Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
2019-05-10MaShoutaoShangXianyongLiLuminXueChangyongLiJieSunLanyi
Ma Shoutao; Shang Xianyong; Li Lumin; Xue Changyong; Li Jie; Sun Lanyi
(1. State Key Laboratory of Heavy Oil Processing, College of Chemical Engineering,
China University of Petroleum (East China), Qingdao 266580;
2. School of Resources and Chemical Engineering, Sanming University, Sanming 365004)
Abstract: In this work, the process simulation of pressure-swing distillation (PSD) and extractive distillation (ED) using ionic liquid (IL) 1-butyl-3-methylimidazolium acetate ([bmim][OAc]) as the entrainer for separation of ethyl acetateethanol-water mixture is performed. The design parameters of the two distillation processes are optimized with the minimum total annual cost (TAC) serving as the objective function. The results show that the TAC saving of ED process is 35.27% in comparison with that of PSD process in the case of achieving the same purity and yield of ethyl acetate.In addition, the dynamic controllability of ED process is further studied. The traditional two-point temperature control structure is proposed for the ED process, and it works pretty well while taking into account the disturbances in both feed rate and feed composition.
Key words: ionic liquids; extractive distillation; simulation; process control
1 Introduction
Ethyl acetate is an important chemical solvent with excellent solubility, which is commonly used in coatings,synthetic fibers and other production processes[1].At present, ethyl acetate is prepared by reactive distillation method using ethanol and acetic acid as the raw materials[2]. If reactive distillation is the only alternative, high purity ethyl acetate cannot be obtained due to the formation of azeotrope composed of ethyl acetate, ethanol, and water. Therefore, some special distillation processes, such as azeotropic distillation(AD)[3], extractive distillation (ED)[4]and pressure-swing distillation (PSD)[5], have been used to solve this problem.PSD takes advantage of its characteristic that the composition of azeotrope has a significant shift with pressure to achieve separation, and then it is applicable to the azeotropic mixture that is sensitive to pressure. PSD is used in the literature[6]for the separation of ethyl acetateethanol-water ternary azeotrope since the azeotropic composition is sensitive to pressure. ED as a method for separating azeotrope has also received widespread attention. Luyben[7]made a comparison of ED and PSD for the separation of acetone and methanol. The results indicated that ED process had lower costs compared with PSD process. Zhang, et al.[8]employed five hydrophilic ILs as entrainers for the separation of ethyl acetateethanol-water ternary azeotrope, which showed that the hydrophilic ILs 1-allyl-3-methylimidazolium chloride([Amim]Cl) and bromide ([Amim]Br) had the highest extraction efficiency and the purity of ethyl acetate could reach 99.27%. However, the addition and recovery of entrainer will undoubtedly increase the cost of ED process. Hosgor, et al.[9]compared the two processes(ED and PSD) for separating the methanol-chloroform mixture and explored the controllability of PSD process.It is found that the PSD process was significantly more economical than the homogeneous ED process. Therefore,the selection of separation method is determined by the properties of the azeotropic system. As for the ethyl acetate-ethanol-water ternary azeotrope, it is meaningful to study whether PSD or ED is better for the separation.So these two processes are investigated in this work,and the design variables of PSD and ED are optimized with a minimum TAC serving as the objective function in the case of achieving the same purity and yield of ethyl acetate. It is worth mentioning that the selection of entrainer is one of the key factors to ensure the required separation performance and energy saving. Traditional organic solvents have disadvantages related with volatilization, pollution and high energy consumption,while ILs have outstanding physicochemical properties covering low vapor pressure, low melting point, and high chemical and thermal stability[10]. Therefore, ED with ILs serving as solvents has become a promising alternative method for the separation of azeotropic mixture, such as ethanol dehydration and extractive desulfurization[11].
In this paper, 1-butyl-3-methylimidazolium acetate([bmim][OAc])[12]is used as the entrainer for the ED of ethyl acetate-ethanol-water ternary mixture. In order to explore the best separation scheme, TAC of the ED process is calculated and compared with that of the PSD process which is also used for the separation of ethyl acetate-ethanol-water mixture. The feeding conditions for the two distillation processes are taken from Yang’s work[6]. In addition, the dynamic controllability of the ED process is further studied. The steady-state and dynamic simulations of ED process with ILs used as the entrainer can provide a reference for the separation of ethyl acetateethanol-water mixture in the industry.
2 Process Simulation and Optimization
Based on the literature report[6], the azeotropic composition of ethyl acetate-ethanol-water under atmospheric pressure with (w(ethyl acetate) of 0.7769,w(ethanol) of 0.1404, and w(water) of 0.0827) is selected as the feed composition for the PSD and ED processes.The mass fraction of ethyl acetate should be no less than 0.999 and its recovery ratio must be more than 0.9999.The purity of ethanol is 0.90 (mass fraction) with its recovery ratio of 0.98. The thermodynamic model NRTL is used to describe the non-ideality of the liquid phase,while the vapor is assumed to be ideal in the PSD and ED processes.
TAC is a key indicator to evaluate the economic performance of chemical processes. According to Douglas’s report[13], TAC includes the operating cost(OC) and the capital cost (CC), with the main cost estimation formulas and design parameters presented in the literature. In this work, the payback period is assumed to be three years with an operating time of 8000 h/year.
2.1 Optimization of PSD process
The azeotropic composition of ternary mixtures under different pressure is obtained from the literature[6]. The NRTL model is adopted for the PSD simulation in this work. Since the components of ethyl acetate, ethanol and water have been included in the component database of process simulators such as Aspen Plus, the binary interaction parameters are obtained as fix values from APV VLE-IG in the Aspen Plus database. The PSD process is designed and shown in Figure 1. The ternary mixture is fed into the high-pressure distillation column(B1) and ethyl acetate is obtained at the bottom of B1.The product at the top of B1 is fed into the atmospheric column (B2) and the ethyl acetate-ethanol-water mixture at the top of B2 is mixed with the feed stream prior to being fed into B1. The ethanol-water mixture is fed into the third distillation column (B3). 90% of ethanol and 96% of water are obtained at the top and bottom of B3,respectively.
According to the simulation results of PSD process for separation of the ethyl acetate-ethanol-water mixture[6],the initial value of design parameters are established.There are multiple design variables in the PSD process,such as the reflux mass ratio (RR), the number of stages(Nstage), the feed stage (Nfeed), etc. The design specifications are added in order to ensure the purity and recovery ratio of products. The optimization of the separation process is implemented based on the sequential iterative optimization procedure. Nfeedis used as the inner loop,while Nstageis used as the outer loop, and TAC under different operating parameters is obtained thereby.
Figure 1 shows the optimization results of PSD process for separation of the ethyl acetate-ethanol-water ternary mixture. The high-pressure column B1 is operated at 5 bar, and the column B2 is operated under atmospheric pressure. The minimum TAC is 37.187×104$, which includes a CC of 42.141×104$ and an OC of 23.140×104$.The purity and recovery ratio of product streamis maintained at the desired value.
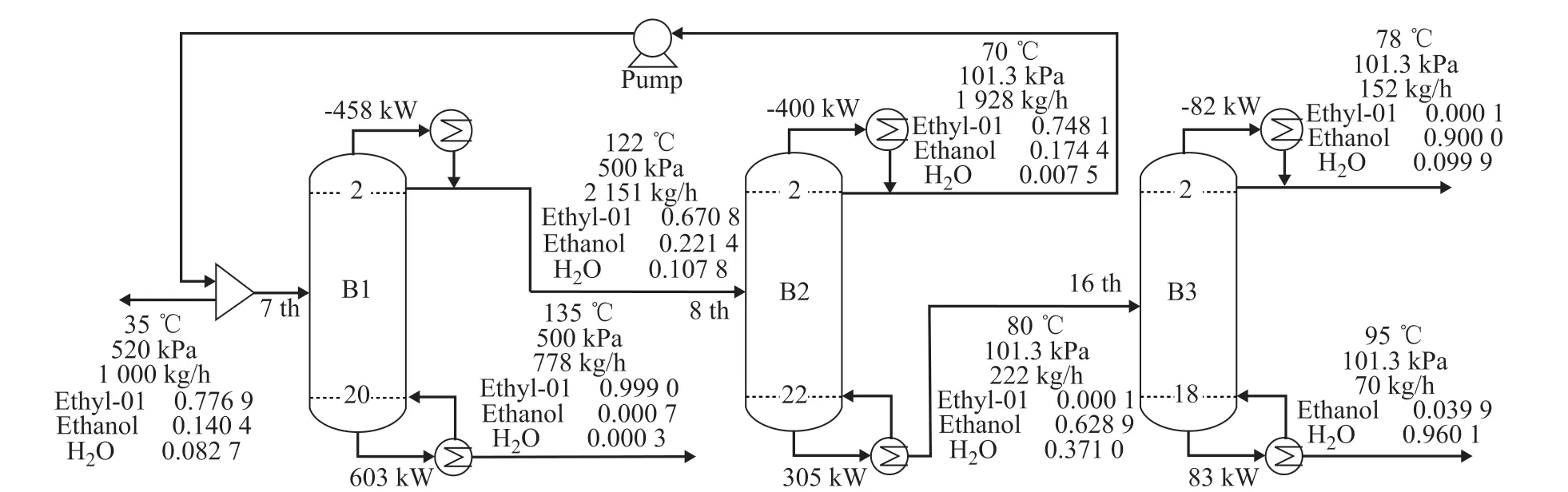
Figure 1 The PSD process for the separation of ethyl acetate-ethanol-water mixture
2.2 Optimization of ED process
In this section, the ED process, which uses [bmim][OAc]as the entrainer, is performed for the separation of ethyl acetate-ethanol-water ternary mixture. The NRTL binary interaction parameters for ethyl acetate-[bmim][OAc],ethanol-[bmim][OAc], and water-[bmim][OAc], which are taken from Zhang’s work[12]for all the species involved in this study, are used for the ED process simulation by Aspen Plus. The binary interaction parameters of ethyl acetateethanol, ethyl acetate-water, and ethanol-water are fixed as values retrieved from APV84 VLE-IG in the Aspen Plus database. Besides, some thermodynamic properties, such as critical properties and boiling points, are predicted and implemented into process simulation in Aspen Plus[14]. As regards [bmim][OAc], which is selected as the entrainer, the critical properties (Tc, Pcand Vc), the normal boiling temperature (Tb), and the eccentric factor (ω) are calculated and shown in Table 1.
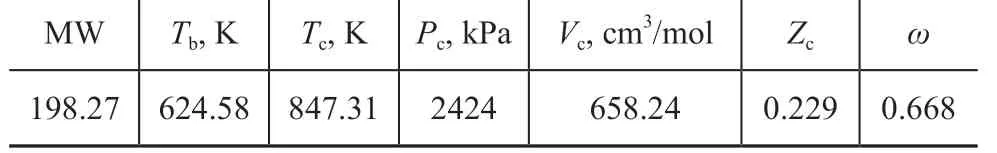
Table 1 The parameters of [bmim][OAc]calculated byliterature[14]
As shown in Figure 2, the ethyl acetate-ethanol-water ternary azeotrope is fed into the bottom of ED column T1, while [bmim][OAc]is fed into the top of column T1.Ethyl acetate is obtained at the top of column T1, and the mixture of ethyl acetate-ethanol-water-[bmim][OAc]is fed into the flash tank. The entrainer [bmim][OAc]can be obtained and recycled at the bottom of flash tank since it is much less volatile than either ethyl acetate or ethanol.The ethanol-water mixture is fed into the distillation column T2 after heat exchange, and ethanol can be obtained at the top of column T2.
The operating parameters of column T2 are not optimized since these parameters are obtained in the PSD process.There are also many design variables in the ED process,such as RR, the number of stages of T1 (NT1stage), the feed stage of T1 (NT1feed), the solvent feed stage (Nsolvent),the solvent-to-feed mass ratio (S/F), etc. Similarly to the PSD process optimization, the design specifications are also added to ensure the same purity and recovery ratio of products. The ED process is optimized in order to obtain the minimum TAC, and the optimization procedure is shown in Figure 3, in which NT1feedand Nsolventare used as the inner loop, and S/F is used as the outer loop.
2.2.1 Optimization of ED column T1
The design specifications are added, and RR is adjusted to ensure the purity and recovery ratio of ethyl acetate at the top of ED column T1. NT1feedand Nsolventare obtained by changing RR and the amount of product at the top of T1.TAC is calculated and shown in Figure 4. It can be seen that when NT1satgeis 23, Nsolventis 5 and NT1feedis 14, then TAC is a minimum.
2.2.2 Optimization of S/F ratio
S/F ratio (mass ratio) is optimized when the operating parameters of ED column T1 are obtained. The design specifications are added, and also RR and the amount of product at the top of T1 are changed in order to ensure the purity and the recovery ratio of ethyl acetate. TAC is calculated by changing the S/F ratio (mass ratio), when NT1stage, NT1feedand Nsolventare fixed. As shown in Figure 5,TAC is a minimum when the S/F value (mass ratio) is 0.62.
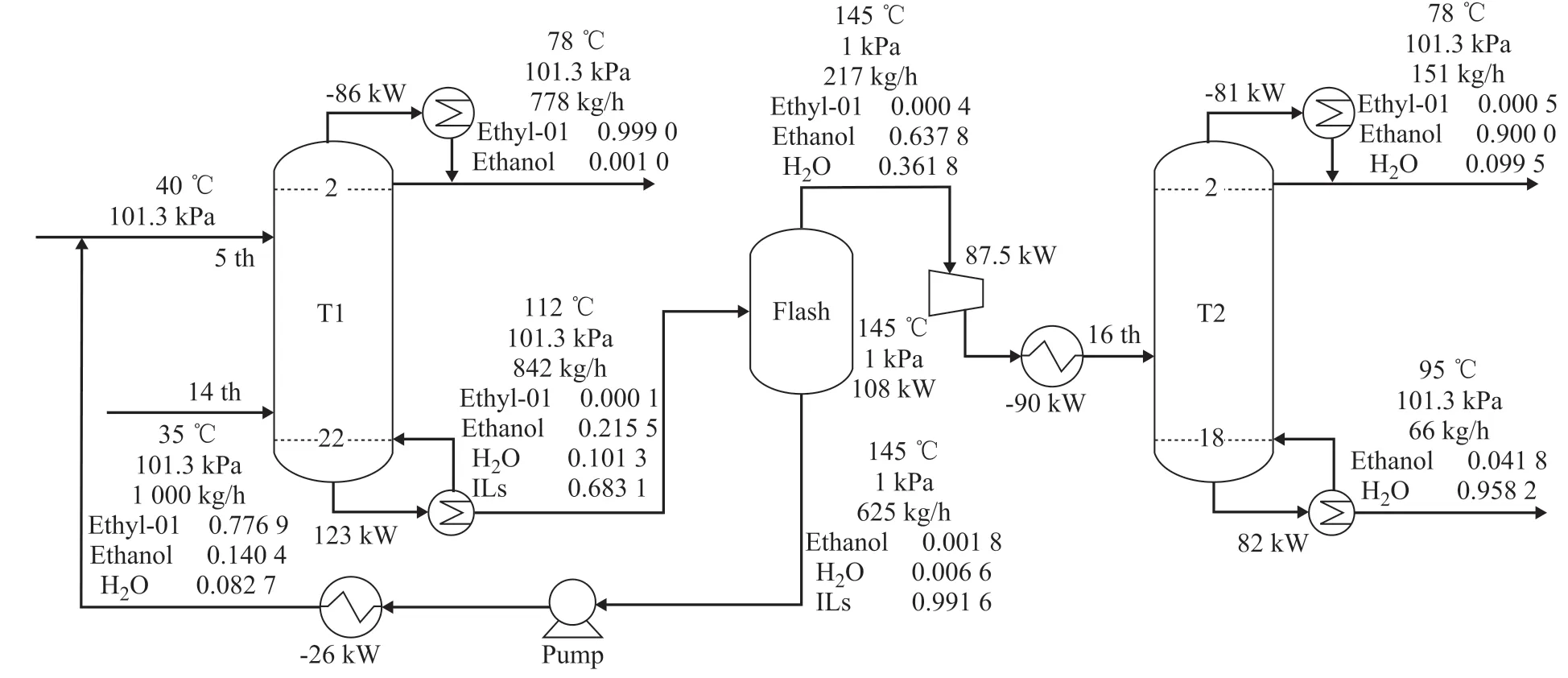
Figure 2 The ED process using [bmim][OAc]as entrainer
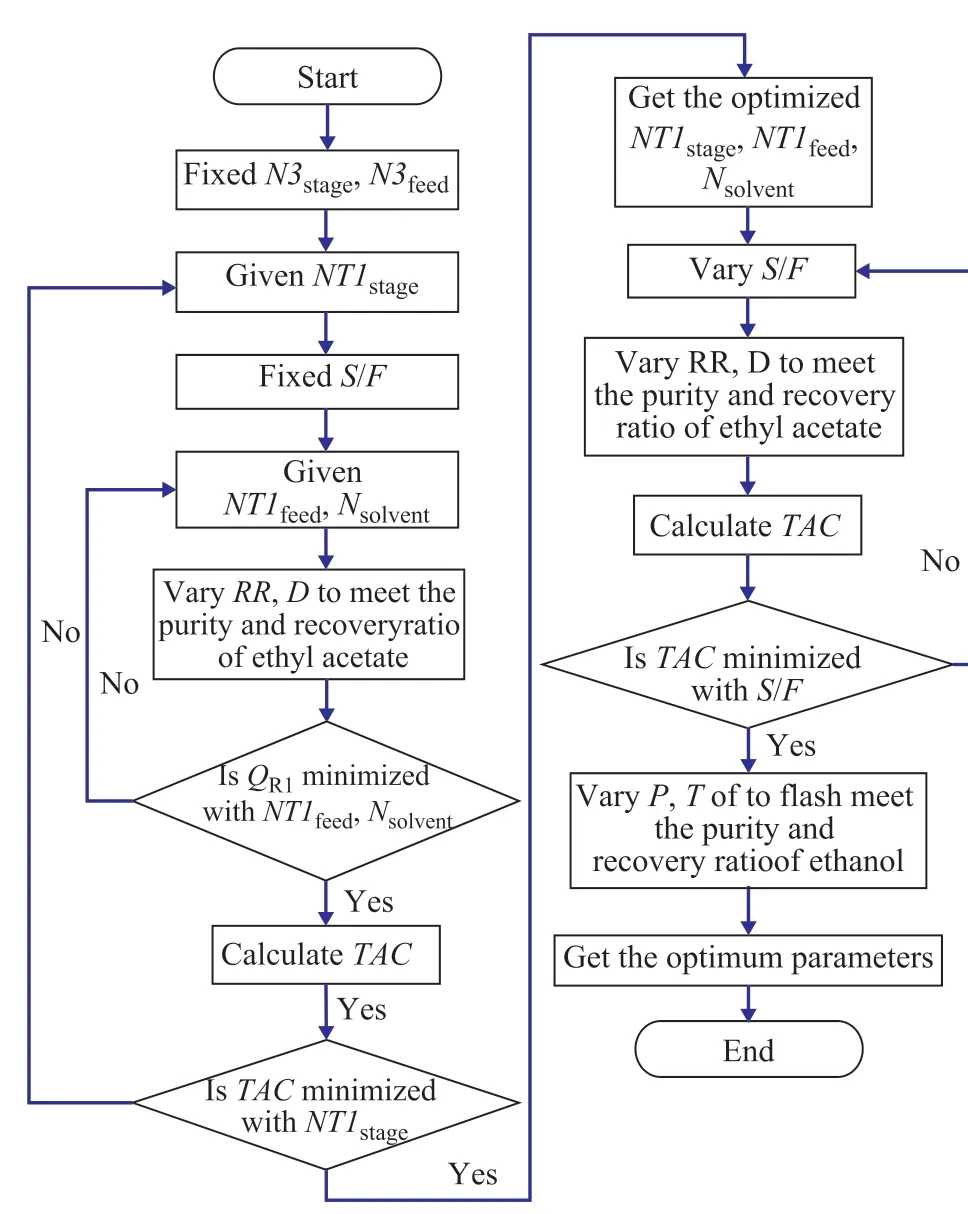
Figure 3 Sequential iterative optimization procedure for ED process
2.2.3 Optimization of flash tank
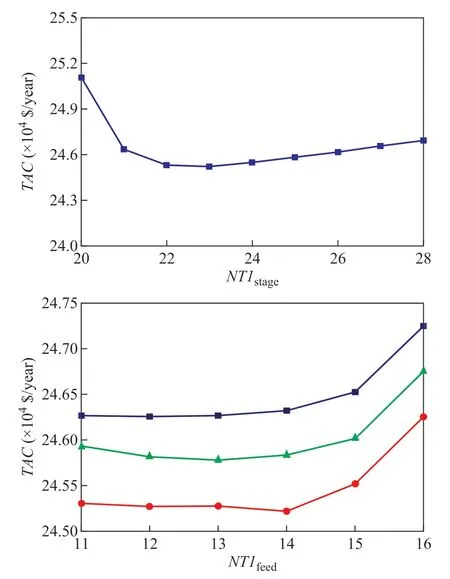
Figure 4 TAC versus the number of stages and feed stage of ED column T1■—Nsolvent=4; ●—Nsolvent=5; ▲—Nsolvent=6
The operating conditions of flash tank can directly affect the recovery ratio of [bmim][OAc]. Figure 6 shows the relationship between the temperature of flash tank (Tflash),the heat duty of flash (Qflash), and the purity of [bmim][OAc].It can be seen that the purity of [bmim][OAc]and Qflashincrease with an increasing Tflash. Therefore, Tflashshould be higher than 140 C to ensure the purity of [bmim][OAc] and make the energy consumption as low as possible.
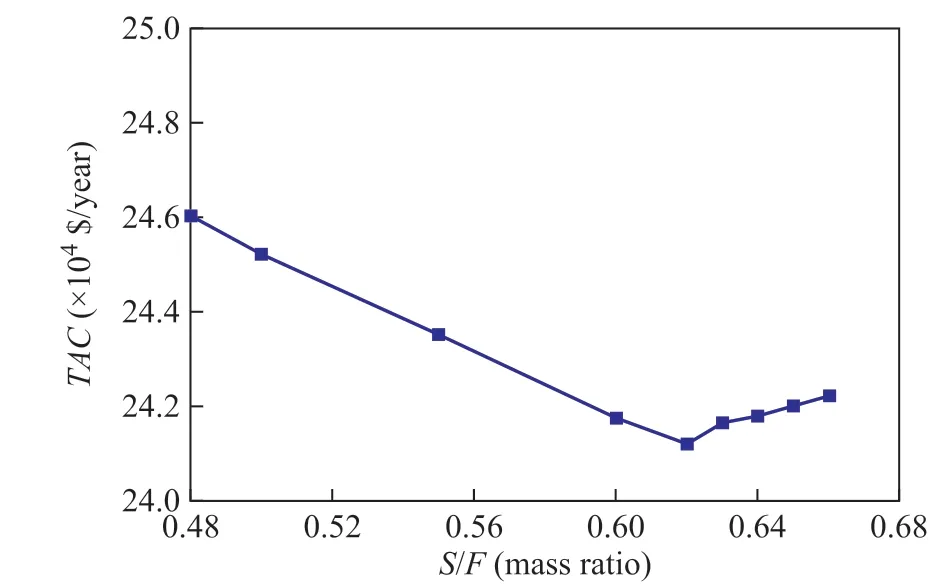
Figure 5 TAC versus solvent to feed mass ratio (S/F)

Figure 6 The relationship between the Qflash, Tflash and massfraction of [bmim][OAc]
Figure 7 shows the relationship between the pressure of flash tank (Pflash), the purity of [bmim][OAc]and Qflash. It can be seen that Pflashhas an insignificant effect on Qflash,while Pflashhas a significant effect on the purity of [bmim][OAc]. Therefore, Pflashis set at 1 kPa in order to ensure the purity of [bmim][OAc].

Figure 7 The relationship between Qflash, Pflash and massfraction of [bmim][OAc]
Doherty and Malone[15]gave a guideline on the appropriate temperature of entrainer before entering ED column,specifying that the temperature of entrainer should be by 5—10 C lower than that of the distillate. Therefore, the temperature of the recycled entrainer is set to be 67 C,which is by 10 C lower than the temperature at the top of ED column. In addition, a one-stage ejector is used since the flash tank is operated under low pressure in the stage of entrainer recovery. The formula for calculating the cost of vacuum is obtained from the literature[15]. The operating parameters of ED process with a minimum TAC are shown in Figure 2.
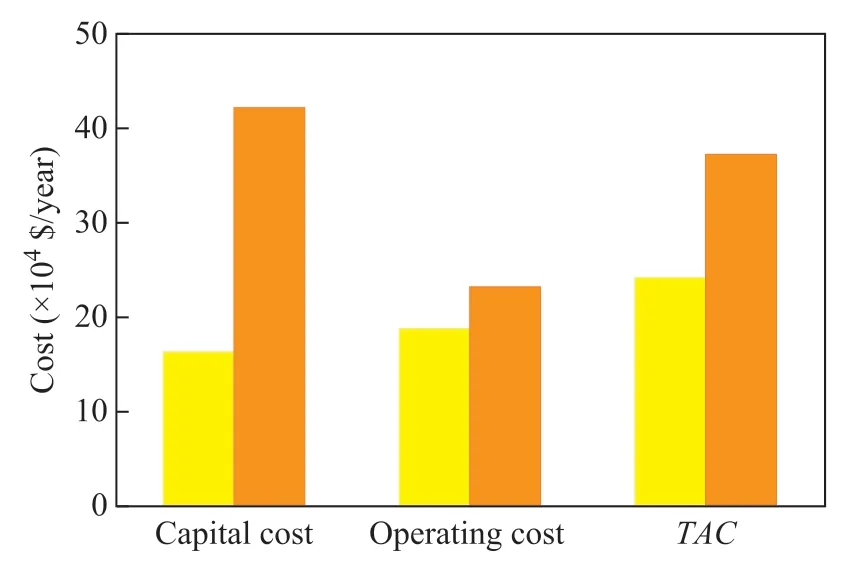
Figure 8 Comparison of TAC between PSD process and ED process■—ED-ILs; ■—PSD
2.3 Comparison of PSD and ED processes
Figure 8 shows the TAC values of the PSD and ED processes in the case of achieving the same purity and yield of products. It is found that the CC and OC of ED process are reduced by 62.16% and 18.95%, respectively,as compared with those of PSD process. TAC of ED process is reduced by 35.27% as compared with that of PSD process. Judging from the perspective of CC,there is one more distillation column in the PSD process in comparison with the ED process. So the energy consumption of the condenser and reboiler of PSD process is greater than that of ED process, which leads to the increase of TAC in the PSD process. As for the OC of the two processes, although the energy consumption of the PSD process is greater than that of the ED process, the cost of vacuum in the ED process also makes up a large portion of OC, making the advantages of OC less obvious than that of CC. However, the OC of ED process is still lower than that of the PSD process. The comprehensive analysis indicates that the ED process using [bmim][OAc]as the entrainer is more economical than the PSD process for separation of the ethyl acetate-ethanol-water mixture.
3 Control System Design
The process with optimal design parameters may not have good controllability. Despite the success in study on the dynamic characteristics of the process, problems in the course of operation will be found[16]. Ideally, the design and operability are highly related objectives, they should be considered together to provide a best possible operational process design. However, it is a challenge to consider both economic and operational design. Therefore, it is important to run a posteriori operability analysis so that the designer can gain an insight on potential controllability problems.This is precisely the purpose of the effort, which is associated with the study on dynamic control. This section provides a dynamic control study on the process of ED by using ILs as the entrainer because of its lower TAC as compared with that of the PSD process, and it can provide a reference for the industrial application of ILs used as the entrainer for the separation of ethyl acetate-ethanol-water ternary azeotrope.
Upon considering the wide use of temperature controllers in chemical industry, a temperature control scheme is adopted in the ED process. The sensitive stage selection of ED column is firstly carried out. Based on the openloop sensitivity analysis shown in Figure 9, when RR and the reboiler duty of ED column (QED) show a change of ± 0.1%, the temperature of stage 6 and stage 18 in ED column are efficiently influenced. Therefore,the temperature of stage 6 is controlled by RR and the temperature of stage 18 is controlled by QR. Since the temperature distribution of the ethanol-water separation column is gentle, the temperature of the first stage and the temperature of the last stage are controlled by RR and QR,respectively.
Appropriate valves and pumps are added in the steadystate simulation. There are 5-min liquid holdups for the reflux tank, sump, and flash tank, when the liquid accounts for a half volume of the vessel[17]. After adding the necessary valves and pumps, the whole steady-state simulation is exported into the Aspen Dynamics.
All the flow loops are conducted by PI controllers with the normal settings covering: KC= 0.5, and τI= 0.3 min,
while P-only controllers are used in all level loops.with KCbeing equal to 2 and τIequating to 9 999 min.All pressure controllers are proportional-integral withKC=20 and τI=12 min. In addition, the relay feedback test is used to calculate the gain and integration time of the temperature controller, and one minute of dead time is adopted in order to compensate for the delay in temperature response.
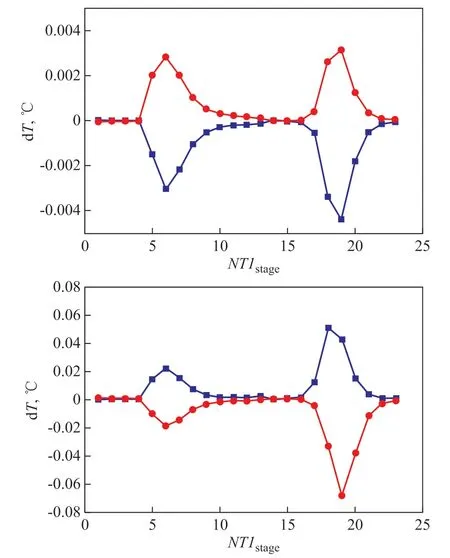
Figure 9 Temperature gains of different stages with ±0.1%change of RR and QR in ED column(a)■—RR+0.1%; ●—RR-0.1%; (b)■—QR+0.1%; ●—QR-0.1%
3.1 Control structure
3.1.1 Control structure of ED column
The pressure at the top of ED column is controlled by the duty of condenser (reverse acting). The level of reflux tank is controlled by the distillate (direct acting).The level of column is controlled by the bottom stream(direct acting). The temperature of stage 6 in ED column(TED6) is controlled by adjusting the reflux rate (direct acting), which can ensure the purity of ethyl acetate.The temperature of stage 18 in ED column (TED18) is controlled by manipulating the heat input of the reboiler in ED column (reverse acting), which can ensure the ratio of ethyl acetate recovered. In order to avoid the dry-plate phenomenon caused by the reduction of reflux rate, a high selector is added and set to a minimum value (one-half of the reflux rate), which can ensure that ED column could maintain a certain amount of reflux rate.
In addition, the temperature of the entrainer is directly related to the result of extraction. Therefore, the temperature of the recycled entrainer is controlled by the duty of the cooler (reverse acting). A ratio control of the feed flow rate to entrainer is used to meet the amount of entrainer needed in the ED process, when the flow rate of entrainer changes.
3.1.2 Control structure of flash tank
The temperature and pressure of the flash tank need to be controlled since they are directly related to the effect of entrainer recovery. The pressure is controlled by the flow rate of vapor product at the top of flash tank (direct acting). The temperature is controlled by manipulating the heat input of flash tank (reverse action).
3.1.3 Control structure of ethanol-water separation column
The pressure at the top of column is controlled by the duty of condenser (reverse acting). The level of reflux tank is controlled by the product flow rate at the top of column (direct acting). The bottom level of column is controlled by the flow rate of liquid product at the bottom of column (direct acting).The temperature of stage 2 in T2 column (T22) is controlled by adjusting the reflux rate (direct acting), which can ensure the purity of ethanol. The temperature of stage 18 in T2 column (T218) is controlled by manipulating the heat input of the reboiler in the column (reverse acting), which can ensure the ratio of ethanol recovery.
The control structure of ED process is established and is shown in Figure 10. After that, ±10% of feed flow rate and ±10% of ethyl acetate composition disturbances are introduced in the control structure of ED process to evaluate the effectiveness.
3.2 Control results
In this section, the ±10% of feed flow rate disturbances are applied to the ED system after 0.5 h of steady operation in order to test the performance of control structure, with the control results shown in Figure 11.
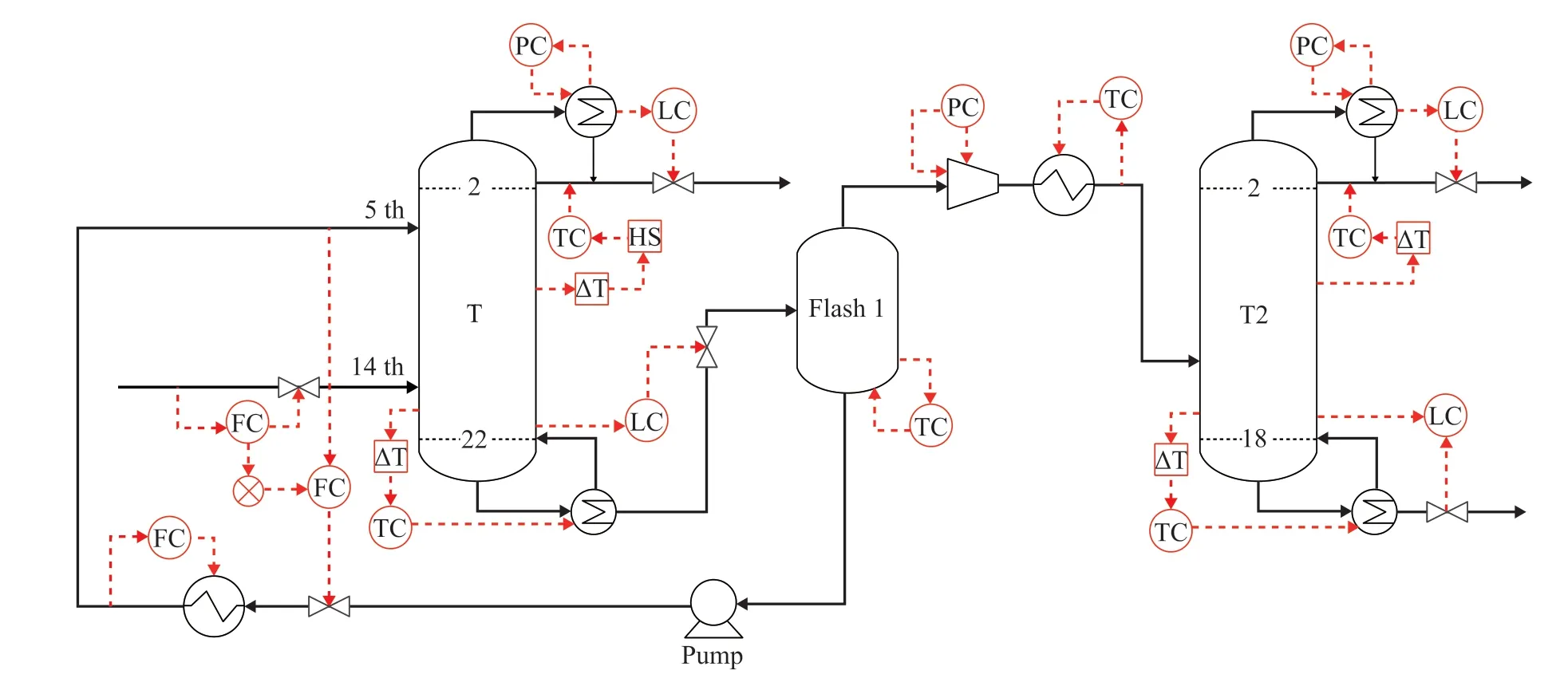
Figure 10 Control structure of ED process
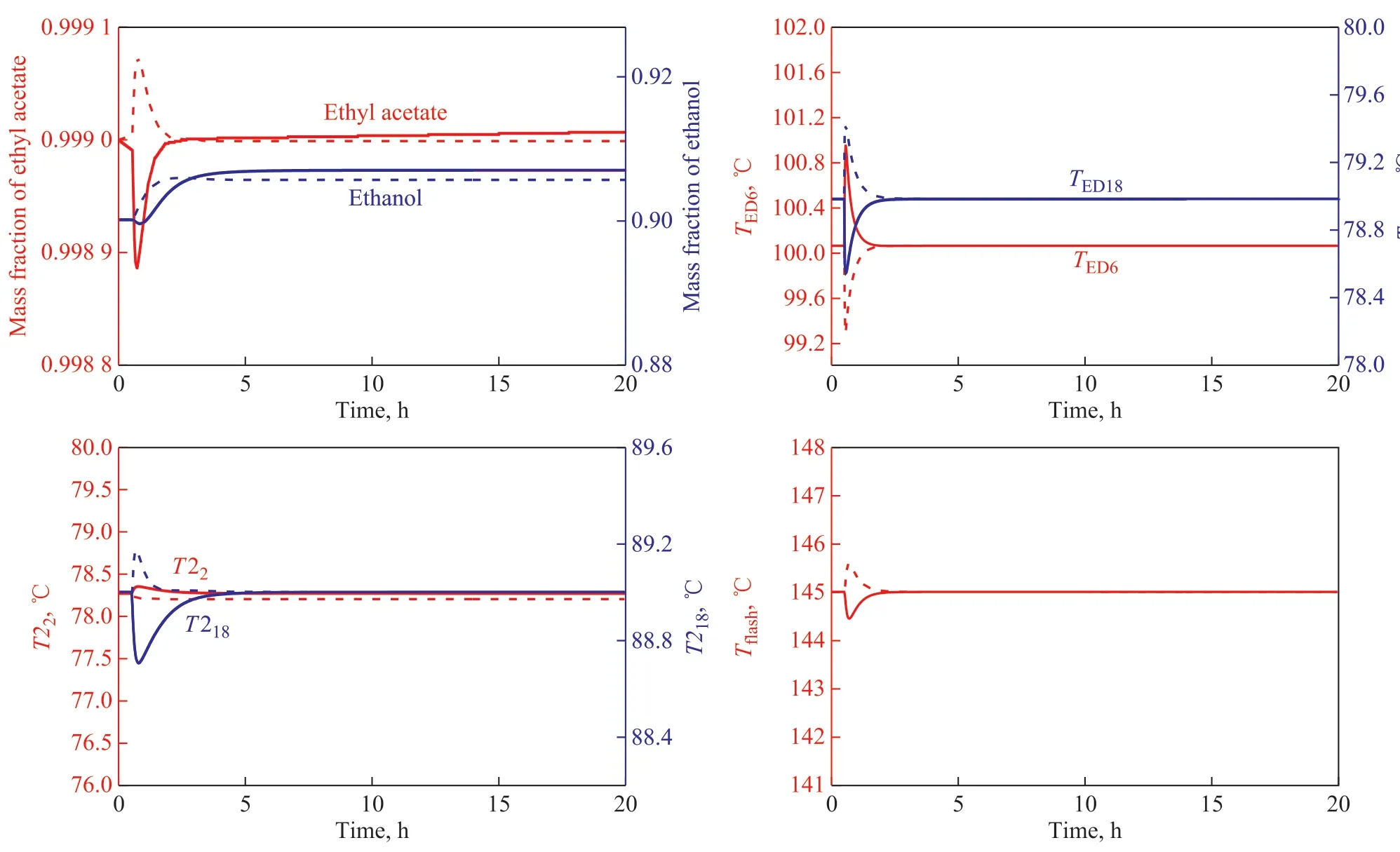
Figure 11 Dynamic performance for ±10% feed flow rate disturbances of the control structure in ED process—F+10%;—F+10%;—F-10%;—F-10%
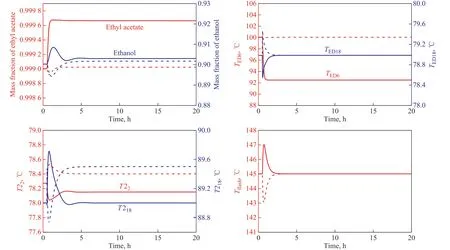
Figure 12 Dynamic performance for ±10% of ethyl acetate composition disturbances of the control structure in ED process—Z+10%;—Z+10%;—Z-10%;—Z-10%
Judging from the control results shown in Figure 11, it can be seen that when the feed flow rate changes by ±10%,the temperatures of the sensitive stages deviate from the set value at first, and then RR of ED column and QEDrespond quickly. The temperatures of the sensitive stages in T1 and T2 columns recover quickly and then stabilize to the vicinity of the set value. The purity of ethyl acetate and ethanol is stable at set value after 4 h, which can meet the product purity requirements. The corresponding temperature and pressure controllers, which are added to the flash tank, can maintain the high purity and yield of entrainer. Similarly, ±10% of ethyl acetate composition disturbances in the feed are introduced after 0.5 h of steady operation. The results are shown in Figure 12.It can be seen from the control results shown in Figure 12 that when the composition of ethyl acetate in the feed changes by ±10%, the control structure can also work pretty well. When the composition of ethyl acetate increases by +10%, the temperature of the stage 6 in the ED column does not return to the vicinity of the set value, and the purity of ethyl acetate at the top of the T1 column can be as high as 99.97%. The main reason is that when the composition of ethyl acetate increases by +10%, more ethanol-water mixture is extracted in T1 column and the relative volatility increases since the S/F value (mass ratio) is constant. Thus the purity of ethyl acetate at the top of the ED column increases,and the temperature of the stage 6 reduces below the set value. The reflux rate of ED column continuously decreases to maintain the temperature of stage 6, and the purity of ethyl acetate, ethanol and recycled entrainer are all stable after 3 hours and can satisfy the purity requirements.
4 Conclusions
The processes for separation of ethyl acetate-ethanolwater ternary azeotrope are studied in this paper. The simulation and optimization of PSD process and ED process, which use ILs as the entrainer, are performed and compared. The results show that the TAC of the ED process is reduced by 35.27% as compared with that of the PSD process, which demonstrates the feasibility and economy of ILs used as the entrainer.In addition, the control structure of the ED process for the separation of ethyl acetate-ethanol-water ternary azeotrope is further studied based on the optimal design parameters. Two-point temperature control structures are adopted, which can effectively resist the disturbances in both feed flow rate and feed composition in the ED system. The steady-state and dynamic simulation results, which are presented in this paper, can provide a reference for the separation of ethyl acetate-ethanol-water ternary azeotrope using ILs as entrainer in the industry.
Acknowledgements:This work was supported by the National Natural Science Foundation of China (Grant No. 21676299 and 21476261). Finally the authors are grateful to the editor and the anonymous reviewers.
杂志排行
中国炼油与石油化工的其它文章
- Modeling of Continuous Cross-flow Microfiltration Process in an Airlift External-loop Slurry Reactor
- Effects of Nitrogen-Containing Biodegradation Enhancers on Sorption of n-Hexadecane in Soil-Water System
- Novel Approach for Improved Tribological Behavior of Biodiesel Soot in Liquid Paraffin
- Compatibility Evaluation between Direct Coal Liquefaction Residue and Bitumen
- Impacts of Wettability on Immiscible Fluid Flow Pattern-Microfluidic Chip Experiment
- Constant Volume Spray Auto-ignition Study of Alkanes
