Novel Approach for Improved Tribological Behavior of Biodiesel Soot in Liquid Paraffin
2019-05-10LiChuanSongHuiZhangJunWuBoZhangQiangqiang
Li Chuan; Song Hui; Zhang Jun; Wu Bo; Zhang Qiangqiang;
Zhuang Yuan2; Hu Xianguo1
(1. School of Mechanical Engineering, Hefei University of Technology, Hefei 230009;
2. School of Automobile and Transportation Engineering, Hefei University of Technology,Hefei 230009)
Abstract: To improve the tribological behavior of biodiesel soot (BDS) in liquid paraffin (LP), the order of biodiesel soot was increased through thermally oxidized treatment at 500 C, and the oil solubility was then improved through a modification using oleylamine (OLA). The BDS and thermally oxidized oleylamine-modified BDS (T-BDS-OLA)were characterized through various methods including the use of TG, FETEM, Raman spectroscopy, FTIR, and a zeta potentiometer. The tribological properties and mechanisms of the BDS before and after the thermally oxidized treatment modification were investigated using a ball-on-disc reciprocating tribometer, FESEM, 3D laser-scanning microscopy, and Raman spectroscopy. The results showed that T-BDS-OLA has a higher degree of order than the BDS, with an onion-like microstructure. BDS and T-BDS-OLA can both improve the antifriction and antiwear properties of LP at a soot content of 0.1%-0.4%, while T-BDS-OLA in LP shows better antifriction and antiwear properties than BDS. The tribological mechanisms can be attributed to both types of soot acting as spacing and roll bearing between the friction surfaces. In addition, the exfoliated graphitic sheets from T-BDS-OLA can form a carbon lubrication layer providing easy sliding.
Key words: biodiesel soot; liquid paraffin; antifriction and antiwear; carbon onion
1 Introduction
As concerns regarding the continuous consumption of fossil fuels and an enhanced global awareness of energy saving, and the reduction of emissions have drawn much attention, scientists from around the world are striving to develop new clean fuels. Biodiesel is one of the most promising alternative fuels for automotive diesel engines.Biodiesel is a mixture of fatty acid alkyl esters, which is obtained through trans-esterification of animal fat,vegetable oils, or waste cooking oil with methanol[1].Compared with petrochemical diesel, the sulfur and aromatics in biodiesel are lower because its outstanding properties can greatly reduce the content of sulfides and carcinogens in the exhaust gas and reduce the amount of pollutants discharged in the environment. In addition,biodiesel contains 10%-11% of oxygen capable of enhancing the rate of heat release during the combustion process and reducing the amount of emissions (CO, HC,and soot)[2]. However, a portion of the biodiesel soot(BDS) generated during combustion can still contaminate the engine oil in the sump as a consequence brought about by the blow-by gasses[3].
With the extensive application of an exhaust gas recirculation system (EGR) in engines, the amount of soot in the engine oil is on the rise, which leads to an increase in the viscosity of the engine oil and an aggravation of the wear. Su, et al.[4]reported that a high concentration of diesel soot in lubricating oil will break up the lubrication film and block the engine oil entering the frictional region,resulting in a reduction of lubricity of the engine oil.However, Guo, et al.[5]showed that the low concentration of soot (0.01%) as a lubricant additive in lubricating oil not only does not aggravate the wear, but can also display antiwear and friction reduction properties. Moreover,our previous research[6]found that the graphitic layers in BDS are not continuous and are surrounded by amorphous phases. Merchan-Merchan and Lapuerta, et al.[7-8]revealed the presence of highly curved graphene segments in BDS, and that BDS displays more ordered graphite-like structures and lower amorphous carbon concentration than diesel soot. Wei, et al.[9-10]reported that thermally oxidized candle soot and acidified candle soot have a higher order than naked candle soot. Candle soot was heat-treated and modified using a cationic surfactant,which can obviously improve the lubricating properties of the liquid paraffin. Antusch, et al.[11]analyzed the tribological behavior of different soot by means of a pinon-disk tribometer. They found that the wear properties of the soot particles are closely related to their reactivity and the number of defect sites. Hirata, et al.[12]found that defects in the outermost layer of the carbon onions increase the bonding with the counter materials, resulting in inferior tribological properties. Therefore, the higher the order of the BDS is, the better the improved lubricity of the BDS would be. However, there have been few reports on improving the tribological behavior of BDS in lubricating oil by increasing its order.
In this paper, the order of BDS was increased through thermally oxidized treatment and improving the oil solubility through a modification with oleylamine (OLA).The tribological behaviors of BDS (before and after thermally oxidized oleylamine-modified) in liquid paraffin(LP) were evaluated using a ball-on-disc reciprocating tribometer, and the corresponding tribological mechanisms were investigated. The aim of this work is to provide the basis for inhibiting the effects of BDS on the tribological properties of lubricating oil, and to lay a foundation for the use of BDS as solid lubricant additives.
2 Experimental
2.1 Materials and preparation
LP was purchased from the Shanghai Zhongqin Chemical Reagent Co., Ltd. Oleylamine was purchased from the Aladdin Industrial Co., Ltd. All of the chemicals used in the present work were reagents of the analytical grade purity. Biodiesel was produced through the transesterification of soybean oil with methanol in the presence of a self-made green biomass ash catalyst[13].
The physicochemical properties of biodiesel are shown in Table 1. BDS was obtained from the combustion of biodiesel using a self-made soot-capture device[6].A brief description of the BDS collection is given in the literature[14]. The biodiesel sample was burned in a porcelain crucible at normal temperature and pressure.The BDS was collected using a glass slide (BDS capture slide) placed above the biodiesel flame. As the biodiesel burned, the flame deposited BDS on the slide. Until a BDS layer of a few micrometers in thickness was deposited, this layer was then very carefully scraped off the slide and collected in a glass bottle. Finally, the collected BDS was dried under vacuum conditions at 120 C for 3 h and ground to a fine granular powder. 200 mg of BDS were placed in a porcelain burning boat inside a tube furnace (OTF-1 200 X, Hefei Kejing Materials Technology Co., Ltd.) at 500 C for 2 h under an air flow of 40 mL/min and then were cooled down at room temperature. The thermally oxidized BDS is denoted as T-BDS. Then, 100 mg of T-BDS were subject to refluxing in 5 g of oleylamine at 110 C for 12 h. The mixture was purified through repeated washing with ethanol using centrifugation. Finally, the product was dried under vacuum conditions at 120 C for 5 h. The T-BDS modified with oleylamine is denoted as T-BDS-OLA.Two kinds of soot (BDS and T-BDS-OLA) were added into the LP at a mass percentage of 0.1%, 0.2%, 0.3%,0.4%, respectively. The mixtures were then stirred using a glass rod for 15 min, followed by magnetic stirring for 1 h to reduce deviations during the experiment.
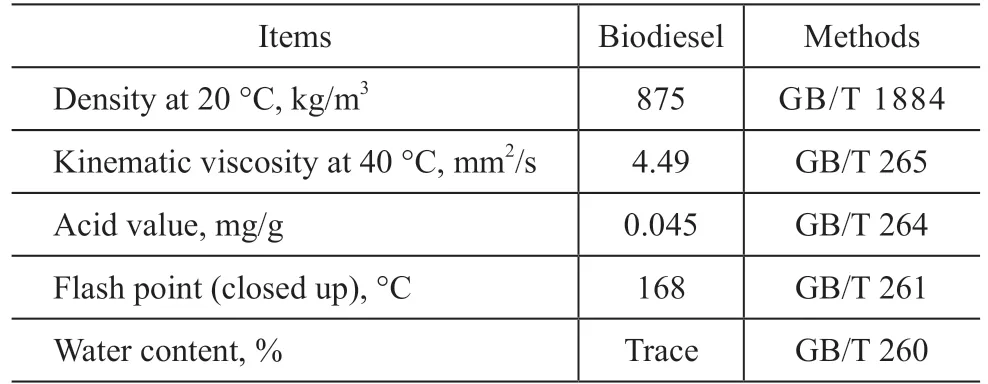
Table 1 Physicochemical properties of biodiesel
2.2 Tribological tests and characterization
Tribological tests were carried out on a ball-on-disc reciprocating tribometer (CFT-I, ZKKH), as shown in Figure 1. The upper ball sample (Φ = 6 mm) and lower plate sample (50 mm×50 mm×5 mm) were made ofGCr15 steel with a hardness of 62―65 HRC. During the frictional experiment, the sliding distance was 5 mm. The equations (1) and (2) for measuring the wear volume of the upper ball sample (Vwear) are shown as follows[15].

where r is the radius of wear scar of the upper ball, h is the height of the wear scar of the upper ball, and R is the radius of the upper ball.

Figure 1 Schematic of the friction pairs of ball-on-disk reciprocating tribometer
A thermogravimetry analysis (TG) of the BDS was carried out using a thermal analyzer (STA449F3,Netzsch)from room temperature up to 700 °C at a ramping rate of 20 °C/min in an air atmosphere. The particle size distribution of the BDS and T-BDS-OLA in LP were determined using a zeta potentiometer (Nano-ZS90, Malven).BDS,T-BDS, and T-BDS-OLA were characterized using a Raman spectroscope(HR Evolution,Horiba Jobin Yvon),a field-emission transmission electron microscope (FETEM,JEM-2 100F,JEOL),and a Fourier-transform infrared spectrometer(FTIR,Nicolet6 700,Thermo Nicolet) at 500 to 4 000 cm-1.The wear zones on the upper balls samples were analyzed using a 3D laser-scanning microscope (VK-X100,Keyence),a scanning electron microscope coupled with an energy dispersive spectroscope(SEM/EDS,SU8 010,Hitachi),and a Raman spectroscope.
3 Results and discussion
3.1 Characterization
Figure 2 shows the TG curve of the BDS, where two weight loss stages can be observed. A slight weight loss could at first be observed at 27 C to 150 C,which corresponded to the removal of absorbed water on the surface. Another dominant weight loss step was observed from 450 C to 675 C, which was caused by the oxidation of BDS. The surface oxidation of the BDS occurred in a temperature range of from 450 C to 500 C.A surface oxidation treatment is beneficial to deriving a significant amount of oxygen-containing functional groups on the BDS surface and changing the degree of order of the BDS[16-17]. Moreover, a deep oxidation of the BDS occurred at a temperature of 500 C to 675 C.Therefore, the thermal oxidation treatment temperature was chosen to be 500 C.
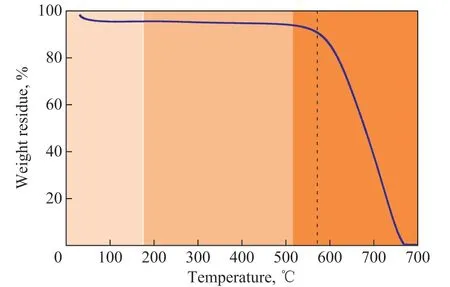
Figure 2 TG curve of BDS
Figure 3 shows the FETEM images of BDS, T-BDS,and T-BDS-OLA. The primary particle size of the BDS,T-BDS, and T-BDS-OLA was~35 nm. Figure 3(a)indicates that the BDS contains some short, disconnected,and concentrically oriented graphitic segments, which are surrounded by amorphous phases. Figures 3(b) and 3(c)show that T-BDS and T-BDS-OLA contain more graphitic segments, which is highly curved, discontinuous, and similar to onion-ring-like structure. Compared with BDS,T-BDS, and T-BDS-OLA exhibit higher-ordered carbon nanostructures. The results indicate that the oxidation treatment at 500 C can improve the degree of order of the BDS[10].
Figure 4 shows the Raman spectra of BDS, T-BDS,and T-BDS-OLA. Herein, a D (disordered graphitic lattice) band and a G (graphitic lattice) band appear at~1 345 cm-1and ~1 590 cm-1, respectively. The ID/IGratio reflects the degree of graphitization disorder of the carbon materials[18], with the decrease in the ratio that is correlated with a decreasing degree of disorder.The results show that the ID/IGratio of T-BDS (2.639)is similar to that of T-BDS-OLA (2.656). However, the ID/IGratio of T-BDS and T-BDS-OLA is lower than BDS(2.937). This means that T-BDS and T-BDS-OLA have a much less degree of graphitization disorder than BDS.These results were confirmed by the FETEM images of the soot samples, as shown in Figure 3.
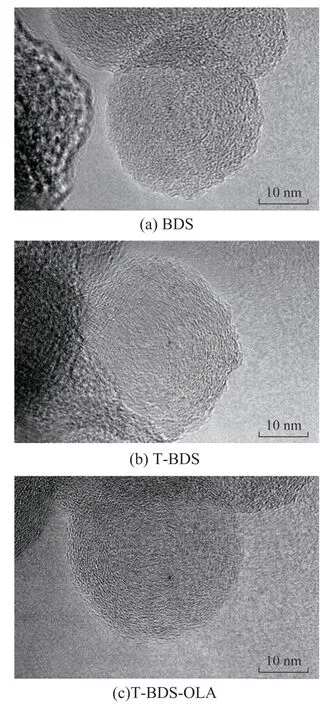
Figure 3 FETEM images of BDS, T-BDS, and T-BDS-OLA
Figure 5 shows the FTIR spectra of oleylamine (OLA),BDS, T-BDS and T-BDS-OLA. As for the BDS, the main features in the spectrum are related to O-H vibrations(3 440 cm-1), C=C vibrations (1 600 cm-1) and C-O-C stretching (1 220 cm-1)[19]. T-BDS showed almost the same FTIR absorption peaks as BDS albeit with an apparent peak of C=O (1 740 cm-1) vibrations. The apparent peak of C=O vibrations indicates that T-BDS has been oxidized.Furthermore, the FTIR absorption peaks of T-BDS-OLA show features of OLA at 2 858 cm-1and 2 926 cm-1(-CH3and -CH2- stretching)[9], indicating that OLA has been effectively adsorbed on the surface of the soot particles and that the surface of T-BDS-OLA contains long carbon chain lipophilic groups.

Figure 4 Raman spectra of BDS, T-BDS, and T-BDS-OLA

Figure 5 FTIR spectra of OLA, BDS, T-BDS,and T-BDS-OLA
Figure 6 shows the particle size distribution of BDS and T-BDS-OLA in LP based on a zeta potentiometer analysis. The results show that the average particle size of BDS in LP was 203.1 nm within a range from 122.4 nm to 342.0 nm, and that the average particle size of T-BDSOLA in LP was 146.2 nm within a range from 78.82 nm to 255.0 nm. This means that the average particle size of T-BDS-OLA in LP is smaller than that of BDS, which is caused by the fact that the lipophilic group of T-BDS-OLA has impeded the particles aggregation and stabilized them in the LP[20].
3.2 Tribological behavior
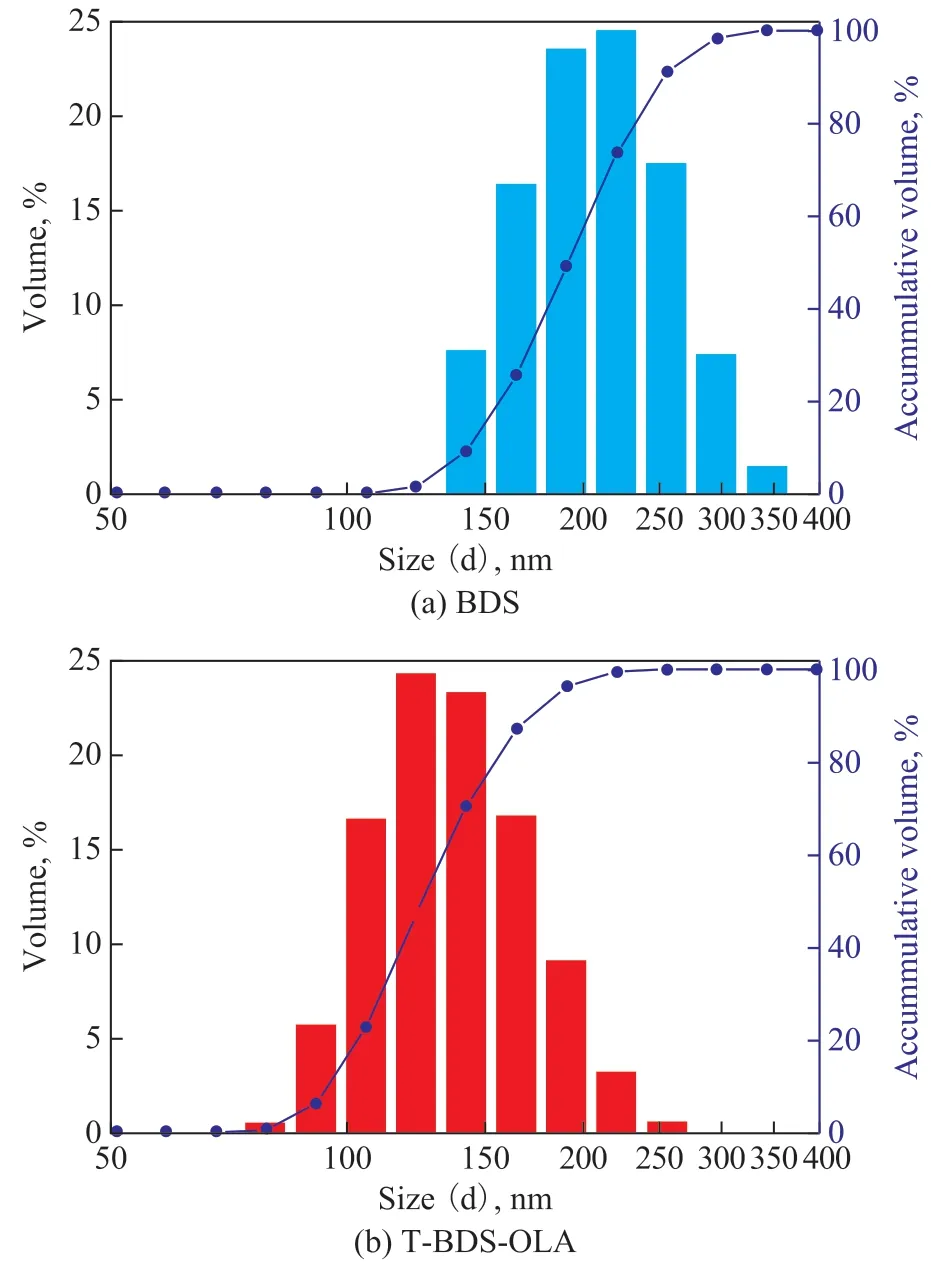
Figure 6 Particle size distribution of BDS and T-BDS-OLA in LP
Figure 7 show the variations in mean friction coefficient and mean wear volumes lubricated with different amounts of the two types of soot in LP, respectively,under conditions covering a load of 100 N, a speed of 50 mm/s, and a sliding time of 30 min. As shown in Figure 7(a), with an increase in content of the two types of soot, the mean friction coefficient of the soot in LP showed a decreasing trend. The mean friction coefficient of LP was 0.205. By contrast, the mean friction coefficients of LP with 0.3% of BDS and LP with 0.3% of T-BDS-OLA were 0.154 and 0.149, respectively. When the amount of the two types of soot exceeded 0.3%, their average friction coefficient in LP tended to be stable.The mean wear volumes of the two types of soot in LP are less than that of LP itself, as shown in Figure 7(b).The mean wear volumes were 1.4×106, 0.72×106, and 0.65×106µm3for the pure LP, LP with 0.2% BDS, and LP with 0.2% of T-BDS-OLA, respectively. Moreover,LP with 0.3% of T-BDS-OLA had the lowest mean wear volumes (0.5×106µm3) among LP with different BDS or T-BDS-OLA concentration. However, when the amount of the two types of soot is over a critical level (0.2% for BDS, 0.3% for T-BDS-OLA), the mean wear volumes of the two types of soot in LP showed a slightly increasing trend. This demonstrates that BDS and T-BDS-OLA can result in a reduction in the friction and an improvement in the wear resistance of LP, when the amount of the two types of soot is low (0.1%-0.4%)[21]. However, an excessive amount of both types of soot is not conducive to improving the wear resistance of the soot in LP[3]. In addition, under the same amount of soot adopted, the average friction coefficient and mean wear volumes of T-BDS-OLA in LP were lower than those of BDS. The results confirmed that T-BDS-OLA in LP possesses a better friction reduction performance and wear resistance than BDS in LP at different amount of soot adopted thereby.
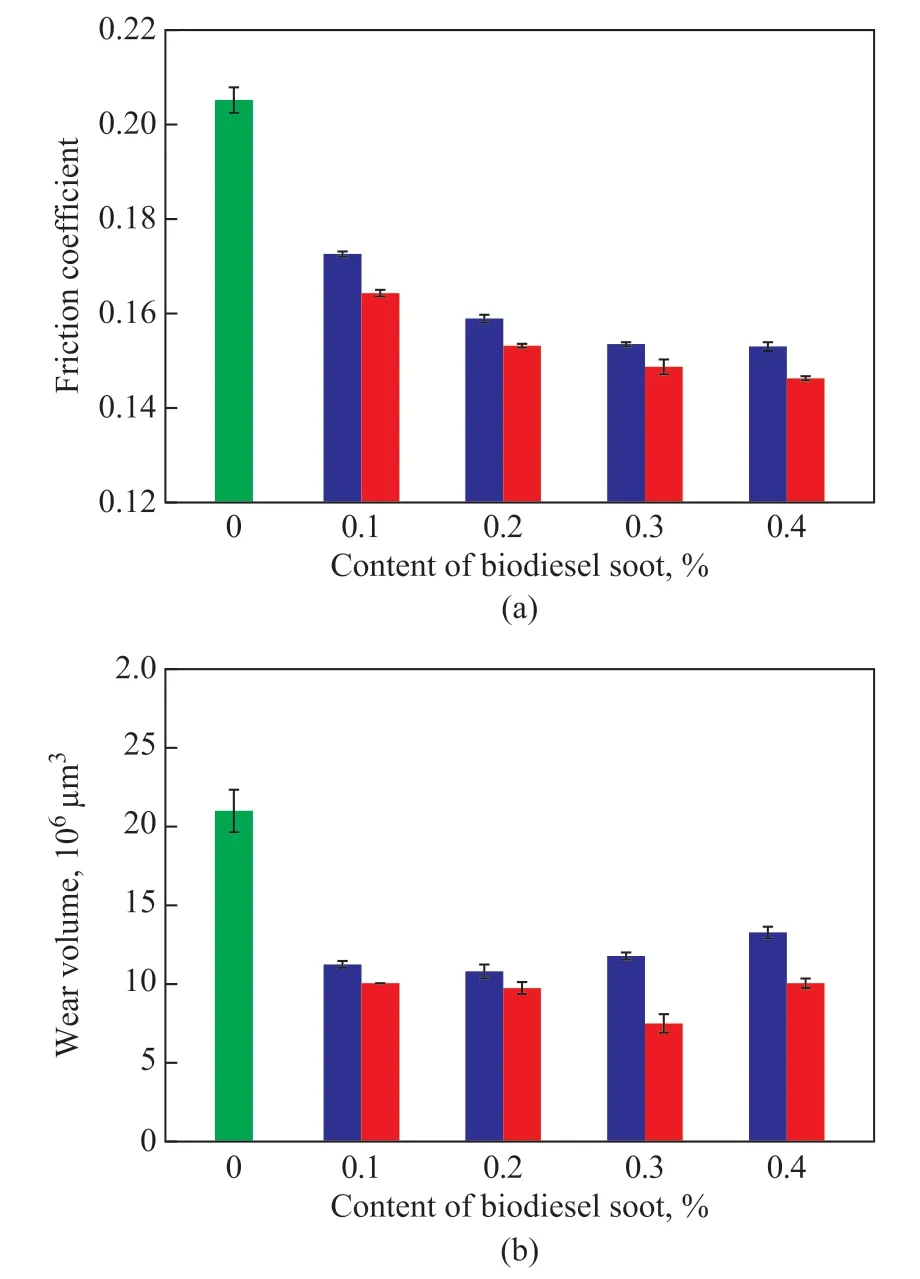
Figure 7 Variations of (a) mean friction coefficient and (b)mean wear volumes when lubrication was achieved with different amounts of the two types of soot in LP at 100 N and 50 mm/s for 30 min■—LP; ■—LP+BDS; ■—LP+T-BDS-OLA
Figure 8 shows the variations in mean friction coefficient and mean wear volumes lubricated with 0.3% of the two types of soot in LP under different loads along with a speed of 50 mm/s, and a sliding time of 30 min. As shown in Figure 8(a), when the load is increased, the average friction coefficient of LP at first exhibits a slight decrease and then increases. When the load exceeds 100 N, the average friction coefficient of LP remains mostly stable.Moreover, the average friction coefficients of the two types of soot in LP are less than that of the LP itself,particularly at a high load (100 N or 150 N). The mean wear volumes of the two types of soot in LP are also remarkably decreased in comparison with the LP itself,as indicated in Figure 8(b). This may occur due to the formation of a lubricant film by LP under a low load.However, with an increase in the load, the lubricant film is liable to destruction, resulting in an enhancement of the direct joggle effect between the micro-convex bodies on the friction surface and the aggravation of friction and wear[9]. The two types of soot can improve both the antifriction and antiwear properties of LP, and were verified to have better antifriction and antiwear properties under a higher load (100 N or 150 N) as compared with the case tested under a lower load (20 N or 50 N).In addition, when the BDS was replaced with T-BDSOLA, the average friction coefficient and the mean wear volumes of the LP were further improved. When the load was 100 N, the mean friction coefficients of LP containing BDS and T-BDS-OLA were reduced by 24.9% and 27.3%respectively, as compared to LP itself. As for the addition of BDS and T-BDS-OLA, the mean wear volumes of LP were decreased by 43.9% and 64.3%, respectively, under an applied load of 100 N. This finding shows that T-BDSOLA mixed with LP possesses better antifriction and antiwear properties than the BDS in LP upon being tested under different loads.
3.3 Analysis of worn surface
Figure 9 shows the characterization results of the wear zones for LP, LP containing 0.3% of BDS, and LP containing 0.3% of T-BDS-OLA, respectively, under conditions covering a load of 100 N, a speed of 50 mm/s,and a sliding time of 30 min. As shown in the FESEM image in Figure 9(a), many large spalling pits occurred in the wear zones lubricated with LP, and Figure 9(b) shows that many furrows and small pits can be observed in the wear zones lubricated with BDS in LP. In addition, the FESEM image of the wear scars lubricated with T-BDSOLA in LP provides no proof of the pits, and only slight furrows can be seen in Figure 9(c). These results are confirmed through 3D microscopic images and profiles of the wear zones. When LP alone was tested, the wear scar was the largest, and the profile curve was both the deepest and the broadest (Figures 9(a’) and 9(a’’)). When BDS was added to LP, the wear scarring decreased, and the profile curve became narrow and shallow (Figures 9(b’)and 9(b’’)). Figures 9(c’) and 9(c’’) show that the wear scarring of T-BDS-OLA in LP decreased, and the profile curve became much narrower and shallower than BDS in LP.This indicates that severe spalling wear has occurred in the wear zones lubricated with LP. The BDS can improve the bearing capacity of an LP film, and can fill in the friction surface to avoid direct contact between the micro-convex bodies[22], leading to reduction of the spalling wear. Meanwhile, BDS can also roll on the friction surface. Nevertheless, the aggregation of BDS can aggravate the abrasive wear[23]. The lipophilic group of T-BDS-OLA can inhibit such aggregation, thereby reducing the abrasive wear. These characterizations are described in Figures 5 and 6 presented above. Apart from spacing and rolling on the surface, T-BDS-OLA has a carbon onion-ring like structure, which is prone to exfoliation into individual graphitic sheets, which can produce an extraordinary lubrication effect[24]. These results are consistent with the trends shown in Figures 7 and 8.
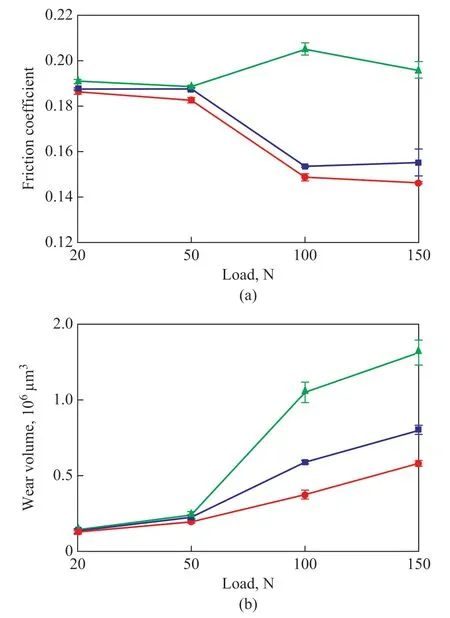
Figure 8 Variations of (a) mean friction coefficient and (b)mean wear volumes when lubrication was achieved with 0.3% of the two types of soot in LP at different loads and 50 mm/s for 30 min■—LP; ■—LP+BDS; ■—LP+T-BDS-OLA
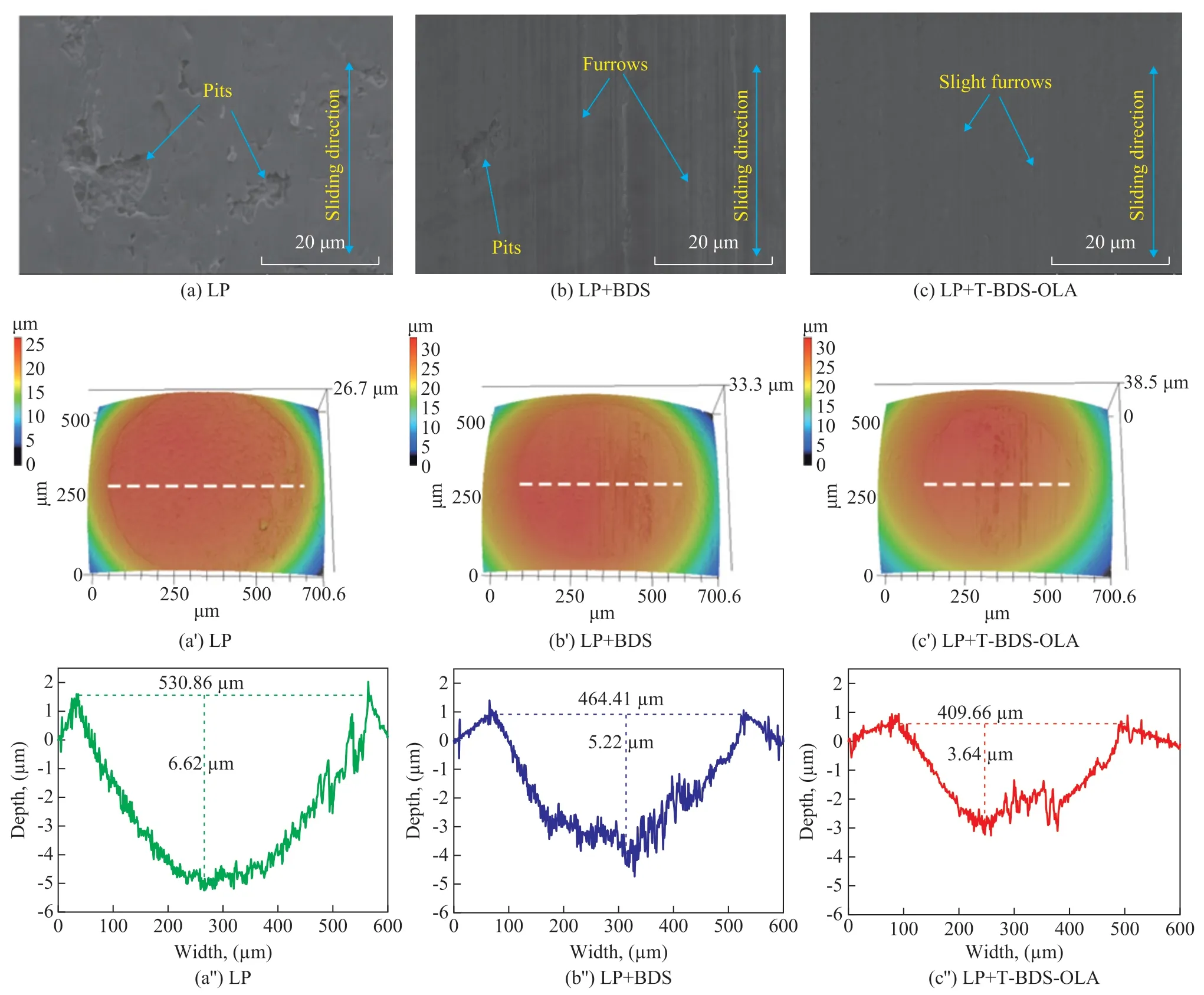
Figure 9 (a, b, c) FESEM images, (a’, b’, c’) 3D microscopic images, and (a’’, b’’, c’’) profiles of the wear zones of the upper balls for LP, LP containing 0.3% BDS, and LP containing 0.3% T-BDS-OLA at 100 N and 50 mm/s for 30 min
3.4 Lubrication mechanism
To further study the tribological differences between BDS and T-BDS-OLA, a Raman spectroscopic analysis was carried out on the wear zones. Figure 10 shows the Raman analysis results of the wear zones of the upper balls after the tribo-test. As Figure 10 indicates, for the two types of soot in LP used in our study, two typical characteristic peaks of soot at the D and G bands can clearly be seen.This observation suggests that the two types of soot were adsorbed on the surface of the steel substrate to prevent direct contact between the friction pairs[25]. Upon combining the results in Figure 4, the ID/IGratio of the wear zone lubricated with BDS in LP (2.927) is shown to be similar to the ID/IGratio of BDS (2.937). However, the ID/IGratio of the wear zone lubricated with T-BDS-OLA in LP (2.900) was larger than that of T-BDS-OLA (2.656).This indicates that the degree of order of BDS showed little change after the tribo-test. However, the degree of order of T-BDS-OLA showed a larger change. This can be explained through the exfoliation of graphitic sheets from T-BDS-OLA, which is responsible for the better lubricating performance compared with BDS[26].
Based on the observations described above, the lubrication mechanisms of BDS and T-BDS-OLA in LP are elucidated as follows. Firstly, both types of soot can fill in the friction interfaces to avoid direct contact between the micro-convex bodies during the tribological test[27]. Secondly, both types of soot can roll on the friction surface, thereby improving the friction-reducing properties. However, BDS can aggregate more easily than T-BDS-OLA, and the aggregation of soot particles causes an increase in abrasive wear[28-29]. Furthermore,under a high load and shear action, some of the graphitic sheets are exfoliated from T-BDS-OLA to form a carbon lubricating layer between the rubbing surfaces, which can slide easily and prevent contact between the surfaces,thereby helping reduce the friction and wear of the interacting surfaces[25-30].
4 Conclusions
In summary, the thermally oxidized treatment conducted at 500 C can increase the order of BDS, and a modification using oleylamine can thereby improve its oil solubility. The FETEM and Raman spectra revealed that the degree of order of T-BDS-OLA was better than that of BDS. The graphitic layers in T-BDS-OLA are similar to a carbon onion-ring structure. The C=O vibrations and-CH3and -CH2- stretching in the FTIR spectra suggest that T-BDS is oxidized and that the surface of T-BDS-OLA contains long carbon chain lipophilic groups. The average particle size of T-BDS-OLA (146.2 nm) in LP is smaller than that of BDS (203.1 nm).BDS and T-BDS-OLA can both improve the antifriction and antiwear properties of LP when they are used at low concentration (0.1%-0.4%). Moreover, T-BDS-OLA blended with LP has better antifriction and antiwear properties than BDS added to LP. The tribological mechanisms can be summarized as follows: 1) During a tribological test, BDS and T-BDS-OLA both enter the friction interfaces and prevent the direct contact between the friction pairs. 2) The two types of soot can act as ball bearings between the contact areas. 3) Exfoliated graphitic sheets from T-BDS-OLA can form a carbonlubricating layer between the friction surfaces that can slide easily and avoid contact between the friction surfaces. Thus, T-BDS-OLA in LP demonstrates better tribological properties.
Acknowledgments:This work was supported by the National Natural Science Foundation of China (Grant No. 51675153)and the Major Science and Technology Special Project in Anhui(Grant No. 17030901084), which are gratefully acknowledged.We thank Professor Kunhong Hu, Dr. Enzhu Hu and Mr. Lei Wang of Hefei University and Mr. Bangbang Wang of Hefei University of Technology for their assistance in the experimental analyses and discussion.
杂志排行
中国炼油与石油化工的其它文章
- Novel NiMo Catalysts Supported on Sol-Gel Nanosized HY Zeolite-Alumina Composites for Hydrodesulfurization of Diesel
- Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis
- Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes
- Phosphorous-Modified Carbon Nanotube-Supported Pt Nanoparticles for Propane Dehydrogenation Reaction
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
