Constant Volume Spray Auto-ignition Study of Alkanes
2019-05-10WangJunYangHeSongHaiqingTianHuayuWangPengfei
Wang Jun; Yang He; Song Haiqing; Tian Huayu; Wang Pengfei
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Spray auto-ignition experiments were carried out in a constant volume combustion chamber for some pure alkanes (n-paraffins with different chain length, cyclohexane, n-butyl cyclohexane, and isooctane) and blends of n-decane with Standard Blended Fuel (isooctane/n-heptane) and product gasoline. Test results showed that the reaction activity of n-paraffins was relatively high. Meanwhile, the auto-ignition characteristics differed significantly with the molecular structures of alkanes. Adding different volume fractions of n-decane to Standard Blended Fuel and product gasoline could improve the fuel reaction activity at varying degree. Finally, functional groups effects were used to simulate the relationship between the molecular topology and the auto-ignition quality.
Key words: spray auto-ignition; reaction activity; functional group; alkane
1 Introduction
Ignition is a vital factor, because it is closely related to thermal efficiency, power performance, and emissions of an engine. The ignition of fuels involves the ignition delay, the heat release rate, the flame propagation, etc.There have been a number of experimental apparatus including the constant volume combustion chambers(CVCC), the rapid compression machines (RCM), and the shock tubes, which can characterize such ignition properties. These experimental data can provide important information for understanding the combustion kinetics and designing the efficient internal combustion engines.Auto-ignition property of fuels is important to compression ignition (CI) diesel engine, and it is defined by cetane number (CN). Diesel fuel with high CN has high reactivity and corresponding short ignition delay(ID) period. There have been a number of research reports on the ignition characteristics of alkanes in the diesel distillation range[1-6]. Not only for diesel engine,the auto-ignition also plays a significant role in spark ignition (SI) gasoline engine. Auto-ignition of the end gas mixture could cause knocking combustion, which would damage the engines severely[7]. Therefore, it is necessary to study the auto-ignition properties of gasoline molecules. The ignition properties of nine heptane isomers[8-9]and cyclohexane with different side chains[10]were investigated. The results showed that molecular structures of alkanes were closely related to the autoignition properties. The higher branching degree would bring about the lower ignition activity.
The composition of gasoline is a continuous ‘hydrocarbon spectrum’, in which linear alkanes with higher carbon numbers (C10—C12) have higher reactivity. Thus, they are poor antiknock components. Even if their content is very low in gasoline, it will produce a large amount of active free radicals under certain conditions to cause combustion of other components, which would lead to knocking.Therefore, it is of great significance to study the effect of long linear paraffin component content on the ignition properties of gasoline. Some research groups have mixed high-octane components with high-cetane components[11]to study the ignition and combustion characteristics in order to seek the chemical kinetic interactions between different components.
In this paper, the auto-ignition of some alkanes with various molecular structures was investigated in a constant volume combustion chamber system. In particular, the ignition properties of n-heptane, isooctane and n-decane were studied in detail by changing the initial combustion temperature. Furthermore, the influence of n-decane content on the auto-ignition of tricomponent blends and product gasoline was studied.Finally, the effect of functional group of fuel molecules on the ignition characteristics was explored preliminarily in order to reveal the relationship between molecular structure and ignition.
2 Experimental
2.1 Test fuels
Solvent fuels of n-hexane and cyclohexane were purchased from the Beijing Chemical Works. n-Heptane, n-octane,isooctane, n-decane, n-dodecane and n-tetradecane were purchased from the Tianjin Damao Chemical Reagent Factory, and n-butyl cyclohexane was purchased from the Tokyo Chemical Industry Co, Ltd. (TCI). Their purity,molecular formula, and molecular weight are listed in Table 1. Finished product gasoline with a research octane number (RON) of 93 was chosen as the object gasoline, the physicochemical properties of which are displayed in Table 2. 7% of n-heptane and 93% of isooctane were mixed by volume to obtain a Standard Blended Fuel (SBF) with a RON rating of 93.

Table 1 Basic parameters of the solvent fuels
2.2 Experimental equipment
The auto-ignition properties were characterized in a constant volume combustion chamber (Cetane ID 510 instrument) with direct fuel injection into the heated and compressed synthetic dry air[12]. A dynamic pressure sensor was positioned and the pressure wave was produced during the combustion of the fuel. Based on the dynamic pressure curve, the derived cetane number (DCN) was calculated by an equation where the ignition delay (ID) and the combustion delay (CD)were defined. ID represented a period of time between the start of fuel injection and the onset of the initial pressure rise, where the pressure exceeded the initial pressure plus 0.02 MPa. CD was defined as the time period from the start of fuel injection to the mid-point of the combustion pressure curve. To obtain a correct DCN data, experiment should be conducted under specific fuel pressure, coolant temperature, injection time, initial chamber temperature, and pressure. The experiments in this paper were conducted according to the standard method ASTM D7668[12], with the test conditions shown in Table 3.

Table 2 Physicochemical properties of the RON 93 product gasoline
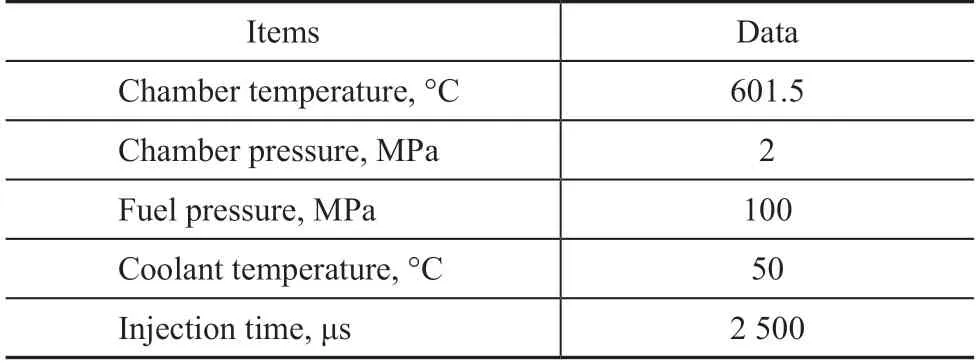
Table 3 Experiment conditions in the CVCC
3 Results and Discussion
3.1 Auto-ignition of pure alkanes
Dynamic pressure curves during combustion process for all the solvent fuels are shown in Figure 1. All the n-paraffins showed a very short ignition and combustion delay time. However, cyclohexane and isooctane had a relatively longer ID and CD time, which resulted in lower cetane number. The detailed data of ID, CD and DCN are listed in Table 4. From the perspective of chemical kinetics, the cyclic structure in cyclohexane and the highly branched structure in isooctane inhibited the low T chain branching reaction[13]. Low T chain branching reaction was very important as it could produce lots of active free radicals exponentially, which would speed up the total reaction rate. When the chain branching was suppressed, the total reaction rate would decline,and in another word, the fuel would have low reactivity.Interestingly, the ignition and combustion delay time of n-butyl cyclohexane was similar to that of n-heptane,which brought about almost the same cetane number.In spite of the existence of cyclic structure with low activity, the n-butyl side chain played an important role in improving the reaction activity.It could be observed that the peak pressure during combustion of fuels with short ID and CD time had pressure fluctuations (Figure 1). However, it was not the case with cyclohexane and isooctane with long ID and CD time. It was supposed that fuels like n-paraffins burned immediately after being injected into the combustion chamber. The pressure increased very fast, which was similar to deflagration. In this situation, the pressure reflected back from the combustion chamber would produce pressure fluctuations. The combustion and pressure increase caused by cyclohexane and isooctane were slow,and as a result the vibration of reflection was smaller.

Figure 1 Chamber pressure of all the tested fuels at a initial temperature of 601.5 oC and an initial pressure of 2.0 MPa


Table 4 ID, CD and DCN of all the tested fuels

Figure 2 Heat release rate of n-heptane, isooctane and cyclohexane at an initial temperature of 601.5 oC and an initial pressure of 2.0 MPa

The rate of heat release (RHR) in the spray experiments could reveal some important information. RHR is defined as the following equation:

where Q is the heat release, P is the chamber pressure,V0is the chamber volume (473 mL), and γ is the specific heat ratio (taken to be as a constant 1.35 for simplicity).The RHRs of n-heptane, isooctane and cyclohexane are shown in Figure 2. Except for n-heptane, isooctane and cyclohexane exhibited distinct two-stage heat release which indicated a two-stage ignition. The first-stage heat release was not sufficient to lead directly to combustion.Some experimental and simulation results[14]illustrated that the first-stage heat release in the two-stage situation was related to cool flame, which referred to a kind of prereaction phenomenon occurring in the fuel ignition delay time region. It was widely believed that the occurrence of cool flame was owing to the accumulation of unstable intermediates, which produced active free radicals at a specific time and resulted in exothermic phenomenon in a short period of time.
Compared with n-heptane, isooctane and cyclohexane had longer auto-ignition delay time that meant longer premixed time with the air in the combustion chamber.However, they had slower combustion rate (Figures 1 and 2). This is evidenced by another point of view that the chemical reactivity of isooctane and cyclohexane was relatively low. It was also one of the factors that longer ignition delay time caused the leaner mixture conditions,which would in turn lead to slower chemical kinetics.
3.2 Auto-ignition of n-heptane, n-decane and isooctane at different chamber temperature
The effect of initial chamber temperature on the autoignition quality of n-heptane, isooctane, and n-decane is exhibited in Figure 3. In order to avoid exceeding the limit of the instrument, the temperature for isooctane was only dropped to 560oC, at which the combustion delay time was already quite long. In general, the combustion delay time increased with a decreasing temperature for all the three fuels. Figure 4 can give more detailed information on ID and CD changes. Except for the ID change of isooctane, all the delay time of the other three fuels increased with a decreasing temperature. The ID change of isooctane presented a negative temperature coefficient (NTC)[13]region, where the reaction activity increased with a decreasing temperature.
The RHRs of the three fuels subjected to combustion at a decreasing temperature are shown in Figure 5. For n-heptane and isooctane, the slower combustion resulted in a reduced heat release rate, which was indicative of the slower combustion rate. Interestingly, for n-decane,as temperature decreased at the initial stage from 640oC to 580oC, the heat release rate increased, and then decreased as the temperature decreased further to 540oC.It implied that n-decane entered a NTC region in this temperature range. Despite the delay in the combustion moment, the combustion rate increased. The NTC was often observed for paraffins. In the NTC region, when the temperature increased, the reaction pathway shifted from chain branching to chain growth, which would reduce the overall reaction rate.
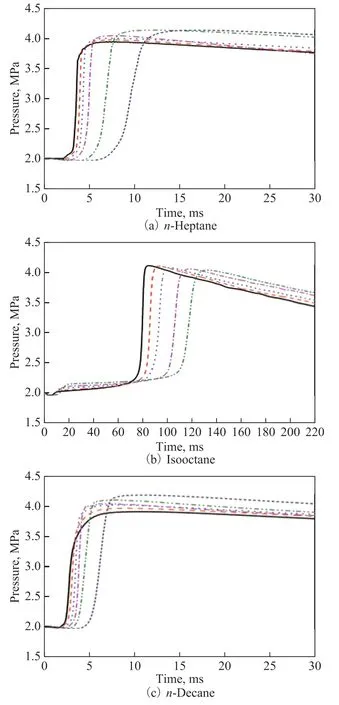
Figure 3 Chamber pressure curves of n-heptane, isooctane,and n-decane at different initial temperature and an initialpressure of 2.0 MPa

3.3 Influence of n-decane content on auto-ignition of tri-component blends and product gasoline
N-heptane and isooctane were reference hydrocarbons in gasoline antiknock tests (RON and MON). They were often blended to be used as an alternative fuel to simulate gasoline. In this part of work, 7% of n-heptane and 93% of isooctane were mixed by volume to form a Standard Blended Fuel (SBF) with a RON rating of 93.In order to investigate the effect of fuel component with high activity on the auto-ignition quality of gasoline,different volume fraction of n-decane was added to SBF.The results of auto-ignition of the tri-component blends are shown in Figure 6. The ignition and combustion delay time was shortened, when an increasing amount n-decane was added. Especially for the combustion delay time, only a small amount of n-decane (0-10%) would cause a significant drop in CD. With a further increase of n-decane content, the decrease of CD became smaller.The SBF without n-decane displayed a two-stage ignition,which could occur owing to the existence of isooctane (as already discussed above). However, with more and more n-decane being blended, the two-stage ignition shifted to the one-stage ignition gradually.
The composition of gasoline was complex, since it contained paraffins, naphthenes, alkenes, and aromatics. Herein, a product gasoline with the same RON 93 as that of SBF was used to study the influence of component with high activity on auto-ignition of product gasoline. As shown in Figure 7,it was almost the same case as SBF. Because of the same

Figure 5 Heat release rate of n-heptane, isooctane, and n-decane at different initial temperature and an initial pressure of 2.0 MPa

RON rating, the product gasoline and SBF had similar main combustion scenario. The ID time was slightly different due to different components in the fuel. With an increasing content of n-decane, the ID and CD time emerged to be the same, indicating a high activity of the fuel.

Figure 4 Ignition delay and combustion delay for n-heptane, isooctane, and n-decane at different initial chamber temperature■—n-heptane; ●—isooctane; ▲—n-decane
Cetane number and octane number could both reflect the activity of a fuel. High cetane number meant low octane number and high activity. Cetane number and octane number were closely related to the composition of a fuel. Figure 8 shows a fairly good linear relationship between DCN and n-decane content for the RON 93 product gasoline with different n-decane content. The results indicated that the highly active fuel like n-decane had a very significant effect on the auto-ignition of gasoline. It almost dominated the reaction process. Gasoline was a kind of fuel that would not be easily subject to self-ignition essentially,however, a small amount of highly active component could cause a sharp drop of knocking resistance.
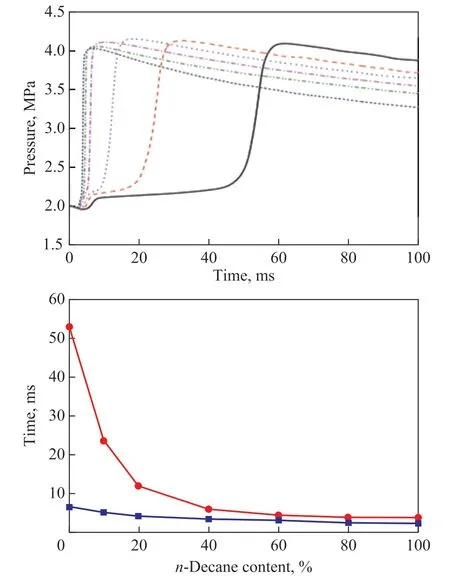
Figure 6 Chamber pressure curves, ignition delay, and combustion delay for the tri-component blends withdifferent n-decane content added to the SBF
3.4 Functional group effect
It is a basic law in the chemical world that structure determines the property, and the property determines the application. How to establish a quantitative relationship between the structure and the property of the fuel has been always attracting great interests. By expressing fuel molecules topologically in the form of functional groups,a quantitative relationship between the auto-ignition property and the fuel structure information has been established as follows[15]:

Figure 7 Chamber pressure curves, ignition delay, and combustion delay for the RON 93 product gasoline with different n-decane content


Figure 8 Linear relationships between DCN and n-decane content for the RON 93 product gasoline with different n-decane content

where, [CH3], [CH2], [CH]and [C]represent the molar number of corresponding groups in 1 mole of fuel molecules. For n-paraffins with different chain length, the ignition delay, the combustion delay time, and the derived cetane number could be formulated in terms of [CH2].
The optimized relationships by maximizing R2are shown below (also shown in Figure 9):

The correlations between ID/CD and [CH2]were not linear, and they were negatively correlated, except for the situation in DCN. This could occur, because more [CH2]would improve the rate of chain branching reactions,leading to shortened ID and CD time.
For the tri-component blends composed of n-heptane,isooctane and n-decane with definite volume fractions,the functional groups could also be used to quantitatively simulate the auto-ignition quality. Chemical characteristics of the tri-component blends are described in Table 5, and the optimized relationships are shown below (also shown in Figure 10):

Due to the same [CH]and [C]used in these blends, [C]was deleted in the equation for simplicity. In the process of optimization, the exponent of [CH3]always approached zero. So, [CH3]would also be left out. It could be observed that the calculated results and the experimental results were very similar. DCN had a linear relationship with [CH2]and [CH]. ID and CD did exhibit proper relationship with [CH2]and [CH]. As [CH2]and [CH]can reflect the branching degree of fuel molecules, it could also be concluded that the lower the degree of branching,the higher the activity.

Figure 9 Optimal correlations obtained by simulating ID,CD, and DCN from functional groups for n-paraffins

Table 5 Chemical characteristics for the tri-component blends with different n-decane content added to the SBF

Figure 10 Optimal correlations obtained by simulating ID,CD and DCN from functional groups for the tri-componentblends with different n-decane content added to the SBF
4 Conclusions
In this work, n-paraffins were ignited immediately after being injected into the combustion chamber with very short ignition and combustion delay. The reactivity of n-paraffins was positively correlated with their chain length. In contrast, isooctane and cyclohexane had very long combustion delay and low cetane number, denoting their low reactivity. For cycloalkanes, the role of pendant group was very important. The n-butyl side chain could greatly increase the reactivity of cyclohexane. Reducing the initial chamber temperature would delay the main combustion moment of n-heptane, isooctane, and n-decane.Hence, adding n-decane to the Standard Blended Fuel and product gasoline with same RON 93 rating would cause a sharp decline in the ignition delay. This suggests that the content of highly active long-chain alkanes should be strictly controlled for engines with special antiknock requirements.The results would also give some information for molecular design of paraffin fuels used in the Homogeneous Charge Compression Ignition (HCCI) engines. Finally, the relationship between molecular topology and auto-ignition properties under this specific experimental condition was studied. The simulated results were very similar to the test results. Further work will be done to verify that if this method of fitting the ignition properties with functional groups is applicable to other fuels.
Acknowledgment:This work is funded by the National Key Research and Development Program (2017YFB0306505).
杂志排行
中国炼油与石油化工的其它文章
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Modeling of Continuous Cross-flow Microfiltration Process in an Airlift External-loop Slurry Reactor
- Effects of Nitrogen-Containing Biodegradation Enhancers on Sorption of n-Hexadecane in Soil-Water System
- Novel Approach for Improved Tribological Behavior of Biodiesel Soot in Liquid Paraffin
- Compatibility Evaluation between Direct Coal Liquefaction Residue and Bitumen
- Impacts of Wettability on Immiscible Fluid Flow Pattern-Microfluidic Chip Experiment
