Effects of Nitrogen-Containing Biodegradation Enhancers on Sorption of n-Hexadecane in Soil-Water System
2019-05-10FanXingyuWuJiangChenBoshuiGuKechengDingJianhuaWuKe
Fan Xingyu; Wu Jiang; Chen Boshui; Gu Kecheng; Ding Jianhua; Wu Ke
(Department of Petroleum, Army Logistics University, Chongqing 401311)
Abstract: Effects of nitrogen-containing biodegradation enhancers (methyl diethanolamine oleate (MDEAO) and oleic diethanolamide (ODEA)) on the adsorption of n-hexadecane in soil were studied by laboratory batch experiments. The partition coefficients (Kd values) of n-hexadecane sorption in soil-water system and those partition coefficients normalized to organic matter (Koc values) were both determined. The adsorption isotherm curve of n-hexadecane in soil-water system was plotted. The measured results demonstrated that Koc values changed in the soil-water system with different enhancers but are relatively invariant for the soil at the same site. The Kd values rose with the increase of the organic matter content in different soil. The average values of Koc in the soil-water system with MDEAO, ODEA, and blank soil were 0.412, 0.252,and 0.309, respectively. The critical micelle concentration of ODEA and MDEAO was 0.7 mg/L and 1.9 mg/L, respectively,denoting that the solubilization capacity of ODEA was much stronger than MDEAO in the soil-water system. Consequently,the adsorption of MDEAO onto the solid surface increased the organic matter content in soil, which could make it more effective in enhancing the n-hexadecane adsorption. On the contrary, ODEA could inhibit the adsorption of n-hexadecane because of its less adsorption rate onto the soil particle surface and higher concentration in the soil-water system. According to the correlation coefficients, it was found that both the Henry linear and the Freundlich nonlinear isotherm sorption models were fitted to the data very well, however the Freundlich model was better than the Henry model.
Key words: methyl diethanolamine oleate; oleic diethanolamide; adsorption isothermal curve; soil
1 Introduction
Since the conventional petroleum-based lubricants have their inherent non-biodegradable nature, the pollution of environment caused by these lubricants cannot be ignored.Specially, the contaminated water-soil system formed by the petroleum-based lubricants could affect the quality of agricultural products and the health of human being.So, a low-cost and environmentally friendly technology for remediating the lubricant-contaminated soil is imperatively needed. Physical remediation, chemical remediation and bioremediation are the main approaches for remediating the contaminated soil. Bioremediation technology, mainly including bioaugmentation and biostimulation, is applied as an effective and eco-friendly method for unraveling the problems related with lubricant contamination of soil[1-3].
Recently, microorganism and surfactant serving as the bio-stimulation approach have been applied in remediating the lubricant-contaminated water and soil[4-6].
The biodegradation enhancer that is synthesized according to the characteristics of soil microbial populations and the properties of petroleum-based lubricants is a kind of surfactants, which can enhance the efficiency of contaminated soil bioremediation dramatically[7-8]. There are two main mechanisms for the promotion of biodegradation, namely: (1) providing nutrients to microorganisms so as to promote the growth and reproduction of microorganisms; and (2) reducing the surface tension of oil and water, so that the enhancer can increase the contact opportunity of microorganisms and hydrocarbon molecules to make lubricants fully degradable[9-12]. However, reducing surface tension between oil and water cannot fully explain the role of enhancer during the bioremediation against lubricant contamination. Therefore, it is of great significance to investigate the sorption behavior of lubricants and understand the acceleration mechanism of enhancer in soil-water system. Meanwhile, the explicit mechanism could provide the theoretical analysis and data support for the activity of enhancer in the bioremediation of contaminated soil. According to the results of previous work and the relevant literature reports, alkanes are the main components of petroleum-based lubricant.
In consideration of the complex composition of lubricants and the accuracy of experimental results, n-hexadecane,the carbon chain length of which is close to the average carbon chain length of lubricant components molecules,was chosen as the model compound of the lubricants in this article.
In the present study, two nitrogen-containing biodegradation enhancers, viz. methyl diethanolamine oleate (MDEAO) and oleic diethanolamide (ODEA), as additives were doped into n-hexadecane. The partition coefficient (Kdvalues) and sorption isothermal curve of n-hexadecane under the effect of these two biodegradation enhancers were investigated by using the laboratory batch experiments in soil-water system. The characteristics of isothermal curve and the effects of two enhancers on the sorption were analyzed.
2 Experimental
2.1 Materials and samples
Biodegradation enhancers included: methyl diethanolamine oleate and oleic diethanolamide. Both of them are synthesized by the research group at the early stage. The structures of the enhancers are shown in Figure 1.

Figure 1 The chemical structures of biodegradation enhancers
n-Hexadecane was purchased from the Kelon Chemical Co., Ltd. with a purity of >98%. Hexane (with a purity of GC grade) used as the solvent in this experiment was purchased from the Fisher Chemical Co., Ltd.
Soil samples in this study were collected from the Beibei district in Chongqing, from the Chaoyang region in Liaoning Province, and from the Golmud region in Qinghai Province, China, respectively. The soil samples were collected at a depth of 10—20 cm beneath the ground surface, which represented the predominant soil type of the relevant site with the diagonal sampling method, and then the soil samples were natural air dried at room temperature for one week. After removing crushed stone, leaves and other massive impurities, the soil samples was then passed through a 2.0 mm stainless steel sieve in order to improve their homogeneity. The soil samples were placed into the dryer for future use.The physicochemical properties of the soil samples are presented in Table 1.

Table 1 Physical and chemical properties of soils
2.2 Experimental methods
2.2.1 Measurement of critical micelle concentration
Critical micelle concentration (CMC) was determined in duplicate using the BYZ-II automatic interfacial tension tester according to the standard platinum ring method. In a 100-mL Erlenmeyer flask, 0.100 g of soil and 20 mL of biodegradation enhancers solution with its concentration ranging from 0 to 20 mg/L prepared by ultrasonic dispersion were added. The soil-water mixtures were then centrifuged at 6 000 g for 10 min at 303 K and the supernatants were tested for the CMC. Each tension test was carried out in two parallel tests, and the error of two parallel tests results should not be greater than 0.02 mN/m,while the average value of the two parallel test results was calculated as the surface tension value of this sample. The interfacial tension versus the biodegradation enhancer concentration was plotted in order to measure the CMC of enhancers in soil-water system.
2.2.2 Batch sorption experiments
A given weight of n-hexadecane dissolved in an appropriate volume of petroleum ether was mixed with a certain quantity of deionized water in beakers, and the mixer would form an emulsion after 30 min of ultrasonic oscillation at 303 K and 40 kHz. The n-hexadecane-water mixture was prepared when the petroleum ether was evaporated. The concentration of n-hexadecane in the prepared mixture was analyzed by gas chromatography(GC). Theoretical concentration of the mixture was 5, 10,20, 40, and 80 mg/L, respectively.
For the sorption experiment, 10 mL of prepared n-hexadecane water mixture, 10 mL of biodegradation enhancers solution and 0.100 0 g of soil were added into an 100-mL Erlenmeyer flask. This approach resulted in the actual concentration of biodegradation enhancers solution and n-hexadecane water mixture equating to 0, 0.5, 2, and 4 mg/L, and 2.5, 5, 10, 20, and 40 mg/L, respectively. The flasks were vortexed for 20 s, and then were placed on a reciprocal shaker at 303 K and 180 r/min for 6 h to reach the sorption equilibrium. The preliminary sorption kinetic experiments indicated that 6 h were sufficient to attain the sorption equilibrium for the n-hexadecane. The suspensions were then centrifuged at 6 000 g for 10 min at 303 K. The n-hexadecane in the aqueous phase was extracted with 10 mL of hexane and the concentration was also analyzed by a gas chromatograph.
2.2.3 GC analysis
Concentrations of n-hexadecane in aqueous phase were analyzed with an Agilent-7890A GC system (Agilent Inc.,USA) equipped with a flame ionization detector (FID)and a HP-5 column. Helium is used as the carrier gas (at a flow rate of 30 cm3/s). The injector temperature was 280 C, and the detector temperature was 300 C. The oven temperature was maintained at 60 C for 1 min and then was ramped to 240 C at a temperature increase rate of 15 C/min and then ramped to 300 C at a temperature increase rate of 10 C/min prior to being held at this final temperature for 1 min.
2.3 Data analysis
The sorbent loading can be calculated by the following equation[13-14]:

where Qe(mg/g) is the sorbent loading, C0and Ce(mg/L)are the initial and the final concentration of the sorbate,respectively, V is the volume of the aqueous phase, and M is the mass of soil in the soil-water system.
The liner distribution and the Freundlich sorption model were researched in the study. Model equations are shown as follows[15-17]:

where Kh, Kfis the Henry coefficient and the Freundlich coefficient, respectively. Qe(mg/g) is the sorbent loading,Ce(mg/L) is the aqueous concentration of the sorbate and n is the Freundlich exponent representing the isotherm non-linearity. Partition coefficients (Kd, L/g) and the partition coefficient that was normalized to organic matter(Koc, L/g) were calculated for describing the sorption ability of the sorbent.

The data were processed using the Origin 9.0 software(OriginLab Inc., USA) and analyzed by the correlation coefficient (R2) in the model fitting.
3 Results
3.1 Determination of critical micelle concentration
The CMC of MDEAO and ODEA was determined using the interfacial tension technique. Surfactant molecules tend to aggregate and form micelle with an increasing concentration in the solution. These micelle lead to an abrupt change of some physicochemical properties of solution such as its interfacial tension. The biodegradation enhancers have the similar character with surfactant in this way. Figure 2 is the exponent relation between the interfacial tension and concentration of two enhancers.Table 2 presents the exponential fitting parameters of interfacial tension in the MDEAO and ODEA solutions.Correlation coefficients are 0.965 and 0.948, respectively,denoting that the exponential model could fit in with the experimental data satisfactorily. As shown in Figure 2,the CMC of MDEAO and ODEA was found at a solution concentration of 1.9 mg/L and 0.7 mg/L, respectively.
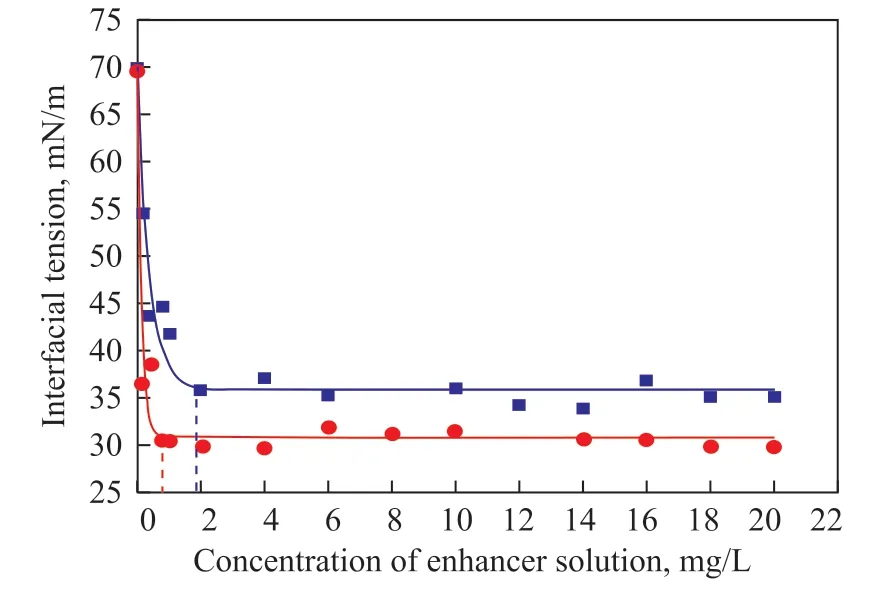
Figure 2 The change of interfacial tension in enhancer solutions■—MDEAO; ●—ODEA

Table 2 The exponential fitting parameters of interfacial tension in enhancer solutions
3.2 Effects of biodegradation enhancers on the sorption of n-hexadecane
3.2.1 Characteristics of n-hexadecane sorption behavior in blank soil and water system
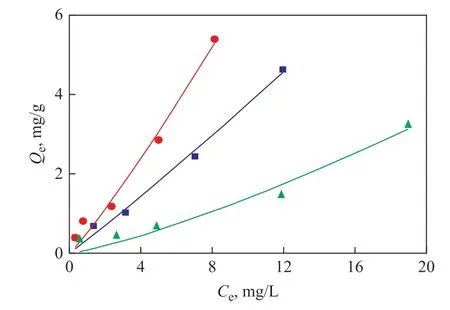
Figure 3 Sorption isotherm curve of n-hexadecane with different sites soil■—Chaoyang; ●—Chongqing; ▲—Golmud
The sorption isotherms of n-hexadecane in soil-water system with different soil samples are shown in Figure 3. All the experiments data were fitted in well with both the liner and the Freundlich models as evidenced by the values of R2shown in Table 3 (R2≥ 0.933) . In the following discussion of the sorption mechanism, the Freundlich sorption model was adopted due to its better fitting performance than the liner one in most cases.As shown in Table 3, with an initial concentration of n-hexadecane varying from 2.5 mg/L to 40 mg/L, the Kdvalues would vary from 0.170 L/g to 0.664 L/g in different site soil. The Kdvalue in the Chongqing soil is the highest among the three soil types, while that of the Chaoyang soil and the Golmud soil decreases in turn. The probable reason is that the organic matter content could result in an increasing adsorption capacity of soil. The higher the organic matter content, the higher the Kdvalues would be.

Table 3 Parameters of sorption isotherm models withdifferent site soil
3.2.2 Characteristics of n-hexadecane sorption behavior in soil-water system with the same enhancer
The Kocand Kdvalues were calculated based on the Freundlich sorption model with the same initial concentration of enhancers (2 mg/L). As shown in Table 4, the Kdvalue in blank soil varying from 0.170 to 0.664 was higher than that varying from 0.061 to 0.540 in soil with the added ODEA, however, it was lower than that varying from 0.224 to 0.903 in soil with MDEAO added thereby. As shown in Figure 4,the Kdvalues of blank soil, the soil with MDEAO and ODEA added versus the organic matter content of three types of soil were plotted. The experimental results were linearly related, which meant that Kocvalues were constant basically in soil-water system of the same enhancer or blank soil. The average Kocvalues in the system with MDEAO, ODEA, and blank soil were 0.412, 0.252, and 0.309, respectively. It is easy to found out that the soil-water system with the added MDEAO could accelerate the sorption of n-hexadecane,and ODEA could restrain the sorption of n-hexadecane on the contrary upon judging from the average values of Kocmentioned above.
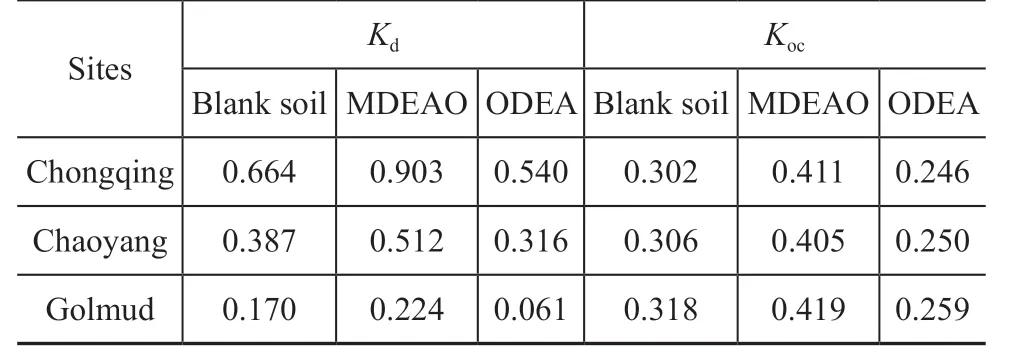
Table 4 Kd and Koc values of soil samples with MDEAO orODEA added and of blank soil

Figure 4 Relationship between Kd and foc with MDEAO,ODEA added and blank soil■—Chongqing; ●—Chaoyang; ▲—Golmud;—blank soil;—MDEAO;—ODEA
The possible reasons[18-19]could be that few micelle of MDEAO were formed due to its relatively higher CMC(1.9 mg/L) when the initial concentration of MDEAO was 2 mg/L. Therefore, MDEAO could just increase the organic matter content of the soil and make it more effective in enhancing n-hexadecane adsorption.Conversely, when the initial concentration of ODEA was 2 mg/L, much higher than the CMC of ODEA (0.7mg/L),a lot of micelle which could improve the solubilization effect of the soil-water system were formed so that ODEA could restrain the sorption of n-hexadecane.
3.2.3 Effects of the different biodegradation enhancers concentration on n-hexadecane adsorption
Sorption isotherms of n-hexadecane with different concentrations of MDEAO and ODEA in Chongqing soil-water system are shown in Figure 5 and the fitting parameters related to the isotherms are presented in Table 5. The concentration of MDEAO and ODEA was 0.5, 2,and 4 mg/L, respectively, with the initial concentration of n-hexadecane varying from 2.5 to 40 mg/L. As shown in Table 5, the Kdvalue of soil-water system containing 2 mg/L of MDEAO is the highest, which is even higher than that with blank soil (0.664 L/g). Conversely, the Kdvalue of system with ODEA varying from 0.641 to 0.400 L/g gradually decreases with an increasing ODEA concentration in aqueous solution.
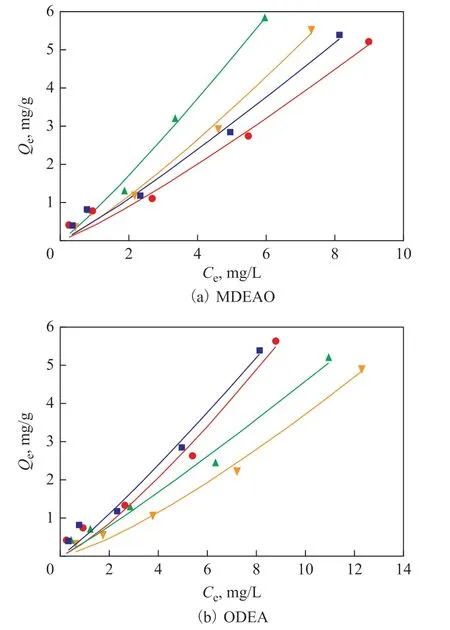
Figure 5 Effects of MDEAO and ODEA on the sorption isotherms of n-hexadecane■—blank soil; ●—0.5 mg/L; ▲—2 mg/L; ▼—4 mg/L
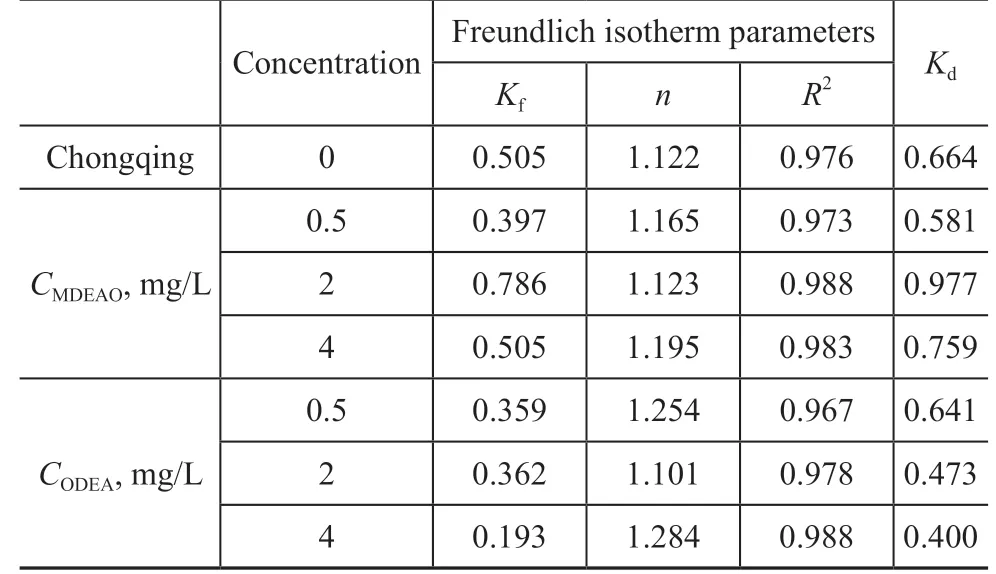
Table 5 The fitting parameters and Kd values of MDEAO and ODEA with different concentration
Since MDEAO and ODEA are similar to non-ionic surfactants, there are two mechanisms of sorption on the surface of soil particles[16,19-21]. One is that the hydrophobic chain of enhancers is directly absorbed through distribution, in which the electrostatic force and hydrophobic interaction force play a significant role.The electrostatic force includes the hydrogen bond, the force between ROH+generated by enhancers hydrolysis and metal silicate with negative electricity in the soil,etc. It can increase the organic matter content due to the enhancers absorbed by soil so that the enhancers can accelerate the sorption of n-hexadecane in soil. The other one is that the enhancers can improve the solubility of n-hexadecane in the aqueous phase for the formation of micellar molecules. Generally speaking, the CMC of enhancers determines the adsorption behavior of n-hexadecane in the soil-water system. At the same initial concentration of enhancers, the enhancer with lower CMC could restrain the organic contaminants from being adsorbed onto the soil and the effect of the enhancer with higher CMC could be boosted as verified by the experimental results.
According to the effects of two enhancers on the sorption of n-hexadecane, the enhancer that inhibits the sorption can be used as a better biostimulation approach for the bioremediation of lubricant-contaminated soil. The inhibition makes the concentration of lubricants in aqueous phase increase so that the contaminants can be fully biodegradable for the acceleration of relevant biochemical reactions rate.
4 Conclusions
(1) The isothermal sorption experiment results of n-hexadecane sorption in the soil-water system with MDEAO, ODEA or blank soil can comply with both the Henry linear and the Feundlich nonlinear isotherm sorption models. The Freundlich isotherm sorption model fitted better in most cases according to the fitting results.(2) The organic matter in the soil is the main sorption source of n-hexadecane. The higher the organic matter content, the higher the sorption capacity of the soil-water system.
(3) The Kocvalues vary in the soil-water system with different enhancers but are relatively invariant for the same site soil. The average values of Kocwith MDEAO,ODEA and blank soil are 0.412, 0.252 and 0.309,respectively, denoting that the effects of MDEAO and ODEA on the sorption of n-hexadecane in soil-water system can involve acceleration and inhibition.
Acknowledgement:The authors gratefully acknowledge the financial support of the Science Foundation for Post-doctoral Researchers, Chongqing, China (project No. Xm2016078)and the Natural Science Foundation of Chongqing (project No.CSTC2017jcyjAX0058).
杂志排行
中国炼油与石油化工的其它文章
- Novel NiMo Catalysts Supported on Sol-Gel Nanosized HY Zeolite-Alumina Composites for Hydrodesulfurization of Diesel
- Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis
- Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes
- Phosphorous-Modified Carbon Nanotube-Supported Pt Nanoparticles for Propane Dehydrogenation Reaction
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
