pathway is more energy-efficient[21~22].Hence,GDH pathway is currently receiving greater attention,and several GDHs from lower organisms have been introduced into plants to improve ammonium assimilation.For example,when a GDH from Escherichia coli was introduced,the transgenic tobacco increased the tolerance to toxic levels of ammonium and biomass production[23].When a gdhA gene from Aspergillus niger was overexpressed in rice,the transgenic lines exhibited significantly increased dry weight,nitrogen content and grain yield compared with non-transformed plants[24].In addition,another NADP(H)-GDH gene CeGDH from Cylindrocarpon ehrenbergii,when introduced into rice,enhanced nitrogen utilization and improved growth and grain yields,especially in low nitrogen fertility[25].Zhou et al and Du et al isolated the fungal NADP(H)-GDH genes PcGDH and SsGDH from Pleurotus cystidiosus and S.sclerotiorum,respectively,and heterologously expressed them in rice[14,18].It is worth noting that both PcGDH and SsGDH transgenic rice plants showed improved nitrogen assimilation and growth quality,especially in low nitrogen fertility,but that there was no apparent difference in grain yields between transgenic rice and wild type plants.These results strongly suggest that ectopic expression of GDHs from lower organisms in higher plants can improve the ammonium assimilation and growth quality,but not necessarily bring higher grain yields.Thus,it would be of great value to isolate and identify from lower organisms new NADP(H)-GDHs which can improve ammonium assimilation and also increase production of rice.
In the present study,an NADP(H)-GDH gene AbGDH was isolated from the fungus Alternaria brassicicola and ectopically expressed in rice to modify the ammonium assimilation by genetic engineering.The kinetic analysis showed that AbGDH was inclined to assimilate ammonium for the interconversion of 2-oxoglutarate and glutamate.Furthermore,hydroponic tests demonstrated that the heterologous AbGDH significantly improved nitrogen assimilation in transgenic rice plants.
1 Materials and methods
1.1 Plant material and growth conditions
Rice of Oryza sativa L.cv.Kitaake and transgenic rice(Ubi::AbGDH)were used in this experiment.Rice seeds were surface-sterilized in 3.0%(V/V)sodium hypochlorite for 30 min and then rinsed three times with sterile water for germination.The positive transgenic seedlings were identified by screening for hygromycin-resistance.The germinated seeds derived from homozygous plants were trans ferred into nutrient solution with two nitrogen concen trations(200 μmol/L and 2 000 μmol/L NH4Cl as the only nitrogen source)[18].Rice seedlings were then cultured in a greenhouse with a 16-h/8-h day/night cycle at 28℃.The nutrient solution was maintained at pH 5.6 and refreshed every 3 d.Rice plants were grown for 20 d,and then their height and dry weight were measured.Total nitrogen content of the whole plants was determined by Kjeldahl analysis[26].
To investigate the agronomic traits of transgenic rice,the 18-d-old seedlings of two transgenic lines(Ubi::AbGDH-16 and Ubi::AbGDH-42)and the nontransformant control were transplanted to a paddy field supplemented with 112.5 kg N/hm2(in the form of urea)as normal nitrogen supply in Changsha,China.All rice plants were grown in early July and harvested in mid-October.Several component traits of yield were evaluated as described previously[27~28],and the yield data were collected from 20 plants in the two middle rows.
1.2 Phylogenetic analysis
The amino acid sequences of AbGDH and its homologs were aligned by ClustalX.Multiple alignments of translated amino acid sequences were performed using ClustalX and MEGA 5.0.Phylogenetic tree was constructed using the neighbor-joining method,length of the branch is proportional to the degree of divergence[18].
1.3 Cloning of AbGDH from A.brassicicola
The full-length cDNA of AbGDH gene was isolated from the A.brassicicola using reverse transcription PCR with degenerate primers(Table 1)according to the method described by Zhou et al[14].The PCR product was verified by sequencing and cloned into the GATEWAY donor vector pGWC[29].Then,the full-length coding sequence of AbGDH was subcloned into the binary vector pCAMBIA1301GW(a slightly modified pCAMBIA1301 vector)via LR reaction as described previously[14].The resulting vector was named pCAMBIA1301GW-Ab-GDH.
1.4 Construction of recombinant prokaryotic expression vector
A forward primer with BamHⅠcleavage site and a reverse primer(Table 1)containing HindⅢcleavage site were used to amplify the AbGDH coding sequence from the pMD-18T vector.The DNA fragment was digested by BamHⅠ and HindⅢ,and ligated into the pCold-TF vector by T4DNA ligase(Takara,Dalian,China).The recombinant plasmid pCold-TF-AbGDH was then transferred into E.coli(strain BL21).Then the fusion protein His-TF-Ab-GDH with His and trigger factor(TF)tags was expressed in E.coli BL21(DE3)and preliminarily analyzed according to the method described by Zhou et al[28].
1.5 Purification and immunoblotting analysis of the recombinant AbGDH protein
To determine NADP(H)-GDH activity of AbGDH,recombinant protein His-TF-AbGDH was purified using a nickel-nitrilotriacetic acid(Ni-NTA)resin(Invitrogen,USA)as described by Zhou et al[14].The temperature was kept constant at 4℃during the process of purification.The Ni-NTA was activated by sterilized water for 3~5 min with gentle rotation,and then the supernatant was discarded.Precipitation with Ni-NTA was washed twice by binding buffer(50 mmol/L Tris,pH 8.0,500 mmol/L NaCl,and 10 mmol/L imidazole).The target protein obtained from the supernatants of the sonication was bound to the activated Ni-NTA for 2 h in binding buffer.The supernatant was discarded.Bound protein was washed with wash buffer(50 mmol/L Tris,pH 8.0,500 mmol/L NaCl,and 20 mmol/L imidazole)for 3 times,5 min each.And then the target protein was eluted with elution buffer(50 mmol/L Tris,pH 8.0,500 mmol/L NaCl,and 250 mmol/L imidazole)for 3 times,5 min each.After centrifugation,the purifiedprotein products were analyzed by SDS-PAGE and stained with Coomassie brilliant blue according to the protocol described by Du et al[18].
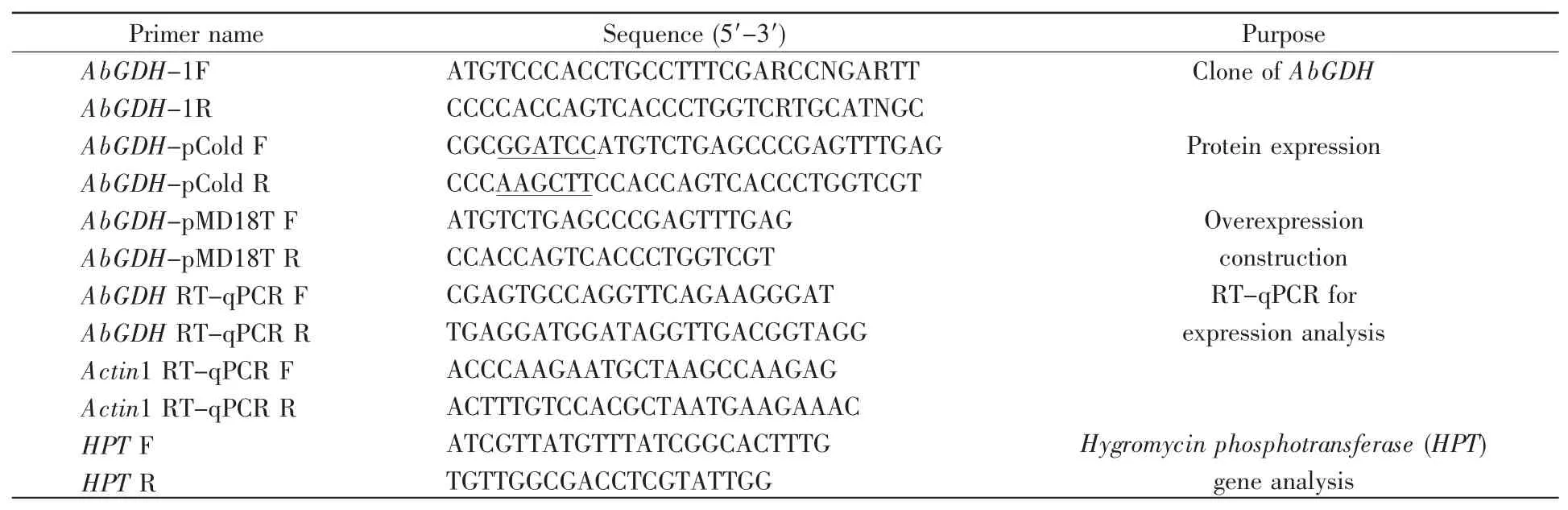
Table 1 Primers for PCR analysis
Immunoblotting analysis was performed according to the method described by Zhou et al[14].Purified protein His-TF-AbGDH was subjected to SDS-PAGE(12%V/V)and transferred to nitrocellulose membrane using ST-I transfer electrophoresis tank(Bio-Rad,USA).The membrane was incubated with an anti-His antibody(Abmart,China),and then probed with a secondary antibody HRP-IgG(Abmart,China).The absorbance was visualized using ChemiDoc XRS(Bio-Rad,USA).
1.6 Genetic transformation of rice and identification of transgenic plants
PCAMBIA1301GW-AbGDH was introduced into Oryza sativa L.cv.Kitaake via Agrobacteriummediated transformation.Genomic DNA was extracted by an extraction method with cetyltrimethylammonium bromide(CTAB)[30]from putative transgenic rice lines and control plants.The transgenic lines were analyzed by PCR amplification of the hygromycin phosphotransferase(HPT)gene with specific primers(Table 1)and hygromycin resistance assay as described by Zhou et al[28].To examine transgene expression,total RNA was extracted from both transgenic and control seedlings,and the cDNA was acquired through the RevertAid First Strand cDNA Synthesis Kit(Fermentas,USA)following the manu facturer’s instructions.Quantitative RT-PCR(qRTPCR)was used to investigate the expression of Ab-GDH by specific primers(Table 1).Rice actin1 was used as an internal control.
1.7 Enzyme activity assay
The NADP(H)-GDH activity of purified recombinant protein His-TF-AbGDH was determined by monitoring the change in absorbance at 340 nm with spectrophotometer according to Noor and Punekar[17].The aminating activity of recombinant protein was measured in a reaction mixture containing 100 mmol/L Tris-HCl,10 mmol/L NH4Cl,10 mmol/L 2-oxoglutarate and 0.1 mmol/L β-NADPH at pH 8.0.To determine the Kmvalue,varied concentrations of 2-oxoglutarate,NH4Cl or glutamate were used.The reduction or oxidation of 1 μmol/L of NADP(H)perminute per milligram recombinant protein at 25℃was defined as one unit of enzyme activity.Additionally,in order to analyze the NADP(H)-GDH activity in plants,soluble proteins were extracted from the roots of control and transgenic rice plants as described by Abiko et al[24],and their respective NADP(H)-GDH activities were measured as described above.
1.8 Subcellular localization analysis
For detection of the subcellular localization of AbGDH,the full length of AbGDH was amplified and subcloned into the pCAMBIA1300-YFP vector.The constructed plasmids were transformed into rice protoplasts as described previously by Zhou et al[31].The fluorescence was then observed with a confocal laser scanning microscopy(Olympus FV1000).
2 Results
2.1 Phylogenetic analysis of AbGDH
With the RT-PCR strategy and sequencing data,the full-length cDNA of NADP(H)-GDH was isolated from A.brassicicola and denoted as AbGDH.The open reading frame of AbGDH is 1 347 bp,encoding a 448-amino acid protein with a calculated molecular mass of 48.8 kD and a pI of 5.89.To study the relationship of NADP(H)-GDH amino acid sequences between A.brassicicola and other organisms(bacteria,fungi and higher plants),the phylogenetic tree was constructed by MEGA 5.0.As shown in Fig.1A,AbGDH has a low homology with NAD(P)(H)-GDHs from O.sativa and Arabidopsis thaliana,while it has a high homology with NADP(H)-GDHs from C.ehrenbergii,A.niger and Eurotium cheralieri.The gdhA from A.niger,CeGDH from C.ehrenbergii and EcGDH from E.cheralieri had previously been identified to enhance nitrogen utilization and increase grain yield in rice[24~25,32].Thus,it can reasonably beassumed that AbGDH played similar roles to CeGDH and EcGDH in improving nitrogen assimilation and grain yield in rice.Additionally,amino acid sequence alignment was performed between AbGDH and other NADP(H)-GDHs from C.ehrenbergii[25],E.coli(NP416275),A.thalia-na(At1g51720)and O.sativa(Os01g37760)using GENEDOC(Fig.1B).As expected,similar to other NADP(H)-GDHs,AbGDH contains the conserved NADP(H)and Glu/2-OG binding domains,indicating that it might regularly function as OsGDH(Os-01g37760)in the nitrogen metabolism in rice.
2.2 AbGDH shows a strong affinity for ammonium in vitro
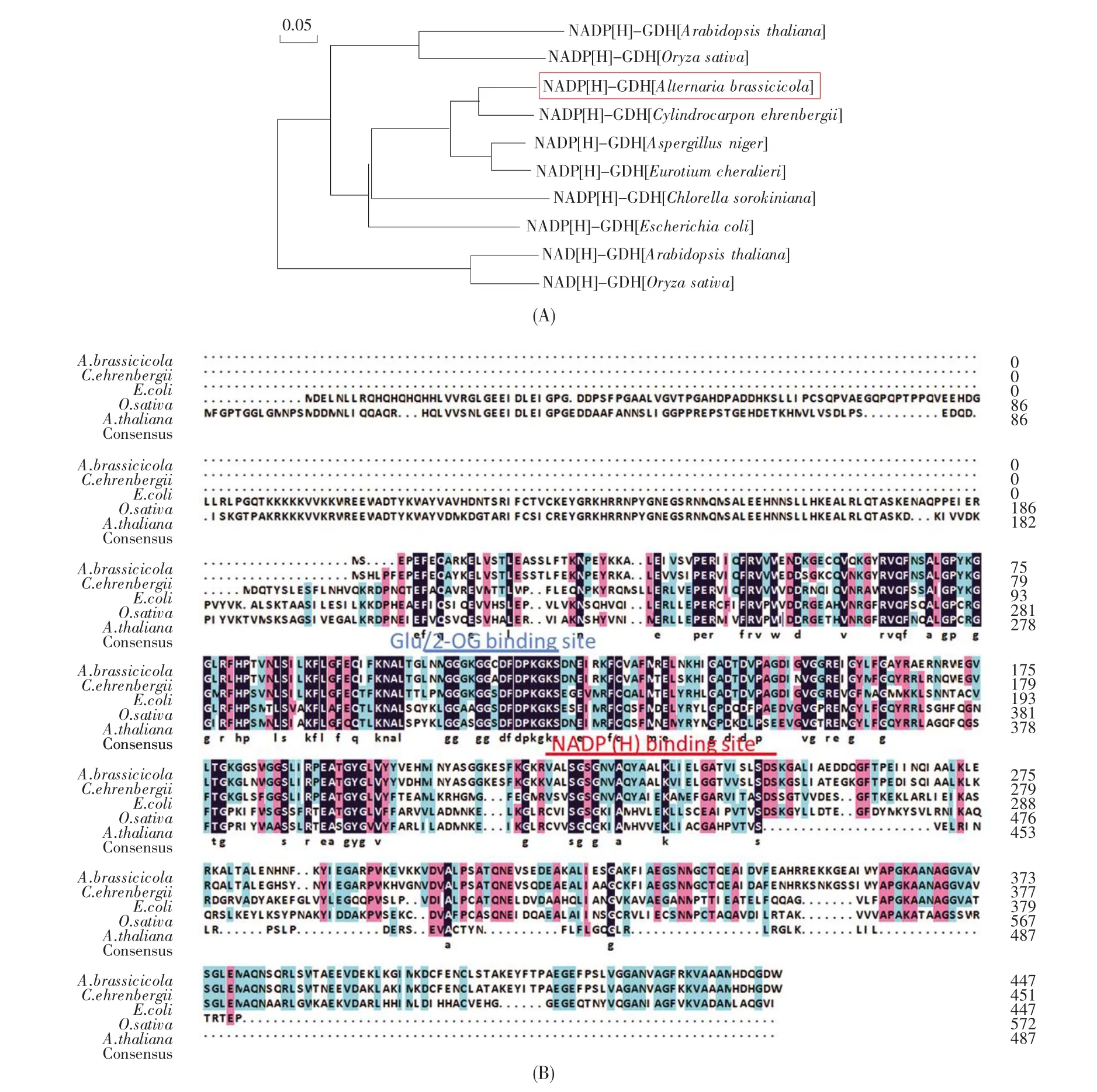
Fig.1 Phylogenetic relationship within bacterial,fungal,and higher plant GDH amino acid sequences(A)Phylogenetic tree of AbGDH.NADP(H)-GDHs from Escherichia coli(NP416275),Chlorella sorokiniana(CAA41635),Aspergillus niger(ACJ03788),Cylindrocarpon ehrenbergii[25],Eurotium cheralieri[32],Arabidopsis thaliana(At1g51720)and Oryza sativa(Os01g37760),and NAD(H)-GDHs from A.thaliana(At5g07440)and O.sativa(Os02g43470);(B)Protein sequence similarity of AbGDH and other representative GDHs from C.ehrenbergii[25],E.coli(NP416275),A.thaliana(At1g51720)and O.sativa(Os01g37760).The identical amino acids are indicated with black shading,and the similar ones are shaded with blue and red.The dashed lines indicate gaps.
To analyze the enzymatic property of AbGDH,the His-TF-tagged recombinant protein His-TFAbGDH was expressed in E.coli BL21(DE3)and purified(Fig.2A).A single polypeptide band consistent with the deduced molecular size of His-TFAbGDH(about 100 kD)was shown in SDS-PAGE.Then the immunoblotting analysis with an anti-His antibody further confirmed the purified recombinant protein His-TF-AbGDH(Fig.2A).Subsequently,to characterize the kinetic property of AbGDH,the Kmvalues of His-TF-AbGDH for glutamate(Fig.2B),NH4+(Fig.2C)and 2-OG(Fig.2D)were determined,respectively.The results demonstrated that the Kmvalues of AbGDH for NH4+(2.144±0.141 mmol/L)and 2-OG(2.690±0.233 mmol/L)were significantly lower than that for glutamate(96.772±0.542 mmol/L),implicating that the affinity of AbGDH for NH4+was markedly higher than that for glutamate.Zhou et al[14]previously reported that the Kmvalue of OsGDH for NH4+was 12.43±1.84 mmol/L,which was notably higher than that of AbGDH.So it was indicated that AbGDH had a predominantly stronger affinity for NH4+than OsGDH,which might make it possible for AbGDH to assimilate NH4+normally even at a very low ammonium concentration.These results showed that introduction of AbGDH into rice might enhance the nitrogen assimilation in transgenic rice.
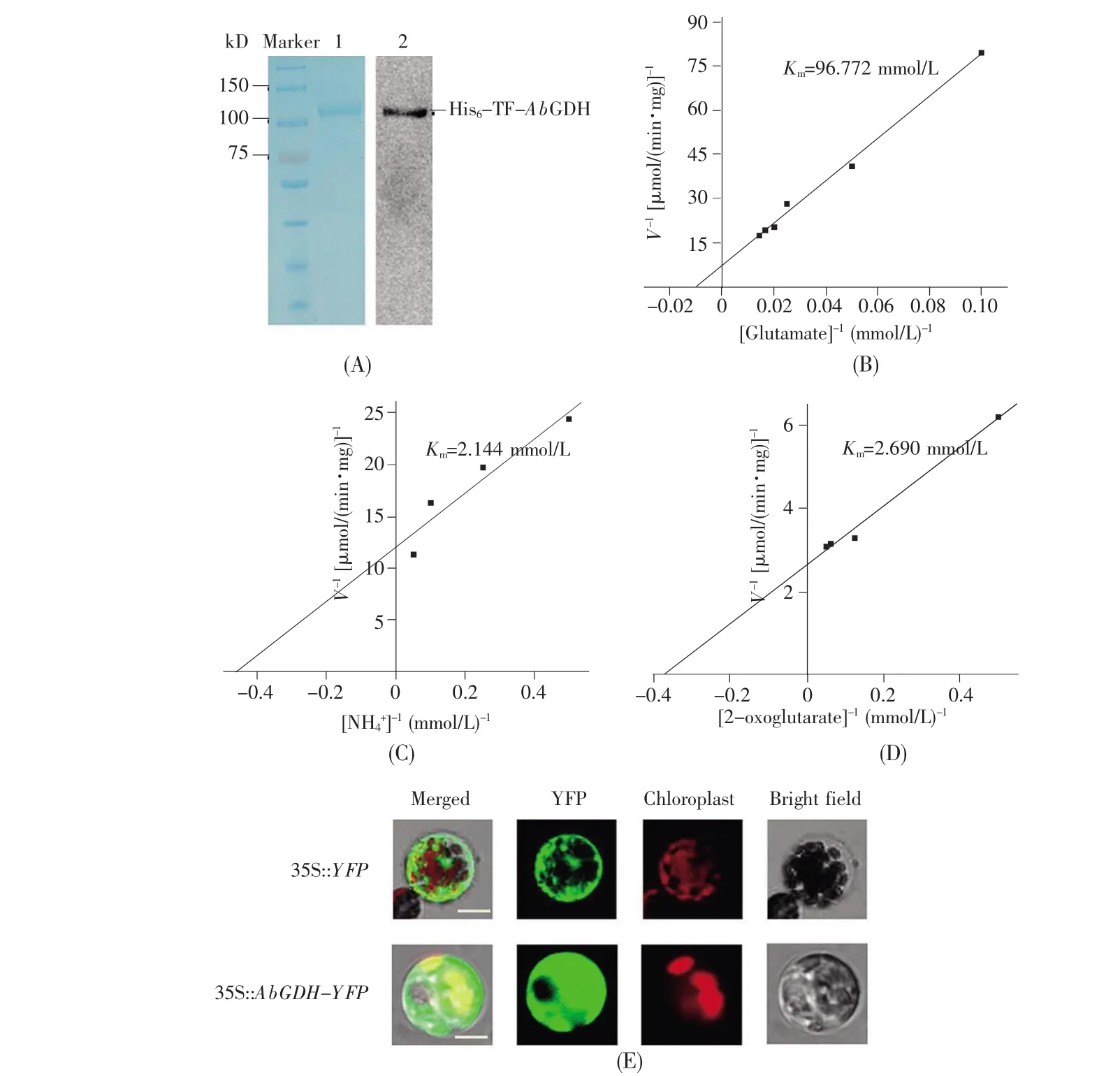
Fig.2 Determination of enzymological properties of AbGDH
To clarify where AbGDH was involved in the ammonium assimilation within a cell,the subcellular localization of AbGDH was analyzed.An AbGDHYFP fusion protein was expressed transiently in rice protoplasts under the control of CaMV35S promoter.With a confocal laser scanning microscopy,it was found that the signal of AbGDH-YFP predominantly located in the cytoplasm,while the signal of YFP alone as a control was widely distributed in the nucleus,plasma membrane and cytoplasm(Fig.2E).It had been reported that another important enzyme,GS protein,involved in the primary ammonium assimilation,was also found to localize in the cytoplasm of vascular bundles of rice leaves[32].Therefore,the introduced AbGDH is believed to mainly co-localize with GS and function in the cytoplasm where ammonium assimilation takes place.
2.3 Expression level and GDH activities of AbGDH transgenic plants
To study the impacts of AbGDH on rice ammonia use efficiency,the AbGDH was subcloned into an over-expression vector(Fig.3A),and subsequently overexpressed in Oryza sativa L.cv.Kitaake.As shown in Fig.3D,an obvious band of about 500 bp could be detected in the transgenic lines while this was not found in the control plants.Then the hygromycin resistance assay was used to further con firm the T-DNA copy number(Fig.3E).What’s more,segregation pattern of T1generation under hygromycin treatment showed that two transgenic lines,Ubi::AbGDH-16 and Ubi::AbGDH-42,carried a sin gle insertion of the heterologous AbGDH(Table 2).Subsequently,PCR and qRT-PCR analyses were conducted to detect the heterologous AbGDH and its transcription levels in transgenic and wild type(WT)plants,respectively.As expected,AbGDH was integrated into the genomic DNA(Fig.3C)and expressed successfully in transgenic plants(Fig.3B).Thus,the two transgenic lines,Ubi::AbGDH-16 and Ubi::AbGDH-42,which were predicted to have a single insertion of the heterologous AbGDH,were selected to perform the subsequent experiments.
To detect whether the heterologous AbGDH alteredthecapabilityofammonium assimilation in transgenic plants,the enzymatic activities of NADP(H)-GDH were measured.Compared with control plants,transgenic lines showed significantly increased aminating activities(Fig.4A).Meanwhile,the affinities for NH4+of total protein in transgenic plants were higher than that in control plants(Fig.4B).These results suggested that the constitutive expression of AbGDH might improve the ammonia assimilation in rice.
2.4 Expression of AbGDH in rice improves nitrogen use efficiency
To determine the nitrogen use efficiency of the transgenic plants,hydroponically grown experiments were performed.The transgenic seedlings grown in 200 μmol/L NH4+nutrient enhanced both plant height and dry weight,as compared with the control seedlings,while there were no obvious differences in 2 000 μmol/L NH4+nutrient between the trans genic and control seedlings(Fig.4C~F),indicating that the heterologous AbGDH could enhance the biomass production at lower concentrations of NH4+supplement in rice.Meanwhile,the nitrogen contents in transgenic plants were found to be much higher than that in control plants,regardless of low or high concentrations of NH4+supplement(Fig.4G).These results indicated that the introduction of the AbGDH could improve the nitrogen use efficiency in rice.
In order to evaluate the effects of over-expression of AbGDH in rice,the control and transgenic rice were grown on a paddy field supplemented with 112.5 kg N/hm2(in the form of urea)as normal nitrogen supply,and the agronomic traits were collected and evaluated at harvest.Similar to the seedlings atthe higher concentration of NH4+supplement,there was no statistically significant difference between the control and transgenic plants in tillering,setting,1 000-seed weight and grain yield under normal nitrogen supply(Fig.4H~K).Similarly,ectopic expression of another fungal GDH gene,CeGDH,derived from C.ehrenbergii in rice had been reported not to affect the grain yield at 112.5 kg N/hm2nitrogen level,but markedly increase the grain yield at 37.5 kg N/hm2nitrogen level[25].It is not certain whether the gene AbGDH could improve the grain yield at lower nitrogen supply like CeGDH.A further investigation should be performed at a paddy field supplemented with 37.5 kg N/hm2in the future.
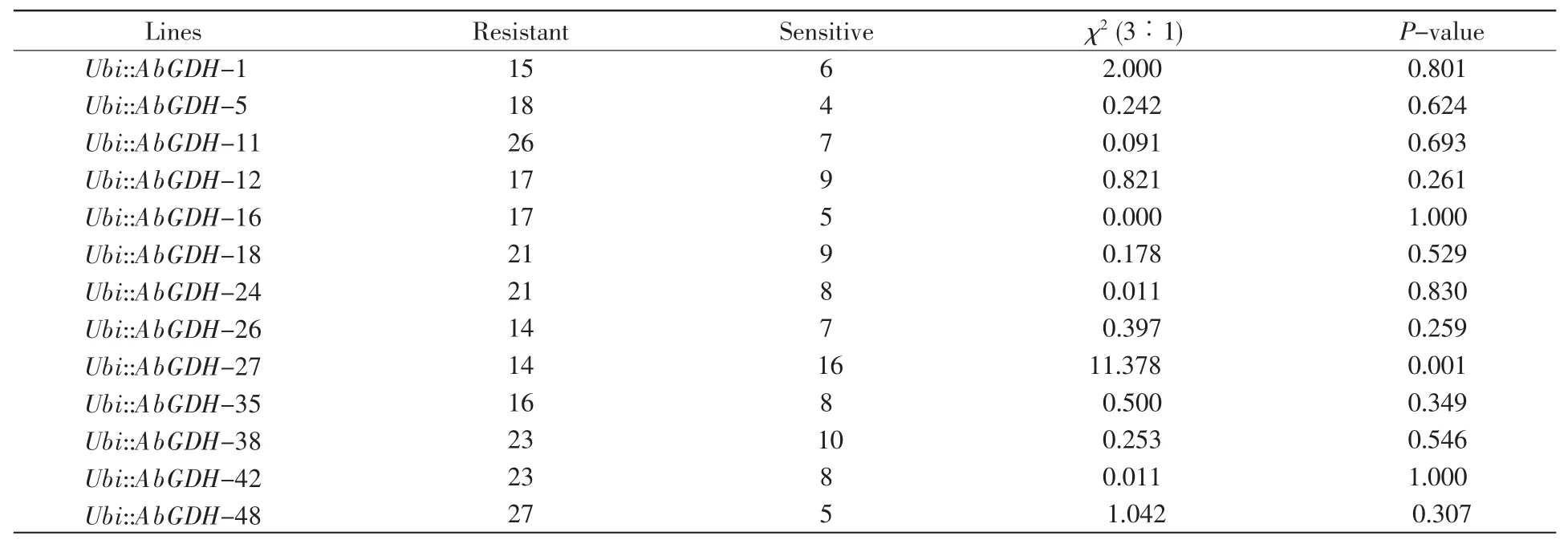
Table 2 Segregation pattern of T1generation under hygromycin treatment
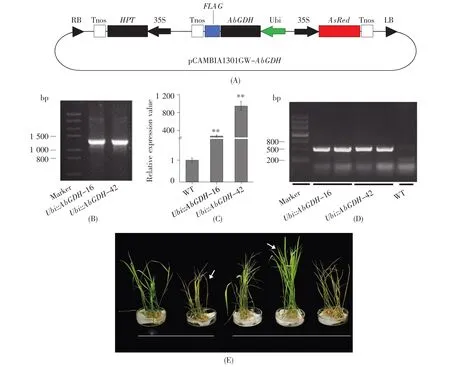
Fig.3 Characteristics of transgenic rice over-expressing the AbGDH gene
3 Discussion
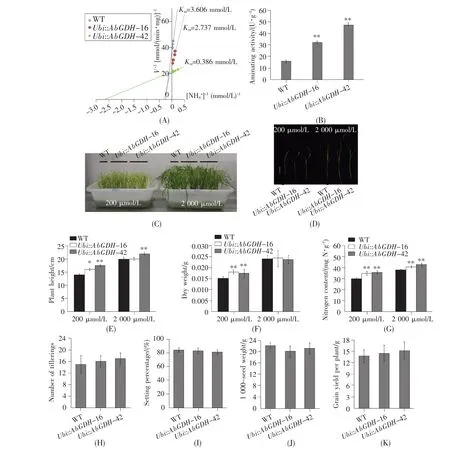
Fig.4 Aminating activity and nitrogen use efficiency of the transgenic plants under different nitrogen concentrations and agronomic traits in field
GDH is a family of enzymes catalyzing reversible deamination of L-glutamate to 2-OG,directly connected to the tricarboxylic acid(TCA)cycle[33].This enzyme family has drawn much attention because assimilation of ammonium via GDH is more energy-efficient than via GS/GOGAT pathway[21~22].Presently,several NADP(H)-GDHs from lower organisms were introduced into plants,and the capacities of ammonium assimilation had been enhanced significantly in transgenic plants[17,24~25,32,34].Phylogenetic analysis of AbGDH showed that AbGDH had a high homology with NADPH-GDHs from A.niger and C.ehrenbergii but a low homology with those from O.sativa and A.thaliana(Fig.1),which indicated that AbGDH might function like gdhA and CeGDH to improve nitrogen assimilation[24~25].Previous studies showed that,compared with lower organisms,GDHs with a quite higher Kmvalue(5.2~70 mmol/L)for NH4+in higher plants could only assimilate NH4+into glutamate at a high NH4+concentration[8,35].Kinetic analysis of AbGDH demonstrated that its Kmvalue for NH4+(2.144±0.141 mmol/L)(Fig.2C)was markedly lower than that of OsGDH(12.43±1.84 mmol/L)[34],indicating that the affinity of AbGDH for ammonium was predominantly higher than that of OsGDH.Collectively,these results suggested that AbGDH could assimilate NH4+normally even at a very low ammonium concentration and has the potential to improve nitrogen uptake efficiency in crop plants.
Owing to the higher affinity for ammonium and more energy-efficiency in ammonium assimilation,several GDHs from lower organisms have been introduced into plants for enhancement of nitrogen uptake efficiency,growth quality and biomass production[23~25,32,36].For example,the transgenic rice plants carrying a gdhA gene from A.niger increased the dry weight,nitrogen content and grain yields under high nitrogen conditions[24].Whereas,the transgenic rice plants carrying CeGDH from C.ehrenbergii enhanced nitrogen utilization,improving growth and grain yields even under low nitrogen conditions[25].In the present study,the nitrogen uptake efficiency was also enhanced in AbGDH transgenic plants,and the growth was promoted at the seedling stage under lower nitrogen conditions(200 μmol/L NH4+),but no obvious difference under higher nitrogen conditions(2 000 μmol/L NH4+)(Fig.4C~G).These results indicated that the introduction of AbGDH could improve the nitrogen use efficiency of rice.
To date,several fungal GDH genes have been introduced into rice cultivars for improving nitrogen assimilation and growth quality[18,24~25,28,32],but only CeGDH and EcGDH can increase the grain yield of transgenic rice under low nitrogen conditions[25,37].Although Zhang et al[38]reported that the gdhA from A.niger could improve the grain yield of a forage rice under low nitrogen conditions,the grain yield of transgenic food rice was found to increase only under high nitrogen conditions and not in low nitrogen fertilizer when the gdhA was introduced into a food rice cultivar[24].In field tests,both CeGDH and Ec-GDH transgenic rice plants exhibited a significant increase of grain yields at 37.5 kg N/hm2but not at 112.5 kg N/hm2nitrogen level[25,32].Similarly,our present results showed that there was no statistically significant difference between the control and Ab-GDH transgenic plants in grain yields under normal nitrogen supply(112.5 kg N/hm2)(Fig.4H~K).Whereas,a further field test should be performed at a paddy field supplemented with lower nitrogen fertilizer such as 37.5 kg N/hm2in the future to investigate whether AbGDH transgenic rice has the same ability as CeGDH and EcGDH ones to improve the grain yield at lower nitrogen supply.
In conclusion,ectopic expression of AbGDH from A.brassicicola can enhance the nitrogen use efficiency and improve the biomass production at seedling stage in rice.
Acknowledgements:
Thanks Dr.Hong Liu for providing the fungus A.brassicicola and assisting in cloning AbGDH(Fujian Agriculture and Forestry University,Fuzhou,China).
