Phosphorous-Modified Carbon Nanotube-Supported Pt Nanoparticles for Propane Dehydrogenation Reaction
2019-05-10LiuJieLiuChangchengDaZhijianZhengHuidong
Liu Jie; Liu Changcheng; Da Zhijian; Zheng Huidong
(1. College of Chemical Engineering, Fuzhou University, Fuzhou 350116;
2. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: The sintering of Pt nanoparticles is one of the main reasons for catalyst deactivation during the high-temperature propane dehydrogenation (PDH) reaction. Promoters and supports have been introduced to prolong the catalyst life.However, it is still necessary to develop novel catalysts with robust stability. Herein, the phosphorus-modified carbon nanotube-supported Pt nanoparticles were employed for the PDH process. Phosphorus modification improves the Pt dispersion, effectively promoting the activity of Pt/P-CNTs. Additionally, the phosphorus-modified CNTs can interact strongly with Pt nanoparticles by improving the electron transfer or hybridization, stabilizing Pt nanoparticles from agglomeration, and significantly enhancing the catalyst stability.
Key words: propane dehydrogenation; phosphorus-modified carbon nanotubes; platinum catalyst; strong metal-support interactions
1 Introduction
Propylene is an important feedstock of the chemical industry, and it is used for making polymers, oxygenates and other chemical intermediates[1-2]. The demand for propylene has been increasing steadily since 1990 due to the rapid development of the downstream production worldwide[3]. In general, propylene is obtained by catalytic cracking and naphtha pyrolysis methods. However, the traditional routes can no longer meet the increasing propylene demands owing to the depletion of oil resources.Among the various new approaches, the dehydrogenation of propane is a promising method to produce propylene[4].Carbon deposits and Pt agglomeration are the main reasons for the low stability of Pt-containing catalysts during the high-temperature propane dehydrogenation (PDH)reaction[5]. Promoters and alternative traditional supports are usually employed to address this problem[6-12], but it is still necessary to develop new catalytic materials for the PDH process.
The tunable acidity[13], the large surface area[14], and the metallic or semi-metallic nature[15]provide carbon nanotubes (CNTs) with great properties for increasing catalyst stability during the PDH process. Su, et al.determined that the heteroatom-modified nanocarbon could effectively stabilize and disperse metal nanoparticles[16]. Recently, the N-, B-, P-, and defect-containing CNTs have been employed to disperse Pt nanoparticles and increase their catalytic performance. Li and his group[17]considered that the enhanced Pt absorption onto the N-doped CNTs was ascribed to the activation of nitrogen-neighbouring carbon atoms. Lin and co-workers[18]further indicated that Pt nanoparticles were anchored on the vacancy defects derived from the N-doping. The enhanced Pt absorption in the B- and P-doped CNTs may also be attributed to the strong hybridization between Pt and heteroatoms[17,19]. Additionally, the implementation of N, B, and P in CNTs can facilitate the dispersion of metal nanoparticles and even promote the formation of oxygen-containing functional groups[20-23]. The new P=O groups are believed to be independent active centres or can be helpful to increasing the activity of the active C=O groups during the PDH reaction[24].In addition, the reason for the addition of P to CNTs in the oxidative dehydrogenation of low alkanes is to block or cover the combustion sites causing COxformation and thus increase the selectivity of the product[25]. P and Pt also have a synergistic effect on promoting the activity,selectivity and durability of CNT catalysts in electrocatalytic reactions[20,26-29]. However, to the best of our knowledge, little research has been reported on the P-modified CNT-supported Pt nanoparticles during the PDH process. Herein, we performed a study on the effect of P on the activity and durability of the Pt-CNT-catalysed dehydrogenation of propane. Two types of supports, the oxygen-containing CNTs and the P-modified oxygen-containing CNTs (P-CNTs), were employed to immobilize Pt nanoparticles for the PDH reaction. Characterization and catalytic assessments were applied to elucidate the role of P.
2 Experimental
2.1 Materials
The CNTs were produced by the Shandong Dazhan New Material Co., Ltd. NH4H2PO4was purchased from the Beijing Yili Fine Chemicals Corporation. The chloroplatinic acid, concentrated sulfuric acid (95%—98%), and nitric acid (65%—68%) were obtained from the Sinopharm Chemical Reagent Co., Ltd. The purity of H2PtCl6and NH4H2PO4was of the analytical reagent grade.
2.2 Preparation of Pt-containing catalysts
Firstly, the pristine CNTs were treated with a mixed solution of concentrated sulfuric acid and nitric acid(VH2SO4:VHNO3= 3:1) in an ultrasonic bath at 25 ºC for 7 h, washed with deionized water to neutral reaction and dried in the air at 120 ºC for 12 h. Then, Pt/CNTs and Pt/P-CNTs were synthesized by the incipient wetness impregnation method at 25 ºC. The NH4H2PO4and H2PtCl6were impregnated onto the CNTs successively to form Pt/P-CNTs. All of the products were dried in the air at 120 ºC for 12 h. Pt/CNTs and Pt/P-CNTs were calcined at 600 ºC in N2for 4 h to get rid of the unstable functional groups before use.
2.3 Characterization
The X-ray diffraction (XRD) patterns were collected on a Philips X’pert X-ray diffractometer with monochromatic Cu Kα radiation (40 kV, 40 mA). The scanning range of 2θ was 10—50º. The X-ray photoelectron spectroscopy(XPS) was performed on a VG ESCALAB 210 instrument employing Mg Kα radiation. All of the binding energies were calibrated by using Cls (284.6 eV) as a reference. The peaks were deconvoluted via the XPSPEAK software. The high-resolution transmission electron microscopy (HRTEM) images were taken using an FEI TECNAI G2 F20 (200 kV) high-resolution transmission electron microscope with the sample mounted on a C-flat TEM grid.
The amount of coke deposits was determined via combustion of the spent catalysts in a STA 409 PC/PG(NETZSCH) thermogravimetric (TG) analyser. Before combustion, the catalyst was pre-treated with flowing air stream (30 mL/min) and N2stream (20 mL/min) at 30 ºC for 10 min. Then, the samples were oxidized from 30 ºC to 800 ºC at a temperature increase rate of 10 ºC/min.The CO2generated was monitored by an on-line mass spectrometer (MS). The amount of deposited coke was calculated from the weight loss of the samples. The Pt and P weight loadings of the as-prepared catalysts were examined on an X-ray fluorescence (XRF) spectrometer MagiX (Philips). The O contents of the catalysts were analysed on an Elementar Vario Micro Cube elemental analyser.
2.4 Catalytic activity measurements
Catalytic assessment was carried out in a stainless steel fixed-bed flow microreactor with an on-line GC-14C gas chromatograph (SHIMADZU, HP-Al/KCL; capillary column: 50 m×0.320 mm × 8.0 μm; flame ionization detector). Before the dehydrogenation reaction, the catalysts were reduced at 580 ºC for 1 h, followed by heating to 600 ºC and feeding with propane. In a typical PDH process, the gas reactant contained 5% propane and a balance of N2(with a total flow rate of 60 mL/min). The operating temperature was 600 ºC with a catalyst loading of 0.2 g at a propane weight hourly space velocity (WHSV) of 1.8 h-1and under a reaction pressure of 0.13 MPa.
The reaction rate of propane (propane rate) and propylene selectivity to products are calculated as follows:
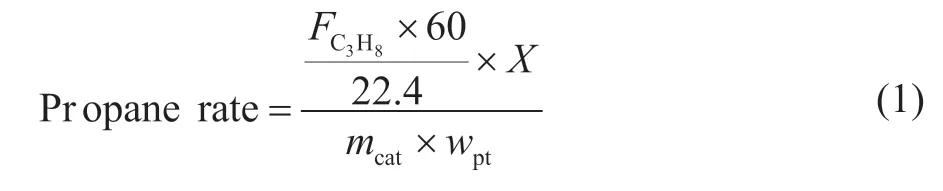
represents the flow rate of propane, X is the conversion of propane, mcatis the catalyst loading, and wPtis the Pt weight loading.

where [Fi]inand [Fi]outare the inlet and outlet flow rates of hydrocarbons, respectively.
A deactivation parameter (D) was used to measure the catalytic stability[30]:

where X0and Xfare the initial and final conversion rates of propane, respectively.
3 Results and Discussion
3.1 Properties of the as-prepared catalysts
Table 1 shows the physicochemical properties of the

Table 1 Physicochemical properties of the as-prepared catalysts
1) Determined by XRF.
2) Calculated from the results obtained on an Elementar Vario Micro Cube elemental analyser.
3) Estimated from the results of N2adsorption-desorption isotherms.
4) Calculated based on the results in Figure 4.
5) Calculated from the results of TG-MS profiles (Figure 5).
6) Estimated from the simulation results of the theoretical computation models in Figure 6.as-prepared catalysts. The content of P is approximately 1.4%, suggesting that the P component has been successfully implemented into the Pt/P-CNTs catalyst. The peaks located at ca. 133.0 eV and 134.8 eV are assigned to C-P-O and C-P-O[31-32], respectively, which is consistent with the above-mentioned result. The amount of O over Pt/CNTs is lower than that over Pt/P-CNTs, which may be caused by the fact that more oxygen-containing groups are introduced along with the doping of P. The content of Pt in Pt/CNTs is close to that in Pt/P-CNTs.After P implantation, the surface area and pore volume of the two catalysts are also similar, implying that P-modification exerts little influence on the texture of the CNTs.
The HRTEM images of Pt/CNTs and Pt/P-CNTs catalysts are shown in Figures 1 and 2. Judging for the fresh Pt/P-CNTs, the size distribution of Pt nanoparticles is in the range of 1.2—2.0 nm (inset of Figure 2b), which is smaller than that of the fresh Pt/CNTs (3.0 nm-4.0 nm,inset of Figure 1a). The inert surface of raw CNTs in this work and the low oxidizing temperature by the mixed acids (25 ºC) make it difficult to introduce more oxygencontaining groups in the CNT supports, further causing difficulty in anchoring Pt nanoparticles.
Figure 3 exhibits the XRD patterns of the as-prepared catalysts. Two major peaks located at 25.8º and 43.0º are indexed to the (002) and (101) reflections of graphite, which suggests that CNTs have a well-organized crystalline structure. The other signals appearing at 39.8º and 46.4º are attributed to the (111) and (200) reflections of Pt (JCPDS 87-0640). Compared with Pt/CNTs, the peak intensity of Pt for Pt/P-CNTs is much lower, which means that the P doping facilitates the dispersion of Pt nanoparticles. Upon combining the XRD and the HRTEM results, it can be safely concluded that the addition of P could facilitate the dispersion of Pt, which is in line with the previous reports[20-21].
3.2 Catalytic performance
Both Pt/P-CNTs and Pt/CNTs catalysts were applied in the PDH reaction, with the corresponding performance exhibited in Figure 4. It can be observed that there is a slight decrease in the propane rate (i.e., the reaction rate of propane)over Pt/P-CNTs during the PDH reaction, which reduces from the initial value of 124 mmol/(g·h), viz. approximately 2 times more than that over Pt/CNTs (68 mmol/(g·h)),to 87 mmol/(g·h). In comparison, however, the propane rate over Pt/CNTs decreases rapidly from 68 mmol/(g·h) to 22 mmol/(g·h). The deactivation parameter (D) is used to measure the catalytic stability, and a low D value suggests a high stability[30]. Hence, the catalytic stability over Pt/
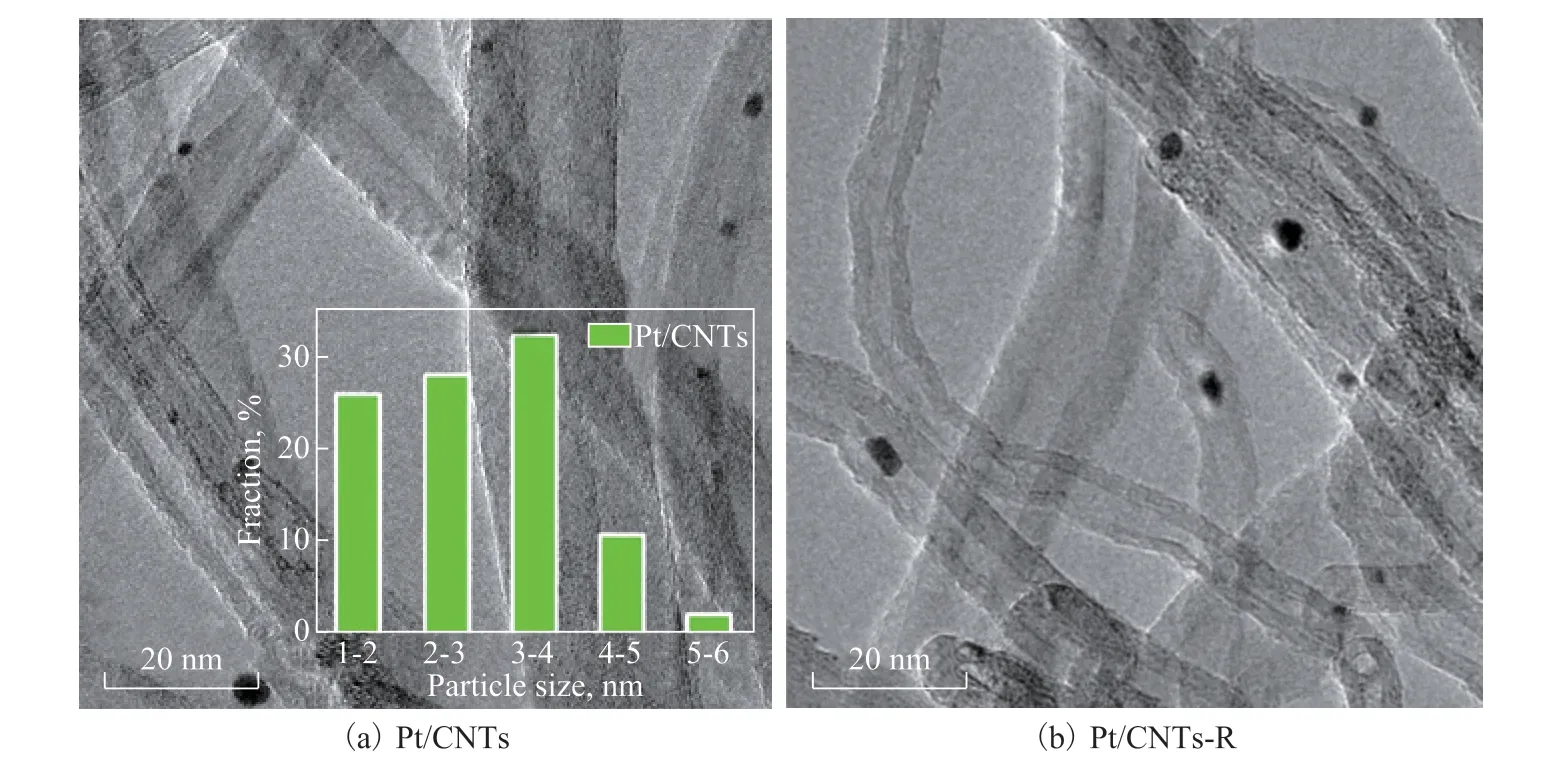
Figure 1 High-resolution transmission electron microscopy images of Pt/CNTs catalyst before and after PDH reaction(The inset is the Pt nanoparticle size distribution, and “-R” represents the spent catalyst.)

Figure 2 High-resolution transmission electron microscopy images of Pt/P-CNTs catalyst before and after PDH reaction: (a, b) Pt/P-CNTs; and (c, d) Pt/P-CNTs-R(The insets refer to the Pt nanoparticle size distribution, and “-R” represents the spent catalyst.)

Figure 3 X-ray diffraction patterns of the as-prepared catalysts: (a) Pt/CNTs; and (b) Pt/P-CNTs
P-CNTs is higher than that over Pt/CNTs (Table 1). Moreover, the propylene selectivity of Pt/P-CNTs is approximately 90%, which is higher by more than 15 percentage points than that of Pt/CNTs (approximately 75%). Generally, the P-modified CNT-supported Pt nanoparticles exhibit superior catalytic activity and stability.
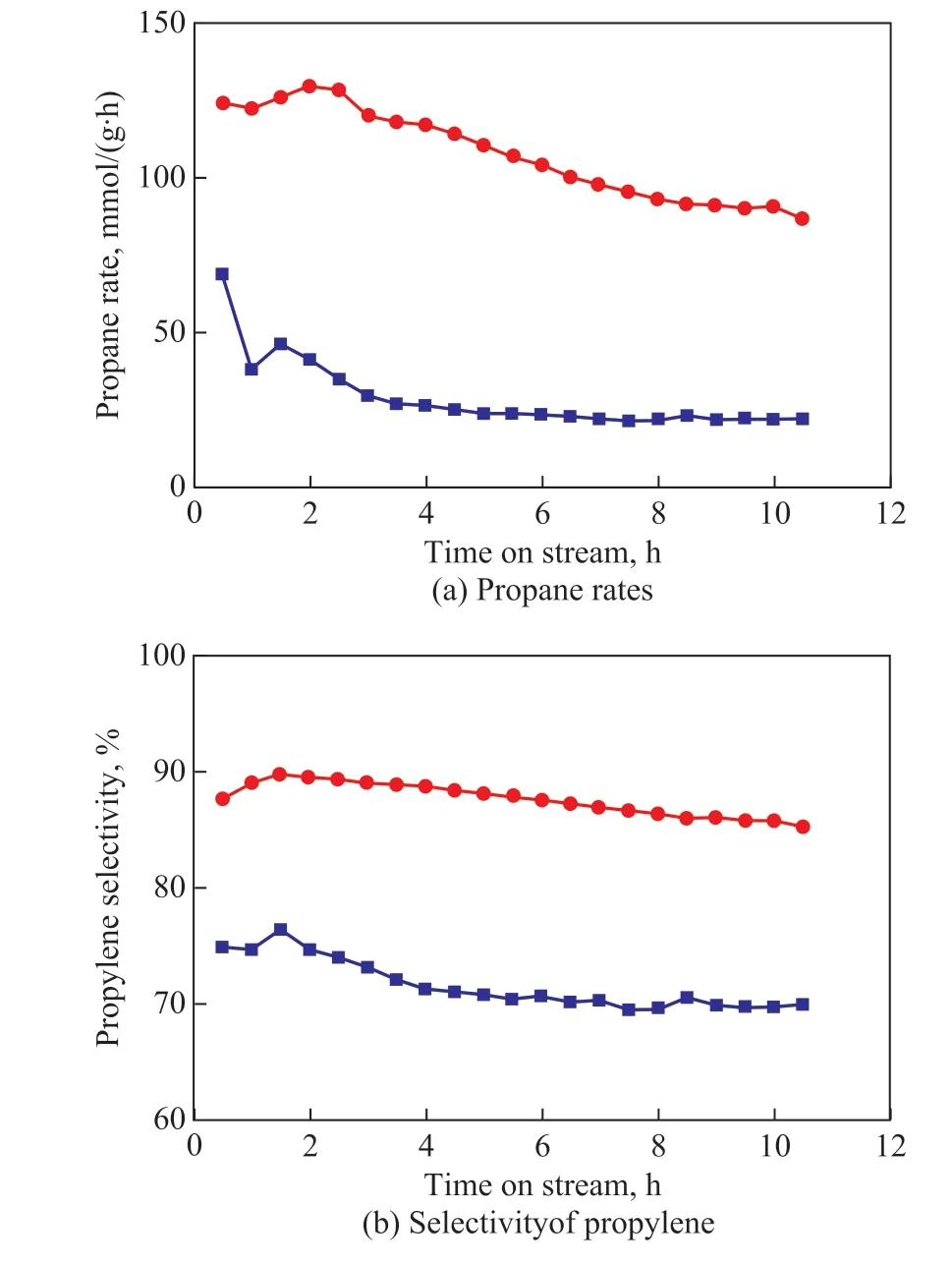
Figure 4 Catalytic activity of the as-prepared catalysts for propane dehydrogenation reaction■—Pt/P-CNTs; ●—Pt/CNTs
The smaller size of Pt nanoparticles for Pt/P-CNTs indicates larger Pt surface areas (Figure 2), which would enhance the accessibility of the active sites to the reactant and thus increase the propane rate. In addition, there is little change in the sizes of Pt nanoparticles over Pt/P-CNTs before and after the PDH reaction, for example, from 1.2 nm to 2.0 nm (Figure 2), suggesting that strong metal-support interactions (SMSIs) may exist between Pt nanoparticles and the P-CNTs support. In contrast, significant agglomeration of Pt nanoparticles over Pt/CNTs occurs after the PDH reaction (Figure 1), which would facilitate the structure-sensitive side-reactions such as hydrogenolysis and carbon deposition, thus causing coke deposition and finally deteriorating the propylene selectivity as well as the catalytic stability (Figure 4 and Table 1). Furthermore, the P doping can block the combustion sites in nanocarbon by inhibiting the formation of electrophilic oxygen species and increase the selectivity for the desired product during the oxidative dehydrogenation reaction. Liu, et al.[33-35]determined that direct dehydrogenation reactions followed the mechanism, which is similar to that of the oxidative dehydrogenation reactions. Hence, the propylene selectivity of Pt/P-CNTs is higher than that of Pt/CNTs.
3.3 Characterization of the catalysts
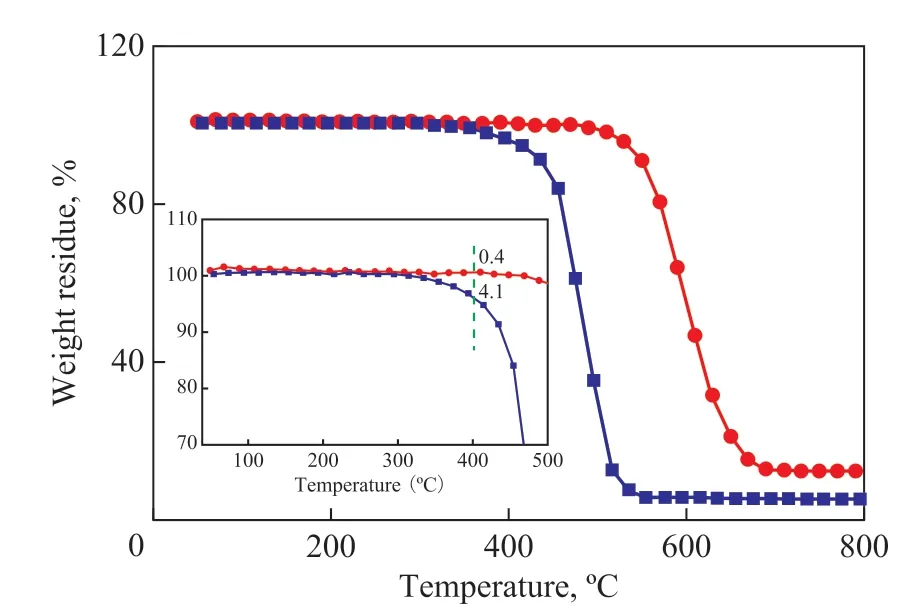
Figure 5 Thermogravimetric analysis-mass spectrometry profiles of the as-prepared catalysts■—Pt/P-CNTs; ●—Pt/CNTs
The amount of coke deposits was analysed via a TGMS method. Shi, et al.[36]thought that the combustion of coke occurring below 400 ºC was attributed to the coke covering the active metal sites, while that burnt above 400 ºC was assigned to the coke located on the surface of the support. It can be seen from Figure 5 that the amount of coke deposits over the Pt/P-CNTs catalyst (0.4%) is remarkably smaller than that over Pt/CNTs (4.1%, Table 1),which is consistent with the results of Figures 1, 2, and 4.To verify the SMSIs for Pt/P-CNTs, the MedeA-VASP software was employed to calculate the absorption energy of Pt nanoparticles over P-CNTs and CNTs supports, respectively, with the results exhibited in Figure 6 and Table 1. The absorption energy of Pt over Pt/P-CNTs is higher than that over Pt/CNTs, which is in agreement with the report made by Tong, et al.[19], indicating that Pt nanoparticles can interact strongly with the P-CNTs support. The binding energy of the Pt 4f electrons for Pt/P-CNTs shifts to a higher value as compared to that for Pt/CNTs (Figure 7), which is in good agreement with the literature information[28]. In addition, the devolution results of Pt 4f in Figure 7 and Table 2 show that the electrons of Pt nanoparticles on P-CNTs are more electrophobic than those of Pt nanoparticles on CNTs, which may be derived from the SMSIs between Pt and P-containing groups[37]. The XPS results may imply that the P doping has an effect on the electronic structure of Pt to enhance the electrical conductivity of the catalyst, which can lead to the SMSIs or even exert an influence on the catalytic performance. Additionally, Tong and colleagues considered that the SMSIs in Pt/P-CNTs might be derived from the hybridization between the hybrid orbitals of Pt and P[19].
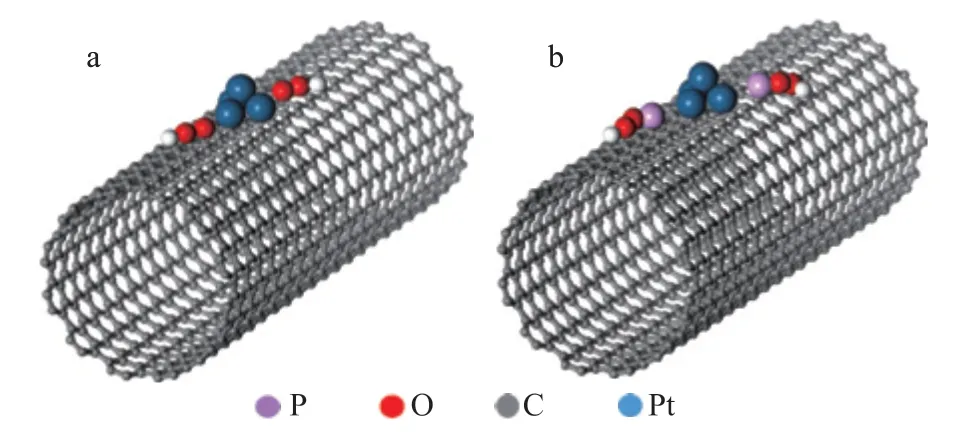
Figure 6 Theoretical computational model of (a) Pt/CNTs and (b) Pt/P-CNTs by MedeA-VASP software (in which the white balls stand for hydrogen atom)
The variations between the fresh and spent Pt/P-CNTs were determined by the XRF and XPS analyses. The content of P over Pt/P-CNTs is 1.4%, which is close to that of Pt/P-CNTs-R (1.1%), and the contents of Pt and O in Pt/P-CNTs are also similar to those in Pt/P-CNTs-R,suggesting that there is no obvious loss of the catalyst.In addition, there is little change in the locations of the binding energy for all the elements in the obtained XPS spectra (Figure 8a). Specifically, the locations and area percentages of Pt with different valence states are almost the same for the fresh and spent Pt/P-CNTs samples (Figure 7 and Table 2). The contents of P=O and C=O groups located at ca. 531.5 eV do not show much reduction after the reaction. Li, et al.[24]and Liu, et al.[34]considered that P=O and C=O groups were the active centres for the PDH reaction. Therefore, the Pt/P-CNTs catalyst exhibits higher stability in the high-temperature PDH reaction, which is consistent with the above-mentioned results.
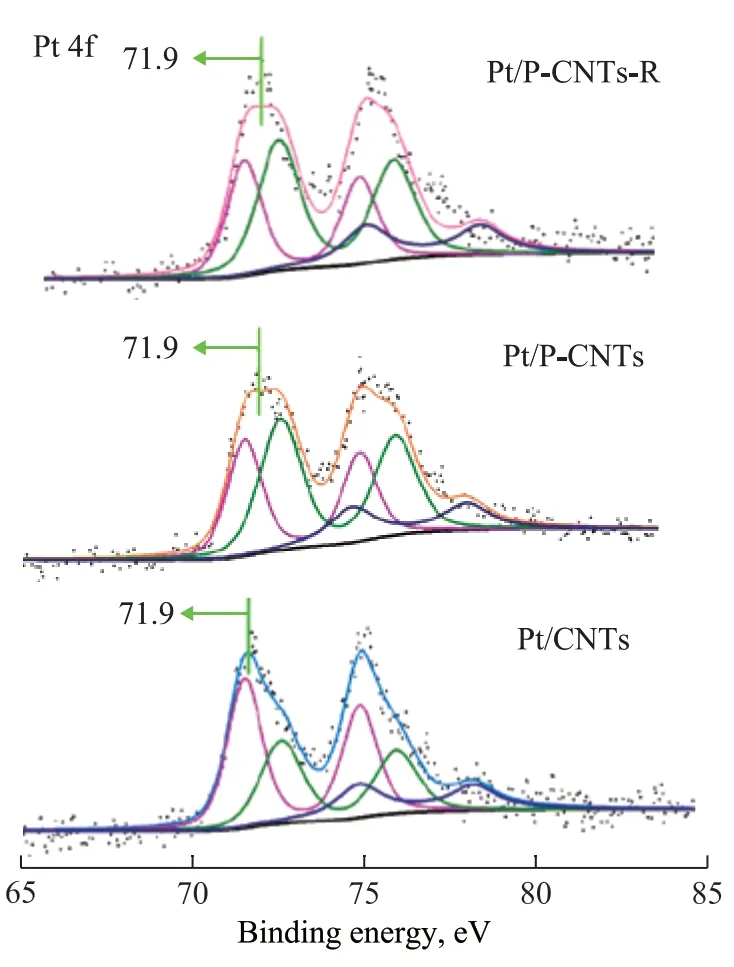
Figure 7 X-ray photoelectron spectra of Pt 4f electrons for Pt/CNTs and Pt/P-CNTs catalysts
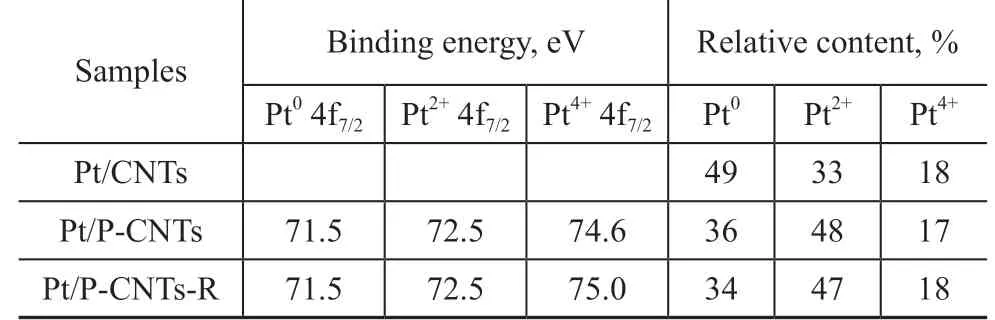
Table 2 Results of X-ray photoelectron spectra for the as-prepared catalysts
4 Conclusions
In general, P-doping in CNTs could effectively promote the dispersion of Pt nanoparticles, enhance the interactions between Pt and the P-CNTs support by improving the electron transfer or hybridization, stabilize the Pt nanoparticles from sintering, and finally improve the catalytic performance as well as the catalytic stability.
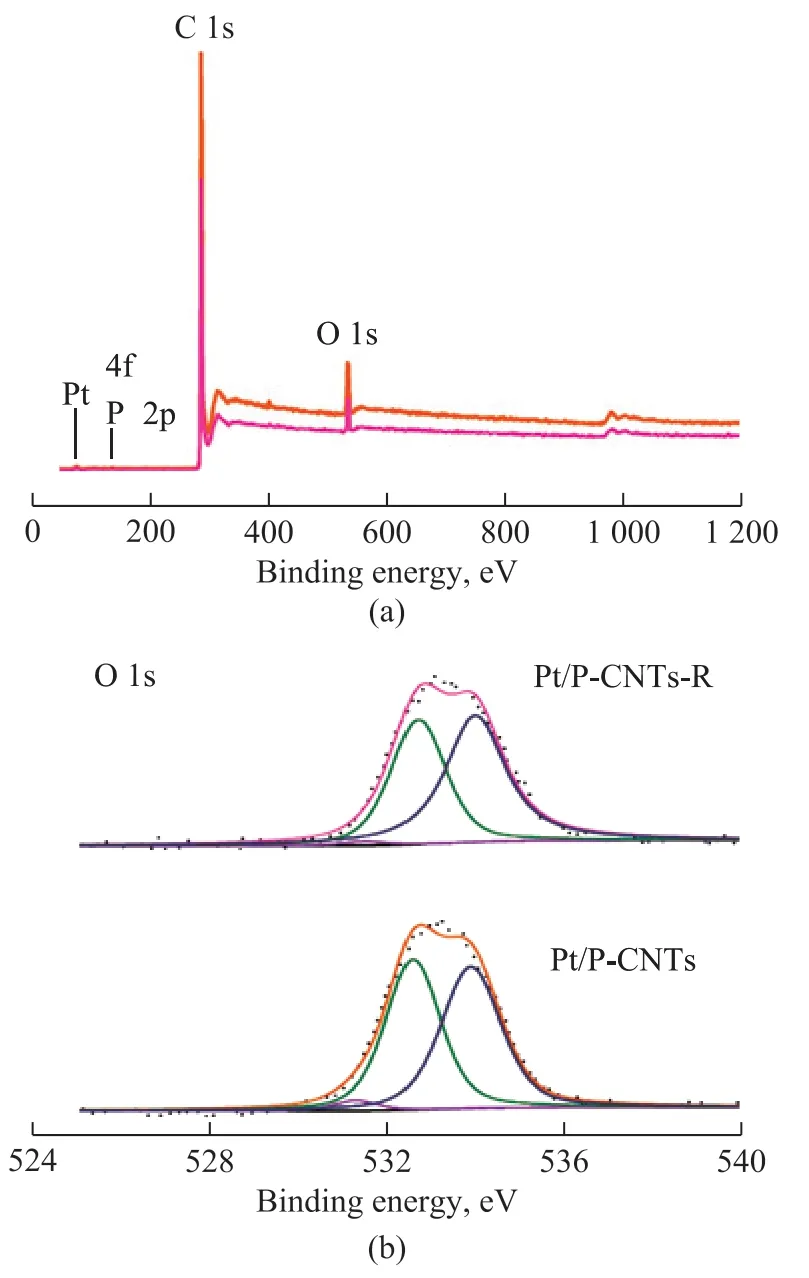
Figure 8 XPS spectra of the fresh and spent Pt/P-CNTs catalysts(a)Pt/CNTs;Pt/P-CNTs-R(“-R” represents the spent catalyst.)
This work provides a new route for preparing the carbon-supported catalysts that can work under harsh reaction conditions.
Acknowledgements:This work was supported by the National Natural Science Foundation of China (Grant 21706036), the State Key Laboratory of Catalytic Materials and Reaction Engineering (RIPP, SINOPEC), the Natural Science Foundation of Fujian Province (Grant 2018J05019). The authors are grateful for experimental help from Dai Zhenyu, Liu Hongyang, and Xie Jingxin.
杂志排行
中国炼油与石油化工的其它文章
- Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes
- Catalytic Cracking Characteristics of Plant Oil for Producing Light Olefins and Light Aromatics
- Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
- Novel NiMo Catalysts Supported on Sol-Gel Nanosized HY Zeolite-Alumina Composites for Hydrodesulfurization of Diesel
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis
